Europe Lymphangioleiomyomatosis Lam Market
Tamanho do mercado em biliões de dólares
CAGR :
% 
 USD
39.90 Million
USD
55.03 Million
2024
2032
USD
39.90 Million
USD
55.03 Million
2024
2032
| 2025 –2032 | |
| USD 39.90 Million | |
| USD 55.03 Million | |
|
|
|
|
Segmentação do mercado europeu de linfangioleiomiomatose (LAM), por tipo de doença (LAM associada à esclerose tuberosa e LAM esporádica), tipo (diagnóstico e tratamento), complicações (pneumotórax, quilotórax, tumor renal, derrame pleural, edema e acúmulo de líquido, e outras), via de administração (oral, parenteral e outras), usuário final (hospitais, clínicas especializadas, centros de diagnóstico, assistência domiciliar e outros), canal de distribuição (venda direta, farmácias hospitalares, farmácias de varejo, farmácias online e outros) - Tendências e previsões do setor até 2032.
Tamanho do mercado europeu de linfangioleiomiomatose (LAM)
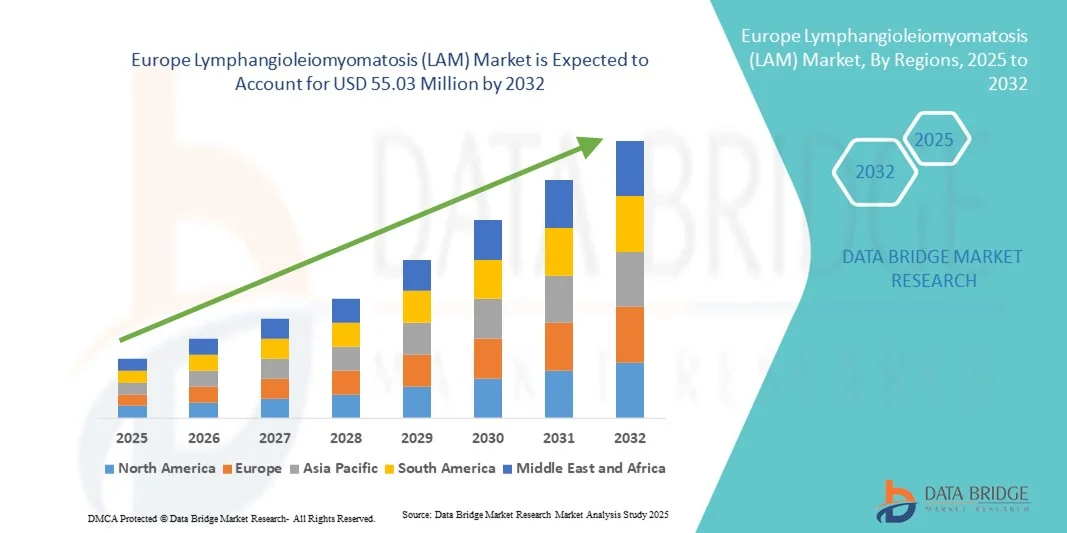
- O mercado europeu de linfangioleiomiomatose (LAM) foi avaliado em US$ 39,90 milhões em 2024 e deverá atingir US$ 55,03 milhões até 2032 , com uma taxa de crescimento anual composta (CAGR) de 4,10% durante o período de previsão.
- O crescimento do mercado é impulsionado principalmente pelo aumento da conscientização e das taxas de diagnóstico de doenças pulmonares raras em países europeus, juntamente com os avanços na medicina de precisão e no diagnóstico molecular.
- Além disso, o aumento das colaborações em pesquisa, o acesso facilitado a medicamentos órfãos e a expansão dos ensaios clínicos para terapias direcionadas estão aprimorando os resultados do tratamento e o manejo dos pacientes. Esses fatores combinados estão acelerando a adoção de terapias inovadoras para LAM, fortalecendo, assim, o crescimento geral do mercado na região.
Análise do Mercado Europeu de Linfangioleiomiomatose (LAM)
- A linfangioleiomiomatose (LAM), uma doença pulmonar rara e progressiva que afeta principalmente mulheres, está recebendo cada vez mais atenção na Europa devido à maior conscientização clínica, aos avanços na pesquisa de doenças raras e às técnicas de diagnóstico aprimoradas, que incluem exames de imagem de alta resolução e testes genéticos.
- A crescente necessidade de terapias eficazes para LAM é impulsionada pela expansão do acesso a tratamentos à base de sirolimus, pelo aumento dos investimentos em programas para doenças raras e pela pesquisa colaborativa apoiada por instituições acadêmicas e empresas farmacêuticas.
- O Reino Unido dominou o mercado europeu de LAM (leucoencefalopatia associada a medicamentos) com a maior participação na receita, de 35,6% em 2024, impulsionado por uma sólida infraestrutura clínica, organizações de pacientes ativas como a LAM Action e significativo apoio governamental para o desenvolvimento de medicamentos órfãos e registros de doenças raras.
- Prevê-se que a Alemanha seja o país com o crescimento mais rápido no mercado europeu de LAM (leucoencefalopatia associada a linfoma) durante o período de previsão, impulsionada pelo aumento da atividade de ensaios clínicos, redes hospitalares avançadas especializadas em doenças pulmonares e pela crescente adoção de abordagens de tratamento personalizadas.
- O segmento de Tratamento dominou o mercado com a maior participação, de 62,3% em 2024, impulsionado pela crescente adoção de terapias direcionadas aos sintomas da LAM e à progressão da doença, juntamente com a crescente disponibilidade de atendimento especializado em hospitais e clínicas especializadas em toda a Europa.
Escopo do relatório e segmentação do mercado europeu de linfangioleiomiomatose (LAM)
|
Atributos |
Principais informações sobre o mercado europeu de linfangioleiomiomatose (LAM) |
|
Segmentos abrangidos |
|
|
Países abrangidos |
Europa
|
|
Principais participantes do mercado |
|
|
Oportunidades de mercado |
|
|
Conjuntos de informações de dados de valor agregado |
Além das informações sobre cenários de mercado, como valor de mercado, taxa de crescimento, segmentação, cobertura geográfica e principais players, os relatórios de mercado elaborados pela Data Bridge Market Research também incluem análises aprofundadas de especialistas, epidemiologia de pacientes, análise de projetos em desenvolvimento, análise de preços e estrutura regulatória. |
Tendências do mercado europeu de linfangioleiomiomatose (LAM)
Crescente foco no diagnóstico precoce e na conscientização sobre doenças raras.
- Uma tendência significativa e crescente no mercado europeu de LAM é a ênfase cada vez maior no diagnóstico precoce por meio de imagens de alta resolução, testes genéticos e registros de pacientes, possibilitando intervenções oportunas e melhores resultados para os pacientes.
- Por exemplo, as redes nacionais de doenças raras no Reino Unido e na França estão implementando protocolos de diagnóstico padronizados para LAM, facilitando a detecção precoce e um melhor controle da progressão da doença.
- Os avanços no diagnóstico molecular e na identificação de biomarcadores estão permitindo que os médicos diferenciem entre LAM esporádica e LAM associada ao Complexo de Esclerose Tuberosa (TSC), aprimorando o planejamento do tratamento personalizado.
- Grupos especializados de defesa dos pacientes e campanhas de conscientização em toda a Europa estão educando profissionais de saúde e pacientes sobre os sintomas da LAM, melhorando as taxas de diagnóstico e reduzindo os atrasos no início do tratamento.
- Essa tendência em direção a uma triagem mais proativa, detecção precoce e conscientização sobre doenças está remodelando as expectativas em relação ao atendimento ao paciente, com empresas e instituições de pesquisa focando cada vez mais na inovação diagnóstica.
- A demanda por soluções de diagnóstico precoce está crescendo rapidamente em hospitais e clínicas especializadas, à medida que médicos e pacientes priorizam a identificação oportuna da LAM para melhorar o manejo a longo prazo e a qualidade de vida.
- A crescente colaboração entre empresas farmacêuticas e instituições de pesquisa está impulsionando o desenvolvimento de kits de diagnóstico avançados e ferramentas preditivas para LAM. A integração de soluções de saúde digital e telemedicina no manejo de pacientes com LAM permite o monitoramento remoto e consultas diagnósticas mais rápidas, acelerando ainda mais a detecção precoce.
Dinâmica do mercado europeu de linfangioleiomiomatose (LAM)
Motorista
Adoção crescente de terapias direcionadas e medicamentos órfãos
- A crescente disponibilidade e adoção de terapias direcionadas, incluindo tratamentos à base de sirolimus , é um fator importante para o mercado europeu de LAM (leucoencefalopatia associada a linfoma), melhorando os resultados para os pacientes e estabilizando a função pulmonar.
- Por exemplo, em março de 2024, o Serviço Nacional de Saúde do Reino Unido (NHS) ampliou o acesso à terapia com sirolimus para pacientes com LAM esporádica, apoiando uma adoção mais ampla de opções de tratamento eficazes.
- O aumento dos investimentos em pesquisa de doenças raras e programas de medicamentos órfãos, tanto por instituições públicas quanto por empresas farmacêuticas, está acelerando o desenvolvimento de novas terapias para LAM.
- A crescente conscientização dos médicos sobre os inibidores de mTOR e outros tratamentos direcionados está levando a um aumento no número de pacientes que recebem a terapia recomendada pelas diretrizes, impulsionando o crescimento do mercado.
- O monitoramento aprimorado do paciente por meio de clínicas especializadas, registros e suporte de telemedicina está facilitando a adesão à terapia e o controle da doença a longo prazo.
- O crescente reconhecimento dos benefícios da terapia direcionada e a expansão das redes de atendimento especializado em toda a Europa estão impulsionando uma maior adoção dos tratamentos LAM em hospitais e clínicas especializadas.
- A expansão de ensaios clínicos e estudos de evidências do mundo real na Alemanha, França e Itália está apoiando a aprovação de novas terapias e aumentando a confiança na eficácia dos tratamentos. Parcerias entre empresas farmacêuticas e grupos de defesa de doenças raras estão aprimorando os programas de acesso e educação para pacientes, impulsionando ainda mais a adesão aos tratamentos.
Restrição/Desafio
Baixa conscientização e altos custos de tratamento
- O conhecimento limitado sobre LAM entre clínicos gerais e alguns pneumologistas continua sendo um desafio crucial, resultando frequentemente em atrasos no diagnóstico e no início do tratamento, o que pode prejudicar o crescimento do mercado.
- Por exemplo, pesquisas na Alemanha e na Itália indicam que muitos pacientes sofrem um atraso no diagnóstico de vários anos devido à falta de familiaridade com doenças pulmonares raras entre os médicos da linha de frente.
- O alto custo do sirolimo e de outras terapias órfãs pode restringir o acesso, principalmente em países com restrições orçamentárias ou cobertura de seguro limitada para doenças raras.
- Embora os programas de apoio ao paciente e os subsídios governamentais estejam melhorando a acessibilidade, o ônus financeiro do tratamento continua sendo uma barreira para alguns pacientes e profissionais de saúde.
- Garantir uma maior conscientização por meio de campanhas educativas, diretrizes clínicas e treinamento médico é crucial para melhorar o diagnóstico precoce e a adesão ao tratamento.
- Superar os altos custos de tratamento por meio de iniciativas de reembolso, programas de assistência ao paciente e apoio político é necessário para facilitar um acesso mais amplo e fortalecer o crescimento do mercado.
- A variabilidade na infraestrutura de saúde entre os países europeus pode criar um acesso inconsistente a cuidados e diagnósticos especializados para LAM, limitando a penetração no mercado. Os desafios regulatórios relacionados à aprovação de medicamentos órfãos e aos procedimentos de reembolso podem atrasar a disponibilidade da terapia, restringindo a expansão do mercado.
Escopo do mercado europeu de linfangioleiomiomatose (LAM)
O mercado é segmentado com base no tipo de doença, tipo de tratamento, complicações, via de administração, usuário final e canal de distribuição.
- Por tipo de doença
Com base no tipo de doença, o mercado europeu de LAM é segmentado em LAM associada ao complexo de esclerose tuberosa (TSC-LAM), LAM esporádica e outras. O segmento de LAM esporádica dominou o mercado com a maior participação na receita, de 58,4% em 2024, devido à sua maior prevalência entre mulheres em idade reprodutiva. Pacientes com LAM esporádica requerem monitoramento contínuo e terapia direcionada, o que sustenta a demanda por tratamento. Extensas pesquisas clínicas, diretrizes de tratamento estabelecidas e registros ativos de pacientes em toda a Europa facilitam o diagnóstico e a intervenção precoces. Hospitais e clínicas especializadas estão cada vez mais focados em LAM esporádica devido à sua natureza progressiva e à necessidade de terapia com inibidores de mTOR. Países europeus como o Reino Unido, a Alemanha e a França lideram a adoção do tratamento e o manejo dos pacientes. O atendimento multidisciplinar, envolvendo pneumologistas, nefrologistas e geneticistas, reforça ainda mais a dominância do segmento.
O segmento de Esclerose Tuberosa com Lamiomiomatose (TSC-LAM) deverá apresentar a taxa de crescimento anual composta (CAGR) mais rápida, de 7,9%, entre 2025 e 2032, impulsionado pela crescente conscientização sobre a ECT entre geneticistas e pneumologistas. Pacientes com TSC-LAM necessitam de cuidados multidisciplinares, incluindo consultas com nefrologistas e pneumologistas. Testes genéticos aprimorados e programas de identificação precoce facilitam o início oportuno do tratamento. O desenvolvimento de diretrizes clínicas focadas em ECT e redes europeias de doenças raras apoiam o crescimento. Campanhas de defesa do paciente e educação estão aumentando as taxas de diagnóstico, contribuindo para a expansão do mercado. Colaborações de pesquisa em andamento para tratamentos de TSC-LAM estão atraindo investimentos em terapias especializadas.
- Por tipo
Com base no tipo, o mercado é segmentado em diagnóstico e tratamento. O segmento de tratamento dominou com uma participação de 62,3% da receita em 2024, impulsionado pela crescente adoção de inibidores de mTOR, como o sirolimus, para estabilizar a função pulmonar. Hospitais e clínicas especializadas são os principais canais de administração do tratamento, e o aumento da atividade de ensaios clínicos impulsiona a adoção. A crescente conscientização sobre a progressão da doença e a disponibilidade de medicamentos órfãos aprovados reforçam a demanda. Os tratamentos para LAM estão cada vez mais integrados a programas de monitoramento do paciente para garantir a adesão. A infraestrutura de saúde da Europa, particularmente na Europa Ocidental, facilita o acesso a terapias avançadas. A terapia de longo prazo para o controle crônico também contribui para o crescimento da receita neste segmento.
O segmento de Diagnóstico deverá apresentar o crescimento mais rápido, com uma taxa de crescimento anual composta (CAGR) de 8,1% entre 2025 e 2032, impulsionado por avanços em tomografia computadorizada de alta resolução, testes de função pulmonar e identificação de biomarcadores. O diagnóstico precoce é crucial na LAM para prevenir danos pulmonares irreversíveis. Hospitais e centros de diagnóstico estão adotando testes genéticos para a diferenciação entre TSC e LAM. Iniciativas europeias para doenças raras promovem a conscientização sobre o diagnóstico e programas de treinamento para médicos. A telemedicina e os laudos digitais aumentam a acessibilidade ao diagnóstico de LAM. O crescente engajamento dos pacientes e as campanhas de conscientização sobre a doença contribuem para uma adoção mais rápida. O aumento do financiamento para programas de diagnóstico de doenças raras também contribui para o crescimento do segmento.
- Por complicações
Com base nas complicações, o mercado é segmentado em pneumotórax, quilotórax, tumor renal, derrame pleural, edema e acúmulo de líquido, entre outras. O segmento de pneumotórax dominou o mercado com uma participação de 41,7% em 2024, visto que o colapso pulmonar recorrente é uma complicação grave que requer hospitalização e intervenção. O manejo do pneumotórax frequentemente envolve cuidados multidisciplinares, contribuindo para custos de tratamento mais elevados. Clínicas e hospitais especializados oferecem monitoramento e procedimentos intervencionistas. A conscientização dos pacientes e a detecção precoce do risco de pneumotórax na LAM estão aumentando em toda a Europa. Diretrizes clínicas padronizadas para o manejo de complicações sustentam a dominância do segmento. Hospitais na Alemanha, França e Reino Unido são líderes em cuidados avançados e intervenção para pneumotórax.
O segmento de quilotórax deverá apresentar o crescimento mais rápido, com uma taxa de crescimento anual composta (CAGR) de 9,2% entre 2025 e 2032, impulsionado pela crescente prevalência de envolvimento linfático em pacientes com LAM. Centros europeus de excelência para doenças pulmonares raras estão expandindo seus serviços para o tratamento do quilotórax. O diagnóstico precoce e as intervenções minimamente invasivas estão impulsionando a adoção dessas práticas. Campanhas de conscientização que destacam essa complicação estão aumentando o engajamento dos pacientes. Clínicas e hospitais especializados estão investindo em técnicas avançadas de tratamento. A pesquisa clínica focada em complicações linfáticas também contribui para o crescimento do segmento.
- Por via administrativa
Com base na via de administração, o mercado é segmentado em oral, parenteral e outras. O segmento oral dominou o mercado com uma participação de 69,1% da receita em 2024, impulsionado pelo uso generalizado da terapia oral com sirolimus para o tratamento a longo prazo. A administração oral é preferida devido à conveniência, à adesão do paciente e à adequação ao tratamento ambulatorial. Hospitais, clínicas especializadas e serviços de assistência domiciliar facilitam o gerenciamento da terapia oral. As políticas de reembolso europeias e os programas de assistência ao paciente apoiam a sua adoção. A administração oral integra-se facilmente aos programas de monitoramento para ajustes de dose. Os médicos frequentemente preferem a terapia oral pela sua facilidade de uso em planos de tratamento de doenças crônicas.
O segmento de administração parenteral deverá apresentar o crescimento mais rápido, com uma taxa de crescimento anual composta (CAGR) de 8,5% entre 2025 e 2032, impulsionado por terapias injetáveis para casos avançados ou pacientes intolerantes à via oral. A administração parenteral é normalmente utilizada em ambiente hospitalar sob supervisão especializada. Inovações em produtos biológicos e terapias direcionadas contribuem para esse crescimento. Ensaios clínicos na Alemanha, França e Itália apoiam a expansão do mercado. Farmácias hospitalares e clínicas especializadas estão oferecendo cada vez mais a administração parenteral. A telemedicina e os programas de apoio ao paciente facilitam a adesão ao tratamento.
- Por usuário final
Com base no usuário final, o mercado é segmentado em hospitais, clínicas especializadas, centros de diagnóstico, assistência domiciliar e outros. O segmento de hospitais dominou o mercado com uma participação de 53,4% em 2024, impulsionado pela concentração de instalações especializadas no tratamento e monitoramento da LAM. Os hospitais oferecem atendimento integrado, incluindo diagnóstico, tratamento e gerenciamento de complicações. Eles servem como centros primários para ensaios clínicos e pesquisas sobre doenças raras. Os hospitais europeus colaboram cada vez mais com grupos de defesa de pacientes em programas de educação e adesão ao tratamento. Infraestrutura avançada e modelos de atendimento multidisciplinares sustentam a dominância desse segmento. Os hospitais continuam sendo a opção preferencial para casos graves ou complexos de LAM.
O segmento de Clínicas Especializadas deverá apresentar o crescimento mais rápido, com uma taxa de crescimento anual composta (CAGR) de 9,0% entre 2025 e 2032, impulsionado pelo número crescente de centros focados em doenças pulmonares raras. As clínicas especializadas oferecem atendimento direcionado a pacientes com LAM, frequentemente proporcionando monitoramento a longo prazo e terapia personalizada. A adoção de soluções de saúde digital e telemedicina amplia o alcance dos serviços. As clínicas estão integrando abordagens multidisciplinares, incluindo consultas de nefrologia e genética para casos de LAM associada à esclerose tuberosa (TSC). Iniciativas de conscientização do paciente estão impulsionando a demanda por serviços de clínicas especializadas. A colaboração com hospitais e redes de doenças raras fortalece as capacidades das clínicas e melhora os resultados para os pacientes.
- Por canal de distribuição
Com base no canal de distribuição, o mercado é segmentado em licitação direta, farmácias hospitalares, farmácias de varejo, farmácias online e outros. O segmento de farmácias hospitalares dominou o mercado com uma participação de 47,8% da receita em 2024, visto que os hospitais continuam sendo o principal ponto de dispensação de inibidores de mTOR e outras terapias LAM. As farmácias hospitalares garantem o armazenamento adequado, o monitoramento da adesão ao tratamento e o aconselhamento do paciente. Elas estão intimamente ligadas a ensaios clínicos e programas de tratamento. As farmácias hospitalares europeias estão expandindo sua capacidade para atender à crescente demanda dos pacientes. Elas também oferecem treinamento sobre administração de medicamentos e gerenciamento de efeitos colaterais. A integração com clínicas especializadas aumenta a eficiência da distribuição e a adesão do paciente ao tratamento.
O segmento de farmácias online deverá apresentar o crescimento mais rápido, com uma taxa composta de crescimento anual (CAGR) de 10,3% entre 2025 e 2032, impulsionado pela crescente adoção digital, serviços de entrega em domicílio e melhor acesso a medicamentos para doenças raras. As plataformas online oferecem conveniência para pacientes em áreas remotas. A integração da telemedicina com as farmácias online aprimora a adesão ao tratamento e o acompanhamento. Iniciativas de educação do paciente e modelos de entrega direta ao paciente impulsionam ainda mais a adoção. A crescente conscientização sobre tratamentos para doenças raras incentiva a compra online. Sistemas digitais de gerenciamento de prescrições auxiliam na entrega segura e oportuna de medicamentos.
Análise Regional do Mercado Europeu de Linfangioleiomiomatose (LAM)
- O Reino Unido dominou o mercado europeu de LAM (leucoencefalopatia associada a medicamentos) com a maior participação na receita, de 35,6% em 2024, impulsionado por uma sólida infraestrutura clínica, organizações de pacientes ativas como a LAM Action e significativo apoio governamental para o desenvolvimento de medicamentos órfãos e registros de doenças raras.
- Pacientes e médicos no Reino Unido se beneficiam de instalações de diagnóstico avançadas, centros de tratamento especializados e acesso a medicamentos órfãos, o que, em conjunto, melhora o controle da doença e os resultados para pacientes com LAM.
- Essa ampla adoção é ainda mais reforçada pela alta conscientização entre os pneumologistas, pelo crescente número de ensaios clínicos e pelas iniciativas nacionais para doenças raras, consolidando o Reino Unido como um centro fundamental para o diagnóstico e tratamento da LAM na Europa.
Análise do Mercado de Linfangioleiomiomatose (LAM) no Reino Unido
O Reino Unido dominou o mercado europeu de LAM (linfoma de Hodgkin) com a maior participação de mercado, de 35,6% em 2024, impulsionado por uma infraestrutura de saúde bem estabelecida, forte apoio governamental a programas para doenças raras e redes ativas de defesa dos pacientes, como a LAM Action. Hospitais e clínicas especializadas no Reino Unido oferecem instalações de diagnóstico avançadas, incluindo exames de imagem de alta resolução e testes genéticos, permitindo a detecção precoce e o manejo eficaz da doença. A ampla disponibilidade da terapia com sirolimus e outros tratamentos direcionados contribui para a alta taxa de adoção. Iniciativas nacionais para doenças raras e programas de registro facilitam o diagnóstico oportuno e o monitoramento de longo prazo dos pacientes.
Análise do Mercado de Linfangioleiomiomatose (LAM) na Alemanha
Espera-se que o mercado alemão de LAM (linfangioleiomiomatose) cresça a uma taxa composta de crescimento anual (CAGR) considerável, impulsionado pela crescente conscientização sobre doenças pulmonares raras, infraestrutura de saúde avançada e aumento da atividade de ensaios clínicos. Hospitais e clínicas especializadas alemãs oferecem serviços abrangentes de diagnóstico e tratamento, apoiando o cuidado de pacientes com LAM esporádica e associada à esclerose tuberosa (TSC-LAM). A adoção de inibidores de mTOR e o manejo multidisciplinar envolvendo pneumologia, nefrologia e genética estão impulsionando o crescimento do mercado. Iniciativas de pesquisa e o apoio governamental a programas para doenças raras aprimoram o acesso à terapia. A educação do paciente e o monitoramento baseado em registros facilitam o diagnóstico precoce e a adesão ao tratamento. O mercado se beneficia de políticas de reembolso robustas e redes bem estabelecidas para doenças raras.
Análise do Mercado de Linfangioleiomiomatose (LAM) na França
O mercado francês de LAM está em crescimento devido à presença de centros especializados em LAM, ao acesso a terapias avançadas e à crescente conscientização dos pacientes. Hospitais e centros de diagnóstico estão integrando exames de imagem de alta resolução e testes genéticos para a detecção precoce da LAM. A pesquisa colaborativa e as Redes Europeias de Referência para doenças pulmonares raras melhoram os resultados para os pacientes. O apoio governamental às políticas para doenças raras e o reembolso facilitam o acesso a medicamentos órfãos. Ensaios clínicos e programas de apoio ao paciente aumentam a adesão ao tratamento. Campanhas de conscientização direcionadas a médicos e pacientes melhoram as taxas de intervenção precoce e contribuem para a expansão geral do mercado.
Análise do Mercado Italiano de Linfangioleiomiomatose (LAM)
O mercado italiano de LAM deverá crescer de forma constante, impulsionado pela expansão da infraestrutura de saúde, pelos registros de pacientes e pelo aumento do acesso a medicamentos órfãos. Hospitais e clínicas especializadas concentram-se no diagnóstico precoce e no acompanhamento a longo prazo de pacientes com LAM. Terapias direcionadas, especialmente o sirolimo, são cada vez mais adotadas devido à sua eficácia comprovada na estabilização da função pulmonar. Centros italianos participam ativamente de redes de pesquisa em doenças raras para promover o acesso ao tratamento. Campanhas de conscientização e treinamento para pneumologistas reduzem os atrasos no diagnóstico. A telemedicina e o monitoramento digital de pacientes aprimoram a adesão ao tratamento e o acompanhamento pós-tratamento.
Participação de mercado na linfangioleiomiomatose (LAM) na Europa
O setor europeu de linfangioleiomiomatose (LAM) é liderado principalmente por empresas consolidadas, incluindo:
- Pfizer Inc. (EUA)
- Novartis AG (Suíça)
- Takeda Pharmaceutical Company Limited (Japão)
- Johnson & Johnson Services, Inc. (EUA)
- GSK plc (Reino Unido)
- AstraZeneca (Reino Unido)
- Laboratórios Dr. Reddy's Ltda. (Índia)
- Zydus Pharmaceuticals, Inc. (Índia)
- Intas Pharmaceuticals Ltd (Índia)
- Apotex Inc. (Canadá)
- Amneal Pharmaceuticals LLC (EUA)
- Corporação Terumo (Japão)
- Hikma Pharmaceuticals plc (Reino Unido)
- TransMedics, Inc. (EUA)
- XVIVO Perfusão AB (Suécia)
- Taj Pharmaceuticals Limited (Índia)
- Morgan Scientific Inc. (EUA)
- Sun Pharmaceutical Industries Ltd (Índia)
- Sanofi (França)
- Boehringer Ingelheim International GmbH (Alemanha)
Quais são os desenvolvimentos recentes no mercado europeu de linfangioleiomiomatose (LAM)?
- Em março de 2025, a Fundação LAM destacou um novo projeto de pesquisa: “Direcionando as interações entre fibroblastos e células endoteliais na patogênese da LAM usando modelos de esferoides 3D e transcriptômica espacial”, sinalizando uma crescente colaboração europeia em pesquisa molecular avançada e modelos translacionais voltados para novas abordagens terapêuticas na LAM.
- Em janeiro de 2025, um estudo publicado na Frontiers in Medicine intitulado “Características e desfechos de pacientes com LAM” analisou uma coorte de pacientes europeus e forneceu dados atualizados do mundo real sobre a progressão da doença, a adesão ao tratamento e os desfechos, reforçando a necessidade de melhor monitoramento e centros especializados na Europa.
- Em abril de 2024, uma pesquisa publicada na revista European Radiology demonstrou que um protocolo de tomografia computadorizada de tórax com dose ultrabaixa, utilizando um filtro de prata e reconstrução por aprendizado profundo, pode reduzir a dose de radiação em cerca de 85%, mantendo a precisão diagnóstica na avaliação de cistos LAM. Este é um avanço importante para um monitoramento de rotina mais seguro na Europa.
- Em fevereiro de 2024, a Aliança para Linfangiomatose e Doença de Gorham publicou um relatório intitulado "Navegando pelo Complexo Mundo das Anomalias Linfáticas: Avanços da Pesquisa em Doenças Pulmonares", que destacou novas perspectivas sobre a ligação da LAM com distúrbios linfáticos, mapeando mutações genéticas no gene TSC2 e descrevendo como a pesquisa sobre LAM na Europa agora oferece implicações mais amplas para anomalias linfáticas complexas.
- Em maio de 2021, um estudo de longo prazo realizado na Espanha e publicado na revista Scientific Reports revelou que mais da metade dos pacientes com LAM tratados com sirolimo mantiveram uma resposta positiva ao longo de cinco anos. Este estudo representa um dos maiores acompanhamentos em condições reais de prática clínica na Europa e reforça a importância da terapia com inibidores de mTOR no tratamento da LAM.
SKU-
Obtenha acesso online ao relatório sobre a primeira nuvem de inteligência de mercado do mundo
- Painel interativo de análise de dados
- Painel de análise da empresa para oportunidades de elevado potencial de crescimento
- Acesso de analista de pesquisa para personalização e customização. consultas
- Análise da concorrência com painel interativo
- Últimas notícias, atualizações e atualizações Análise de tendências
- Aproveite o poder da análise de benchmark para um rastreio abrangente da concorrência
Metodologia de Investigação
A recolha de dados e a análise do ano base são feitas através de módulos de recolha de dados com amostras grandes. A etapa inclui a obtenção de informações de mercado ou dados relacionados através de diversas fontes e estratégias. Inclui examinar e planear antecipadamente todos os dados adquiridos no passado. Da mesma forma, envolve o exame de inconsistências de informação observadas em diferentes fontes de informação. Os dados de mercado são analisados e estimados utilizando modelos estatísticos e coerentes de mercado. Além disso, a análise da quota de mercado e a análise das principais tendências são os principais fatores de sucesso no relatório de mercado. Para saber mais, solicite uma chamada de analista ou abra a sua consulta.
A principal metodologia de investigação utilizada pela equipa de investigação do DBMR é a triangulação de dados que envolve a mineração de dados, a análise do impacto das variáveis de dados no mercado e a validação primária (especialista do setor). Os modelos de dados incluem grelha de posicionamento de fornecedores, análise da linha de tempo do mercado, visão geral e guia de mercado, grelha de posicionamento da empresa, análise de patentes, análise de preços, análise da quota de mercado da empresa, normas de medição, análise global versus regional e de participação dos fornecedores. Para saber mais sobre a metodologia de investigação, faça uma consulta para falar com os nossos especialistas do setor.
Personalização disponível
A Data Bridge Market Research é líder em investigação formativa avançada. Orgulhamo-nos de servir os nossos clientes novos e existentes com dados e análises que correspondem e atendem aos seus objetivos. O relatório pode ser personalizado para incluir análise de tendências de preços de marcas-alvo, compreensão do mercado para países adicionais (solicite a lista de países), dados de resultados de ensaios clínicos, revisão de literatura, mercado remodelado e análise de base de produtos . A análise de mercado dos concorrentes-alvo pode ser analisada desde análises baseadas em tecnologia até estratégias de carteira de mercado. Podemos adicionar quantos concorrentes necessitar de dados no formato e estilo de dados que procura. A nossa equipa de analistas também pode fornecer dados em tabelas dinâmicas de ficheiros Excel em bruto (livro de factos) ou pode ajudá-lo a criar apresentações a partir dos conjuntos de dados disponíveis no relatório.