Europe Medical Device Regulatory Affairs Outsourcing Market
Tamanho do mercado em biliões de dólares
CAGR :
% 
 USD
8.31 Billion
USD
21.78 Billion
2025
2033
USD
8.31 Billion
USD
21.78 Billion
2025
2033
| 2026 –2033 | |
| USD 8.31 Billion | |
| USD 21.78 Billion | |
|
|
|
|
Europe Medical Device Regulatory Affairs Outsourcing Market, By Services (Regulatory Affairs Services, Quality Consulting and Medical Writing), Product (Finished Goods, Electronics and Raw Material), Device Type (Class I, Class II and Class III), Application (Cardiology, Diagnostic Imaging, Orthopedic, IVD, Ophthalmic, General and Plastic Surgery, Drug Delivery, Dental, Endoscopy, Diabetes Care and Others), End User (Small Medical Device Company, Medium Medical Device Company and Large Medical Device Company), Country (Germany, U.K., France, Italy, Spain, Netherlands, Russia, Switzerland, Belgium, Turkey, Ireland, Rest of Europe,) Industry Trends and Forecast to 2028
 Market Analysis and Insights: Europe Medical Device Regulatory Affairs Outsourcing Market
Market Analysis and Insights: Europe Medical Device Regulatory Affairs Outsourcing Market
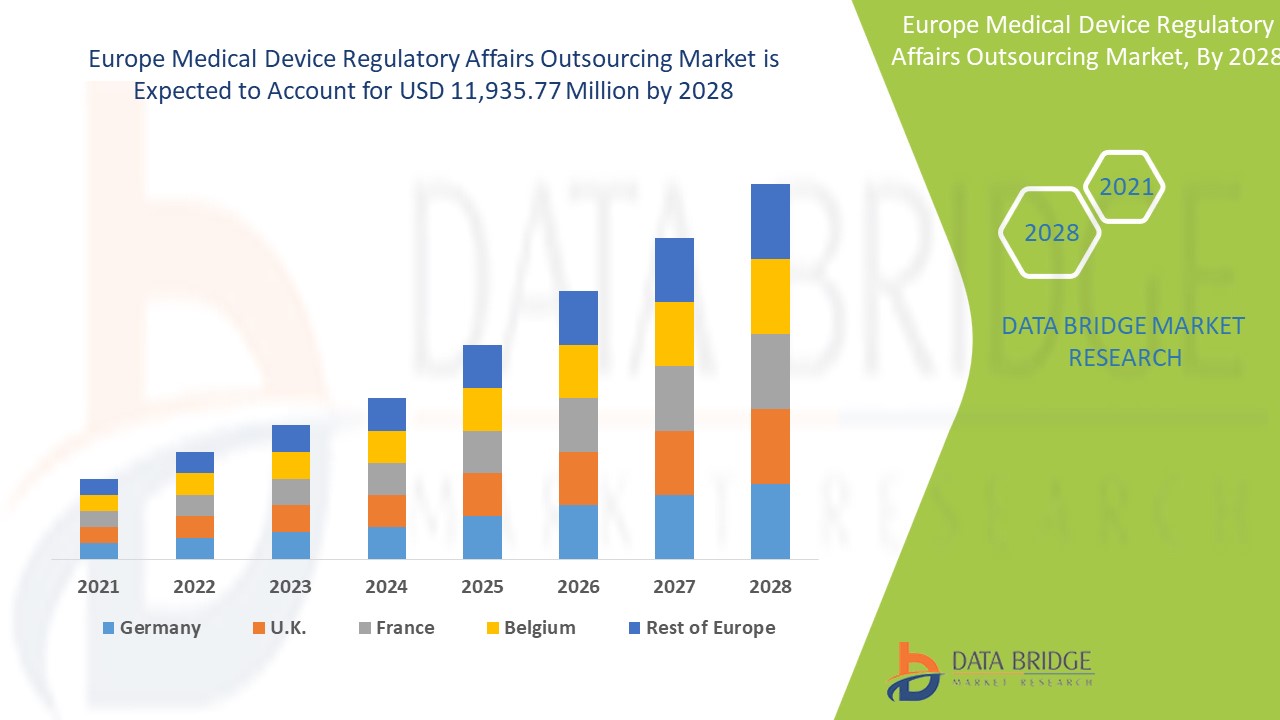
The medical device regulatory affairs outsourcing market is expected to gain market growth in the forecast period of 2021 to 2028. Data Bridge Market Research analyses that the market is growing with a CAGR of 12.8% in the forecast period of 2021 to 2028 and is expected to reach USD 11,935.77 million by 2028. The strategic initiative for geographical expansions is anticipated to drive the growth of the medical device regulatory affairs outsourcing market
Outsourcing is an important part of every pharmaceutical and biotechnology companies’ value chain during research and development (R&D). The regulatory affairs outsourcing services entail medical writing and publication of regulatory documentation by professional medical authors, quality control (QC) auditors and publishers who contribute to high-quality clinical research projects. The demand for regulatory services outsourcing has been fueled by a substantial increase in clinical studies conducted in emerging economies, providing a healthy platform for this industry's growth.
The increasing number of patent expirations acts as driver for its growth in the medical device regulatory affairs outsourcing market. The fluctuation in the prices of various medical devices regulatory affairs services acts as restraint for its growth in the medical device regulatory affairs outsourcing market. The awards and recognition provides excellent opportunity for the medical device regulatory affairs outsourcing market growth. The pandemic outbreak of COVID-19 acts as challenge for the growth of the medical device regulatory affairs outsourcing market.
The medical device regulatory affairs outsourcing market report provides details of market share, new developments, and product pipeline analysis, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, product approvals, strategic decisions, product launches, geographic expansions, and technological innovations in the market. To understand the analysis and the medical device regulatory affairs outsourcing market scenario contact Data Bridge Market Research for an Analyst Brief, our team will help you create a revenue impact solution to achieve your desired goal.
 Medical Device Regulatory Affairs Outsourcing Market Scope and Market Size
Medical Device Regulatory Affairs Outsourcing Market Scope and Market Size
The medical device regulatory affairs outsourcing market is segmented on the based on the basis of services, product, device type, application and end user. The growth among segments helps you analyze niche pockets of growth and strategies to approach the market and determine your core application areas and the difference in your target markets.
- On the basis of services, the medical device regulatory affairs outsourcing market is segmented into regulatory affairs services, quality consulting and medical writing. In 2021, the regulatory affairs services segment is expected to dominate the medical device regulatory affairs outsourcing market because of the increased adoption of regulatory affairs outsourcing by key medical device companies.
- On the basis of product, the medical device regulatory affairs outsourcing market is segmented into finished goods, electronics and raw material. In 2021, the finished goods segment is expected to dominate the medical device regulatory affairs outsourcing market due to the increased adoption of regulatory affairs outsourcing for the finished goods by major medical device companies.
- On the basis of device type, the medical device regulatory affairs outsourcing market is segmented into class I, class II and class III. In 2021, the class I segment is expected to dominate the medical device regulatory affairs outsourcing market because of the rising demand for medical devices worldwide to treat patients with chronic diseases.
- On the basis of application, the medical device regulatory affairs outsourcing market is segmented into cardiology, diagnostic imaging, orthopedic, IVD, ophthalmic, general and plastic surgery, drug delivery, dental, endoscopy, diabetes care and others. In 2021, the cardiology segment is expected to dominate the medical device regulatory affairs outsourcing market because of the increased adoption of regulatory affairs outsourcing for the class III medical devices by key medical device companies.
- On the basis of end user, the medical device regulatory affairs outsourcing market is segmented into small medical device company, medium medical device company and large medical device company. In 2021, the medium medical device company segment is expected to dominate the medical device regulatory affairs outsourcing market due to the rising demand for medical devices worldwide.
Europe Medical Device Regulatory Affairs Outsourcing Market Country Level Analysis
The medical device regulatory affairs outsourcing market is analyzed and market size information is provided by the country, services, product, device type, application and end user as referenced above.
The countries covered in the Europe medical device regulatory affairs outsourcing market report are the Germany, U.K., France, Italy, Spain, Netherlands, Russia, Switzerland, Belgium, Turkey, Ireland, rest of Europe.
The Germany is dominating in the Europe medical device regulatory affairs outsourcing market owing to escalation in healthcare expenditure for the regulatory affairs services segment.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points such as new sales, replacement sales, country demographics, regulatory acts and import-export tariffs are some of the major pointers used to forecast the market scenario for individual countries. Also, presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of sales channels are considered while providing forecast analysis of the country data.
Increasing Geographical Expansion Activities by Medical Device Companies is boosting the Medical Device Regulatory Affairs Outsourcing Market Growth
The medical device regulatory affairs outsourcing market also provides you with detailed market analysis for every country growth in medical device regulatory affairs outsourcing industry. Moreover, it provides detailed information regarding medical device regulatory affairs outsourcing sales, impact of regulatory scenarios and trending parameters regarding medical device regulatory affairs outsourcing market. The data is available for historic period 2011 to 2019.
Competitive Landscape and Medical Device Regulatory Affairs Outsourcing Market Share Analysis
The medical device regulatory affairs outsourcing market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, production sites and facilities, company strengths and weaknesses, product launch, product trials pipelines, product approvals, patents, product width and breadth, application dominance, technology lifeline curve. The above data points provided are only related to the company’s focus related to the Europe medical device regulatory affairs outsourcing market.
The major companies covered in the Europe medical device regulatory affairs outsourcing market report are Parexel International Corporation, North American Science Associates, Inc., SGS SA, Trilogy Writing & Consulting GmbH, Creganna (a subsidiary of TE Connectivity), Intertek Group plc, WuXi AppTec, Charles River Laboratories, Celestica Inc., Freyr, Cactus Communications, Eurofins Scientific, TÜV SÜD, Sterigenics U.S., LLC – A Sotera Health company, TE Connectivity, FLEX LTD., Heraeus Holding, Integer Holdings Corporation, IQVIA, Covance, Plexus Corp., Sanmina Corporation, OMICS International, Tecomet, Inc., East West Manufacturing, Omron Corporation among other global and domestic players. DBMR analysts understand competitive strengths and provide competitive analysis for each competitor separately.
Many contracts and agreements are also initiated by the companies’ worldwide which are also accelerating the medical device regulatory affairs outsourcing market.
For instance,
- In January 2020, Charles River Laboratories announced that it has acquired HemaCare Corporation (HemaCare), a company specialized in cell therapy. The company's strategic acquisition has expanded its product portfolio of early-stage research and manufacturing support solutions leading to increased sales and revenue.
Collaboration, product launch, business expansion, award and recognition, joint ventures and other strategies by the market players is enhancing the company footprints in the medical device regulatory affairs outsourcing market which also provides the benefit for organization’s profit growth.
SKU-
Obtenha acesso online ao relatório sobre a primeira nuvem de inteligência de mercado do mundo
- Painel interativo de análise de dados
- Painel de análise da empresa para oportunidades de elevado potencial de crescimento
- Acesso de analista de pesquisa para personalização e customização. consultas
- Análise da concorrência com painel interativo
- Últimas notícias, atualizações e atualizações Análise de tendências
- Aproveite o poder da análise de benchmark para um rastreio abrangente da concorrência
Metodologia de Investigação
A recolha de dados e a análise do ano base são feitas através de módulos de recolha de dados com amostras grandes. A etapa inclui a obtenção de informações de mercado ou dados relacionados através de diversas fontes e estratégias. Inclui examinar e planear antecipadamente todos os dados adquiridos no passado. Da mesma forma, envolve o exame de inconsistências de informação observadas em diferentes fontes de informação. Os dados de mercado são analisados e estimados utilizando modelos estatísticos e coerentes de mercado. Além disso, a análise da quota de mercado e a análise das principais tendências são os principais fatores de sucesso no relatório de mercado. Para saber mais, solicite uma chamada de analista ou abra a sua consulta.
A principal metodologia de investigação utilizada pela equipa de investigação do DBMR é a triangulação de dados que envolve a mineração de dados, a análise do impacto das variáveis de dados no mercado e a validação primária (especialista do setor). Os modelos de dados incluem grelha de posicionamento de fornecedores, análise da linha de tempo do mercado, visão geral e guia de mercado, grelha de posicionamento da empresa, análise de patentes, análise de preços, análise da quota de mercado da empresa, normas de medição, análise global versus regional e de participação dos fornecedores. Para saber mais sobre a metodologia de investigação, faça uma consulta para falar com os nossos especialistas do setor.
Personalização disponível
A Data Bridge Market Research é líder em investigação formativa avançada. Orgulhamo-nos de servir os nossos clientes novos e existentes com dados e análises que correspondem e atendem aos seus objetivos. O relatório pode ser personalizado para incluir análise de tendências de preços de marcas-alvo, compreensão do mercado para países adicionais (solicite a lista de países), dados de resultados de ensaios clínicos, revisão de literatura, mercado remodelado e análise de base de produtos . A análise de mercado dos concorrentes-alvo pode ser analisada desde análises baseadas em tecnologia até estratégias de carteira de mercado. Podemos adicionar quantos concorrentes necessitar de dados no formato e estilo de dados que procura. A nossa equipa de analistas também pode fornecer dados em tabelas dinâmicas de ficheiros Excel em bruto (livro de factos) ou pode ajudá-lo a criar apresentações a partir dos conjuntos de dados disponíveis no relatório.














