Europe Molecular Diagnostics Services Market
Tamanho do mercado em biliões de dólares
CAGR :
% 
 USD
38.02 Million
USD
67.31 Million
2024
2032
USD
38.02 Million
USD
67.31 Million
2024
2032
| 2025 –2032 | |
| USD 38.02 Million | |
| USD 67.31 Million | |
|
|
|
|
Segmentação do mercado de serviços de diagnóstico molecular na Europa, por tipo de serviço (serviços de reparo de instrumentos, serviços de treinamento, serviços de conformidade, serviços de calibração, serviços de manutenção, serviços de automação escalável, serviços turnkey, serviços de realocação de instrumentos, personalização de hardware, serviços de garantia de desempenho, serviços de design e desenvolvimento, soluções para cadeia de suprimentos, serviços de introdução de novos produtos, serviços de fabricação, serviços ambientais e regulatórios, certificação e auditoria de sistemas de gestão médica, serviços de pesquisa clínica, serviços de consultoria e outros serviços), tecnologia (PCR, PCR em tempo real, sequenciamento de última geração e outras tecnologias), usuário final (hospitais, centros de diagnóstico, instituições acadêmicas e de pesquisa e outros) - Tendências e previsões do setor até 2032
Tamanho do mercado de serviços de diagnóstico molecular na Europa
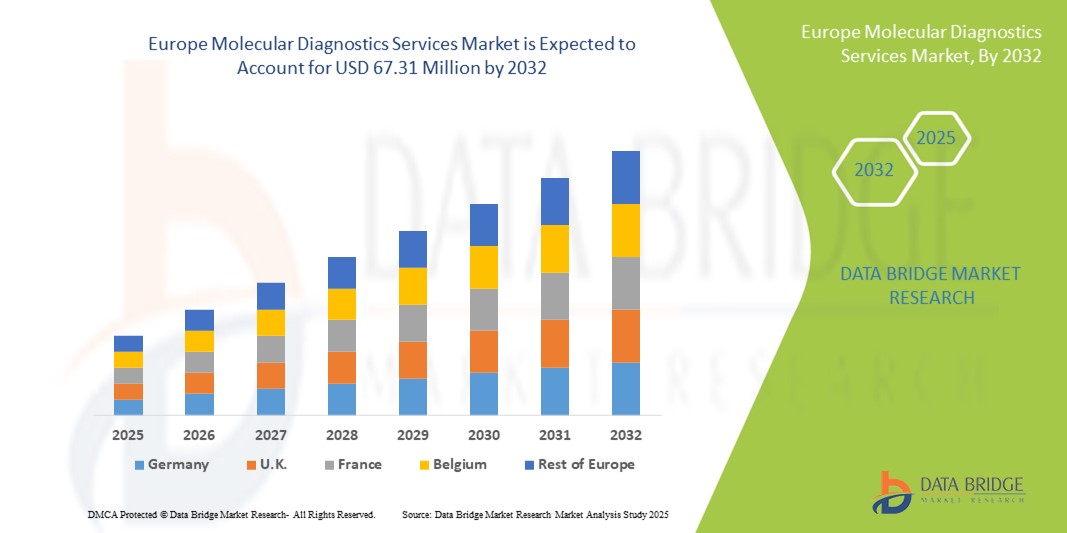
- O tamanho do mercado de serviços de diagnóstico molecular na Europa foi avaliado em US$ 38,02 milhões em 2024 e deve atingir US$ 67,31 milhões até 2032 , com um CAGR de 7,40% durante o período previsto.
- O crescimento do mercado é em grande parte impulsionado pela crescente prevalência de doenças crônicas e infecciosas , pela crescente adoção da medicina personalizada e pelos avanços tecnológicos em técnicas de diagnóstico molecular, como PCR , NGS e ensaios baseados em microarray.
- Além disso, a crescente demanda por detecção precoce de doenças, diagnósticos precisos e soluções de testes de alto rendimento em hospitais, laboratórios de diagnóstico e centros de pesquisa está posicionando os serviços de diagnóstico molecular como um componente crítico da saúde moderna. Esses fatores convergentes estão acelerando a adoção pelo mercado, impulsionando significativamente o crescimento do setor.
Análise de Mercado de Serviços de Diagnóstico Molecular na Europa
- Os serviços de diagnóstico molecular, que abrangem técnicas avançadas como PCR, PCR em tempo real e sequenciamento de última geração , estão se tornando cada vez mais essenciais no ecossistema de saúde da Europa devido ao seu papel na detecção precisa de doenças, aplicações de pesquisa e otimização do fluxo de trabalho laboratorial em hospitais, centros de diagnóstico e instituições acadêmicas.
- A crescente demanda por serviços de diagnóstico molecular é impulsionada principalmente pela crescente prevalência de doenças crônicas e infecciosas, pela crescente adoção da medicina de precisão e pelos avanços tecnológicos que permitem testes mais rápidos, confiáveis e econômicos.
- A Alemanha dominou o mercado de serviços de diagnóstico molecular com a maior participação na receita de 39,6% em 2024, apoiada por infraestrutura avançada de saúde, alta adoção de tecnologias moleculares e forte presença de provedores de serviços líderes, com adoção significativa em hospitais, centros de diagnóstico e instituições de pesquisa
- Espera-se que a Polônia seja o país com crescimento mais rápido no mercado de serviços de diagnóstico molecular durante o período previsto devido ao aumento dos investimentos em saúde, à expansão das capacidades laboratoriais e à crescente demanda por serviços abrangentes, como manutenção, calibração e soluções completas.
- O segmento de Serviços de Manutenção dominou o mercado de serviços de diagnóstico molecular em 2024, com uma participação de mercado de 29,7%, impulsionado pela crescente necessidade de operações laboratoriais ininterruptas, alta confiabilidade de instrumentos de diagnóstico molecular e soluções de serviço econômicas em ambientes clínicos e de pesquisa.
Escopo do Relatório e Segmentação do Mercado de Serviços de Diagnóstico Molecular na Europa
|
Atributos |
Principais insights de mercado da Europe Molecular Diagnostics Services |
|
Segmentos abrangidos |
|
|
Países abrangidos |
Europa
|
|
Principais participantes do mercado |
|
|
Oportunidades de mercado |
|
|
Conjuntos de informações de dados de valor agregado |
Além dos insights sobre cenários de mercado, como valor de mercado, taxa de crescimento, segmentação, cobertura geográfica e principais participantes, os relatórios de mercado selecionados pela Data Bridge Market Research também incluem análises aprofundadas de especialistas, análises de preços, análises de participação de marca, pesquisas com consumidores, análises demográficas, análises da cadeia de suprimentos, análises da cadeia de valor, visão geral de matérias-primas/consumíveis, critérios de seleção de fornecedores, análise PESTLE, análise de Porter e estrutura regulatória. |
Tendências do mercado de serviços de diagnóstico molecular na Europa
Automação Avançada e Soluções Integradas de Laboratório
- Uma tendência significativa e crescente no mercado europeu de serviços de diagnóstico molecular é a crescente adoção de soluções laboratoriais automatizadas e integradas que melhoram a eficiência do fluxo de trabalho e reduzem os erros manuais.
- Por exemplo, plataformas de automação escaláveis permitem o manuseio simultâneo de múltiplas amostras, melhorando o rendimento e mantendo a precisão nos ensaios de PCR, PCR em tempo real e NGS.
- A integração de sistemas de gestão de laboratório controlados por software com instrumentos de diagnóstico permite o monitoramento em tempo real do desempenho do instrumento e do rastreamento de amostras, reduzindo gargalos operacionais
- Essa automação também permite a manutenção preditiva e a detecção de erros, garantindo a prestação ininterrupta de serviços e minimizando o tempo de inatividade do laboratório.
- Prestadores de serviços como a Eurofins e a SGS estão a desenvolver soluções completas que combinam automação com serviços de consultoria e formação, melhorando a eficiência e a conformidade dos laboratórios.
- A tendência para laboratórios de diagnóstico molecular inteligentes, totalmente integrados e automatizados está remodelando os padrões operacionais, impulsionando a demanda por pacotes de serviços abrangentes em hospitais e centros de diagnóstico
Dinâmica do mercado de serviços de diagnóstico molecular na Europa
Motorista
Aumento da demanda devido à crescente carga de doenças e à medicina personalizada
- A crescente prevalência de doenças crônicas e infecciosas, juntamente com a crescente adoção da medicina personalizada, é um fator significativo para o aumento da demanda por serviços de diagnóstico molecular.
- Por exemplo, os hospitais e os centros de diagnóstico dependem cada vez mais dos testes baseados em PCR e NGS para a detecção precoce de doenças genéticas e agentes patogénicos infeciosos.
- A crescente conscientização entre profissionais de saúde e pacientes sobre diagnósticos de precisão está alimentando a demanda por soluções de teste mais rápidas, precisas e confiáveis
- A expansão da infraestrutura de saúde, especialmente em países como a Alemanha, está permitindo uma maior disponibilidade de serviços de diagnóstico molecular em ambientes clínicos e de pesquisa
- Ofertas de serviços abrangentes, incluindo manutenção, calibração e treinamento, aumentam a produtividade do laboratório e a adoção de tecnologias avançadas de diagnóstico
- A necessidade de soluções de diagnóstico molecular rápidas, precisas e escaláveis está obrigando os provedores de saúde a integrar esses serviços como prática padrão em protocolos de gerenciamento de doenças
Restrição/Desafio
Altos custos e desafios de conformidade regulatória
- As preocupações com os altos custos operacionais e de serviço representam um desafio significativo para uma penetração mais ampla no mercado, especialmente para laboratórios de pequeno e médio porte
- Por exemplo, plataformas de automação avançadas e serviços baseados em NGS exigem um investimento inicial significativo, limitando a adoção em instalações com restrições orçamentais.
- Os rigorosos requisitos regulamentares e de conformidade de qualidade na Europa exigem documentação, validação e certificação robustas, aumentando a complexidade operacional
- Enfrentar os desafios de conformidade e, ao mesmo tempo, garantir a prestação de serviços de alta qualidade é fundamental para ganhar a confiança dos laboratórios e das instituições
- A variabilidade nos preços dos serviços e a disponibilidade limitada de pessoal qualificado para procedimentos complexos de diagnóstico molecular podem restringir o crescimento do mercado em algumas regiões
- Superar esses desafios por meio da otimização de custos, suporte regulatório e programas de treinamento para a equipe de laboratório será vital para a adoção sustentada e expansão do mercado.
Escopo do mercado de serviços de diagnóstico molecular na Europa
O mercado é segmentado com base no tipo de serviço, tecnologia e usuário final.
- Por tipo de serviço
Com base no tipo de serviço, o mercado europeu de serviços de diagnóstico molecular é segmentado em Serviços de Reparo de Instrumentos, Serviços de Treinamento, Serviços de Conformidade, Serviços de Calibração, serviços de manutenção, serviços de automação escalável, serviços turnkey, serviços de realocação de instrumentos, personalização de hardware, serviços de garantia de desempenho, serviços de design e desenvolvimento, soluções para a cadeia de suprimentos, serviços de introdução de novos produtos, serviços de fabricação, serviços ambientais e regulatórios, certificação e auditoria de sistemas de gestão médica, serviços de pesquisa clínica, serviços de consultoria e outros serviços. O segmento de Serviços de Manutenção dominou o mercado com a maior participação na receita de mercado de 29,7% em 2024, impulsionado pela crescente necessidade de garantir operações laboratoriais ininterruptas. Hospitais, centros de diagnóstico e instituições de pesquisa priorizam os serviços de manutenção para evitar paradas dispendiosas e garantir alta confiabilidade dos instrumentos de diagnóstico molecular. A demanda é ainda suportada por fluxos de trabalho complexos em plataformas de PCR, PCR em Tempo Real e NGS, onde o monitoramento contínuo do desempenho é crítico. Os provedores de serviços também combinam manutenção com serviços de calibração e treinamento, aprimorando a proposta de valor geral. O aumento dos investimentos em infraestrutura laboratorial na Alemanha e em outros mercados maduros fortalece o domínio dos serviços de manutenção. Soluções de manutenção preventiva e de rotina são cada vez mais preferidas para eficiência de custos e conformidade regulatória.
O segmento de Serviços Turnkey deverá apresentar a maior taxa de crescimento, de 24%, entre 2025 e 2032, impulsionado pela crescente adoção em mercados emergentes como a Polônia. Os serviços turnkey oferecem soluções completas, incluindo instalação, design de fluxo de trabalho e treinamento, reduzindo a sobrecarga dos laboratórios na gestão de processos complexos de implementação. Esses serviços são particularmente atraentes para hospitais e centros de diagnóstico que estão adotando novas tecnologias moleculares pela primeira vez. A flexibilidade, a eficiência e a redução do risco operacional oferecidos pelos serviços turnkey estão impulsionando a rápida adoção. A crescente preferência por soluções abrangentes e prontas para uso acelera o crescimento em laboratórios clínicos e de pesquisa.
- Por Tecnologia
Com base na tecnologia, o mercado europeu de serviços de diagnóstico molecular é segmentado em PCR, PCR em tempo real, sequenciamento de nova geração (NGS) e outras tecnologias. O segmento de PCR dominou o mercado com a maior participação na receita, de 35% em 2024, devido ao seu amplo uso para detecção de doenças infecciosas de rotina, testes genéticos e aplicações de pesquisa. A PCR é altamente confiável, econômica e apoiada por protocolos de serviço estabelecidos para calibração, manutenção e reparo. Muitos centros de diagnóstico e hospitais dependem de ensaios baseados em PCR por seus rápidos tempos de resposta e precisão, tornando-os a espinha dorsal do diagnóstico molecular na Europa. A Alemanha e outros países da Europa Ocidental apresentam forte adoção devido à infraestrutura avançada de saúde e redes laboratoriais estabelecidas. A integração com serviços de manutenção, calibração e automação reforça ainda mais a posição dominante da PCR. A ampla familiaridade clínica com fluxos de trabalho de PCR garante demanda contínua entre os usuários finais.
Espera-se que o segmento de Sequenciamento de Nova Geração (NGS) apresente o CAGR mais rápido, de 23% a 25%, entre 2025 e 2032, impulsionado pela crescente adoção em medicina personalizada, testes oncológicos e aplicações de pesquisa. O NGS permite o sequenciamento de alto rendimento, fornecendo insights genéticos abrangentes para diagnóstico de doenças e seleção de terapias. O crescimento é sustentado por crescentes investimentos em treinamento, consultoria e serviços completos para auxiliar laboratórios na implementação de fluxos de trabalho de NGS. A Polônia e outros mercados europeus emergentes estão adotando cada vez mais o NGS devido à crescente conscientização sobre os benefícios da medicina de precisão. Aprimoramentos tecnológicos contínuos e custos decrescentes de sequenciamento estão acelerando a adoção. O NGS está se tornando uma tecnologia preferencial para diagnósticos complexos que exigem informações genômicas abrangentes.
- Por usuário final
Com base no usuário final, o mercado europeu de serviços de diagnóstico molecular é segmentado em hospitais, centros de diagnóstico, instituições acadêmicas e de pesquisa, entre outros. O segmento de Hospitais dominou o mercado, com a maior participação na receita, de 40% em 2024, impulsionado por seus requisitos de diagnóstico de alto volume e pela demanda por testes moleculares confiáveis. Os hospitais exigem ofertas de serviços abrangentes, incluindo manutenção, calibração, treinamento e soluções completas para garantir operações laboratoriais precisas e ininterruptas. A Alemanha e outros mercados europeus maduros possuem redes hospitalares bem estabelecidas que adotam tecnologias avançadas de diagnóstico molecular, como PCR, PCR em Tempo Real e NGS. A integração dos serviços com os sistemas de gestão de laboratórios hospitalares aumenta a eficiência do fluxo de trabalho e reduz o tempo de inatividade operacional. A necessidade dos hospitais de cumprir os padrões regulatórios reforça ainda mais a demanda por soluções completas. O crescente investimento em laboratórios modernos de diagnóstico molecular mantém os hospitais como o segmento dominante de usuários finais.
Prevê-se que o segmento de Instituições Acadêmicas e de Pesquisa (Acadêmicas & Research Institutions) apresente a maior taxa de crescimento, de 23%, entre 2025 e 2032, impulsionado pela expansão das atividades de pesquisa e do financiamento para genômica, doenças infecciosas e pesquisa translacional. As instituições de pesquisa adotam cada vez mais automação escalável, NGS e serviços turnkey para acelerar os cronogramas de estudo e melhorar a confiabilidade dos dados. Mercados emergentes, como a Polônia, estão investindo em infraestrutura de pesquisa avançada, criando alta demanda por serviços de diagnóstico molecular. As instituições acadêmicas se beneficiam de provedores de serviços que oferecem treinamento, consultoria e serviços de design e desenvolvimento para aplicações especializadas. O crescimento é ainda apoiado por iniciativas de pesquisa colaborativa entre universidades e hospitais, promovendo a adoção de tecnologias moleculares de ponta.
Análise regional do mercado de serviços de diagnóstico molecular na Europa
- A Alemanha dominou o mercado de serviços de diagnóstico molecular com a maior participação na receita de 39,6% em 2024, apoiada por infraestrutura avançada de saúde, alta adoção de tecnologias moleculares e forte presença de provedores de serviços líderes, com adoção significativa em hospitais, centros de diagnóstico e instituições de pesquisa
- Hospitais, centros de diagnóstico e instituições de pesquisa na Alemanha priorizam precisão, confiabilidade e ofertas de serviços abrangentes, incluindo manutenção, calibração, treinamento e soluções completas para plataformas de PCR, PCR em tempo real e NGS.
- Essa ampla adoção é ainda apoiada por altos gastos com saúde, fortes estruturas de conformidade regulatória e crescentes investimentos em modernização de laboratórios, estabelecendo serviços de diagnóstico molecular como um componente crítico das operações clínicas e de pesquisa em todo o país.
Visão do mercado de serviços de diagnóstico molecular na Alemanha
O mercado de serviços de diagnóstico molecular da Alemanha dominou o mercado europeu, com a maior participação na receita de 39,6% em 2024, impulsionado por infraestrutura avançada em saúde, alta adoção de tecnologias moleculares como PCR, PCR em Tempo Real e NGS, e crescente demanda por diagnósticos de precisão. Hospitais, centros de diagnóstico e instituições de pesquisa priorizam testes confiáveis, precisos e de alto rendimento, apoiados por serviços abrangentes, incluindo manutenção, calibração, treinamento e soluções completas. O foco do país em inovação, conformidade regulatória e modernização laboratorial fortalece a adoção, enquanto a presença de provedores de serviços líderes garante amplo acesso a tecnologias de ponta em diagnóstico molecular. O domínio da Alemanha reflete sua maturidade tecnológica, redes laboratoriais bem estabelecidas e forte investimento em infraestrutura de saúde e pesquisa, tornando-a um polo importante para serviços de diagnóstico molecular na Europa.
Visão do mercado de serviços de diagnóstico molecular na Polônia
Espera-se que o mercado de serviços de diagnóstico molecular da Polônia seja o de crescimento mais rápido na Europa durante o período previsto, impulsionado pelo aumento dos investimentos em saúde, pela expansão das capacidades laboratoriais e pela crescente adoção de tecnologias avançadas de diagnóstico molecular. Hospitais, centros de diagnóstico e instituições de pesquisa dependem cada vez mais de serviços como manutenção, calibração, soluções completas e treinamento para implementar plataformas de PCR, PCR em Tempo Real e NGS de forma eficaz. O crescimento é sustentado pela modernização da infraestrutura laboratorial, pelo aumento da conscientização sobre medicina de precisão e pela melhoria da conformidade com as normas regulatórias europeias. Os centros emergentes de saúde e pesquisa da Polônia estão acelerando a adoção de serviços de diagnóstico molecular, tornando-se um mercado em rápida expansão na Europa.
Visão do mercado de serviços de diagnóstico molecular na França
O mercado francês de serviços de diagnóstico molecular apresenta crescimento constante, apoiado por uma infraestrutura de saúde de alta qualidade, pela crescente adoção de tecnologias moleculares e pelo foco na detecção precoce de doenças. Hospitais e centros de diagnóstico na França estão cada vez mais integrando serviços como manutenção, calibração, treinamento e suporte consultivo para melhorar a eficiência laboratorial e garantir resultados precisos. A ênfase do país em pesquisa, conformidade regulatória e acreditação laboratorial está impulsionando a demanda por prestadores de serviços profissionais. A França também está adotando soluções inovadoras, como serviços de automação turnkey e escaláveis, para otimizar fluxos de trabalho e reduzir os desafios operacionais. A crescente conscientização sobre medicina de precisão e gestão de doenças crônicas reforça ainda mais a adoção. O mercado se beneficia de fortes iniciativas governamentais que promovem a modernização da saúde e a padronização laboratorial.
Visão do mercado de serviços de diagnóstico molecular do Reino Unido
Espera-se que o mercado de serviços de diagnóstico molecular do Reino Unido cresça a um CAGR notável durante o período previsto, impulsionado pelo aumento da prevalência de doenças infecciosas e genéticas, pela crescente adoção de tecnologias moleculares avançadas e pela demanda por soluções diagnósticas precisas. Hospitais, centros de diagnóstico e instituições de pesquisa estão investindo em serviços abrangentes, incluindo manutenção, calibração, treinamento e soluções completas, para aumentar a produtividade dos laboratórios. O robusto sistema de saúde, as iniciativas de pesquisa e as estruturas regulatórias do Reino Unido impulsionam a adoção pelo mercado. A integração do diagnóstico molecular com programas de medicina de precisão e projetos de pesquisa acadêmica acelera ainda mais o crescimento. A crescente demanda por automação, otimização do fluxo de trabalho e suporte consultivo fortalece a posição dos provedores de serviços. O mercado também é sustentado pela crescente conscientização entre médicos e pacientes sobre a detecção precoce de doenças e abordagens de tratamento personalizadas.
Participação no mercado de serviços de diagnóstico molecular na Europa
O setor de serviços de diagnóstico molecular da Europa é liderado principalmente por empresas bem estabelecidas, incluindo:
- F. Hoffmann-La Roche Ltd (EUA)
- QIAGEN (Alemanha)
- BIOMÉRIEUX (França)
- Illumina, Inc. (EUA)
- Thermo Fisher Scientific Inc. (EUA)
- Agilent Technologies, Inc. (EUA)
- Danaher Corporation (EUA)
- Sysmex Corporation (Japão)
- PerkinElmer (EUA)
- Hologic, Inc. (EUA)
- Fujirebio Europe NV (Bélgica)
- Euroimmun Medizinische Labordiagnostika AG (Alemanha)
- Centogene AG (Alemanha)
- GeneProof sro (República Tcheca)
- altona Diagnostics GmbH (Alemanha)
- Seegene Inc. (Coreia do Sul)
- Aidian Oy (Finlândia)
- Bioeksen Biotech (Turquia)
- LRE Medical GmbH (Alemanha)
Quais são os desenvolvimentos recentes no mercado europeu de serviços de diagnóstico molecular?
- Em novembro de 2024, a Menarini Diagnostics e a Nucleix firmaram um acordo comercial de longo prazo para a distribuição exclusiva do teste EpiCheck para a bexiga na Europa. Este teste de urina não invasivo, com marcação CE, detecta cânceres primários ou recorrentes de bexiga e do trato urinário superior, com o objetivo de transformar o atendimento a pacientes com câncer de bexiga na Europa.
- Em outubro de 2024, a Seegene e a Werfen firmaram um acordo de parceria para compartilhar tecnologia em diagnóstico molecular. Esta colaboração se concentra na integração das tecnologias de diagnóstico da Seegene com os sistemas de automação laboratorial da Werfen, visando aumentar a eficiência diagnóstica e expandir o alcance dos testes moleculares na Europa.
- Em outubro de 2024, a Alveo Technologies iniciou o envio do seu primeiro teste molecular pontual para influenza aviária em aves para a UE. O Painel de Influenza Aviária Alveo Sense fornece resultados rápidos e precisos em campo, apoiando a detecção precoce e medidas de controle na indústria avícola.
- Em julho de 2024, a Roche anunciou a aquisição da tecnologia Point-of-Care da LumiraDx, aprimorando seu portfólio de diagnósticos com uma plataforma multiensaio. Essa aquisição visa expandir o acesso a testes diagnósticos em ambientes de atenção primária e apoia a ambição da Roche de fornecer soluções descentralizadas, incluindo um teste molecular rápido para tuberculose no local de atendimento, em colaboração com a Fundação Bill e Melinda Gates.
- Em julho de 2024, a AB ANALITICA e a SNIBE anunciaram uma parceria de distribuição na Itália para a plataforma Molecision R8 da SNIBE. Esta colaboração visa aumentar a disponibilidade de soluções avançadas de diagnóstico molecular no mercado italiano, apoiando os profissionais de saúde com tecnologias de teste inovadoras.
SKU-
Obtenha acesso online ao relatório sobre a primeira nuvem de inteligência de mercado do mundo
- Painel interativo de análise de dados
- Painel de análise da empresa para oportunidades de elevado potencial de crescimento
- Acesso de analista de pesquisa para personalização e customização. consultas
- Análise da concorrência com painel interativo
- Últimas notícias, atualizações e atualizações Análise de tendências
- Aproveite o poder da análise de benchmark para um rastreio abrangente da concorrência
Metodologia de Investigação
A recolha de dados e a análise do ano base são feitas através de módulos de recolha de dados com amostras grandes. A etapa inclui a obtenção de informações de mercado ou dados relacionados através de diversas fontes e estratégias. Inclui examinar e planear antecipadamente todos os dados adquiridos no passado. Da mesma forma, envolve o exame de inconsistências de informação observadas em diferentes fontes de informação. Os dados de mercado são analisados e estimados utilizando modelos estatísticos e coerentes de mercado. Além disso, a análise da quota de mercado e a análise das principais tendências são os principais fatores de sucesso no relatório de mercado. Para saber mais, solicite uma chamada de analista ou abra a sua consulta.
A principal metodologia de investigação utilizada pela equipa de investigação do DBMR é a triangulação de dados que envolve a mineração de dados, a análise do impacto das variáveis de dados no mercado e a validação primária (especialista do setor). Os modelos de dados incluem grelha de posicionamento de fornecedores, análise da linha de tempo do mercado, visão geral e guia de mercado, grelha de posicionamento da empresa, análise de patentes, análise de preços, análise da quota de mercado da empresa, normas de medição, análise global versus regional e de participação dos fornecedores. Para saber mais sobre a metodologia de investigação, faça uma consulta para falar com os nossos especialistas do setor.
Personalização disponível
A Data Bridge Market Research é líder em investigação formativa avançada. Orgulhamo-nos de servir os nossos clientes novos e existentes com dados e análises que correspondem e atendem aos seus objetivos. O relatório pode ser personalizado para incluir análise de tendências de preços de marcas-alvo, compreensão do mercado para países adicionais (solicite a lista de países), dados de resultados de ensaios clínicos, revisão de literatura, mercado remodelado e análise de base de produtos . A análise de mercado dos concorrentes-alvo pode ser analisada desde análises baseadas em tecnologia até estratégias de carteira de mercado. Podemos adicionar quantos concorrentes necessitar de dados no formato e estilo de dados que procura. A nossa equipa de analistas também pode fornecer dados em tabelas dinâmicas de ficheiros Excel em bruto (livro de factos) ou pode ajudá-lo a criar apresentações a partir dos conjuntos de dados disponíveis no relatório.