Mercado europeu de dispositivos de assistência ventricular esquerda (DAVE), por tipo de produto (bomba cardíaca, controlador, baterias, fios), terapia (terapia ponte para transplante (BTT), terapia alvo, terapia ponte para candidatura (BTC), ponte -Terapêutica de Recuperação (BTR), Faixa Etária (Adulto, Pediátrico), Indicação (Insuficiência Cardíaca Congestiva, Cardiopatia Congénita , Miocardite, Paragem Cardíaca, Arritmias Familiares e Arrítmicas, Cardiomiopatias, Insuficiência Cardíaca Avançada, Outros), Geração (Segunda Dispositivos de geração , dispositivos de terceira geração, dispositivos de primeira geração), durabilidade (longo prazo, intermédio prazo, curto prazo), design (axial, centrífugo), tipo de pulso (não pulsátil, pulsátil), utilizador final (hospitais, laboratórios de cateterismo cardíaco , Clínicas Especializadas, Outros), Canal de Distribuição (Concurso Directo, Vendas a Retalho, Outros), País (Alemanha, França, Reino Unido, Itália, Rússia, Espanha, Turquia, Holanda, Suíça, Bélgica, Irlanda, Resto da Europa) Tendências da Indústria e Previsão para 2029.
Análise de mercado e insights : Mercado europeu de dispositivos de assistência ventricular esquerda (DAVE)
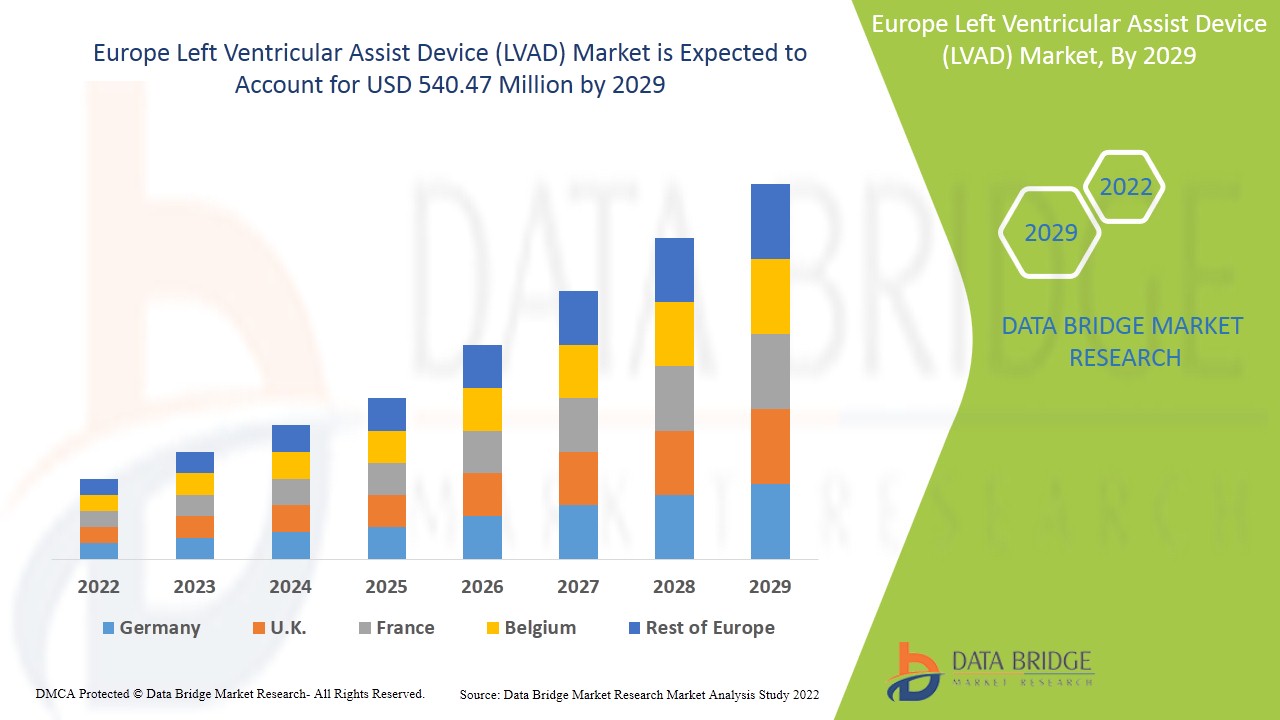
Espera-se que o mercado europeu de dispositivos de assistência ventricular esquerda (DAVE) ganhe crescimento de mercado no período de previsão de 2022 a 2029. A Data Bridge Market Research analisa que o mercado está a crescer com um CAGR de 9,2% no período de previsão de 2022 a 2029 e é deverá atingir os 540,47 milhões de dólares até 2029. Os crescentes avanços tecnológicos no dispositivo de assistência ventricular esquerda atuam como impulsionadores para o crescimento do mercado de dispositivos de assistência ventricular esquerda (DAVE).
Um dispositivo de assistência ventricular esquerda (DAVE) é uma bomba mecânica implantada em doentes com insuficiência cardíaca. Ajuda a câmara inferior esquerda do coração (ventrículo esquerdo) a bombear o sangue do ventrículo para a aorta e para o resto do corpo.
É utilizado em doentes que atingiram insuficiência cardíaca terminal. O DAVE é implantado cirurgicamente, uma bomba mecânica operada por bateria, que ajuda o ventrículo esquerdo (principal câmara de bombeamento do coração) a bombear o sangue para o resto do corpo.
As principais razões que impulsionam o crescimento do mercado europeu de dispositivos de assistência ventricular esquerda são o aumento do número de doentes que sofrem de insuficiência cardíaca e a escassez de dadores cardíacos. Além disso, os DAVE tecnicamente sofisticados (por exemplo, HeartMate III) contribuem para o crescimento do mercado. Além disso, prevê-se que os avanços contínuos nesta disciplina, bem como novas aplicações de terapias inovadoras, abram novos caminhos para o mercado da DAVE. No entanto, estes dispositivos são dispendiosos e apresentam riscos como coágulos sanguíneos e hemorragias, que deverão dificultar consideravelmente a expansão do mercado. Além disso, o aumento da recolha de produtos é um desafio significativo para o mercado.
O relatório de mercado do dispositivo de assistência ventricular esquerda (DAVE) fornece detalhes da quota de mercado, novos desenvolvimentos e análise do pipeline de produtos, impacto dos participantes do mercado doméstico e localizado, analisa as oportunidades em termos de bolsas de receitas emergentes, alterações nas regulamentos de mercado, aprovações de produtos, decisões de estratégia, lançamentos de produtos, expansões geográficas e inovações tecnológicas no mercado. Para compreender a análise e o cenário do mercado do dispositivo de assistência ventricular esquerda (DAVE), contacte a Data Bridge Market Research para obter um resumo do analista, a nossa equipa irá ajudá-lo a criar uma solução de impacto na receita para atingir o objetivo desejado.
Âmbito do mercado e dimensão do mercado do dispositivo de assistência ventricular esquerda (DAVE)
O mercado dos dispositivos de assistência ventricular esquerda (DAVE) está segmentado com base no tipo de produto, terapia, faixa etária, indicação, geração, durabilidade, design, tipo de pulso, utilizador final e canal de distribuição. O crescimento entre segmentos ajuda-o a analisar os nichos de crescimento e as estratégias para abordar o mercado e determinar as suas principais áreas de aplicação e a diferença nos seus mercados-alvo.
O mercado europeu de dispositivos de assistência ventricular esquerda (DAVE) está categorizado em dez segmentos notáveis, tais como tipo de produto, terapia, faixa etária, indicação, geração, durabilidade, design, tipo de pulso, utilizador final e canal de distribuição.
- Com base no tipo de produto, o mercado europeu de dispositivos de assistência ventricular esquerda (DAVE) está segmentado em bomba cardíaca, controlador, baterias e fios. As baterias são ainda segmentadas em recarregáveis e não recarregáveis. Em 2022, espera-se que a bomba cardíaca domine o mercado, uma vez que desempenha as funções do coração sem o substituir e permite que os doentes vivam mais tempo do que aqueles que são tratados apenas com terapêutica médica.
- Com base na terapia, o mercado europeu de dispositivos de assistência ventricular esquerda (DAVE) está segmentado como terapia ponte para transplante (BTT), terapia alvo, terapia ponte para candidatura (BTC), terapia ponte para recuperação (BTR). Em 2022, prevê-se que o setor da ponte para o transplante (BTT) domine o mercado porque ajuda os doentes a esperar por um transplante e evita maiores danos no coração e noutros órgãos até que um dador esteja disponível.
- Com base na faixa etária, o mercado europeu de dispositivos de assistência ventricular esquerda (DAVE) está segmentado em adulto e pediátrico. O adulto é ainda segmentado em 19 a 39 anos, 40 a 59 anos, 60 a 79 anos e acima dos 80 anos. Em 2022, prevê-se que o segmento adulto domine o mercado, uma vez que a maioria dos eventos de insuficiência cardíaca ocorre em adultos. A prevalência é de 1-2% da população europeia, aumentando para mais de 10% entre as pessoas com mais de 70 anos. Isto pode implicar que mais de 10 milhões de pessoas sofram de insuficiência cardíaca.
- Com base na indicação, o mercado europeu de dispositivos de assistência ventricular esquerda (DAVE) está segmentado em insuficiência cardíaca congestiva, doença cardíaca congénita, miocardite, paragem cardíaca, arritmias familiares e cardiomiopatias arrítmicas, insuficiência cardíaca avançada e outras. Em 2022, prevê-se que a insuficiência cardíaca congestiva domine o mercado, uma vez que é o diagnóstico mais comum em doentes hospitalizados com mais de 65 anos. Na Europa, mais de 16 milhões de pessoas foram diagnosticadas com ICC.
- Com base na geração, o mercado europeu de dispositivos de assistência ventricular esquerda (DAVE) está segmentado em dispositivos de segunda geração, dispositivos de terceira geração e dispositivos de primeira geração. Em 2022, o mercado é dominado pelas bombas rotativas de segunda geração, que têm a vantagem de um design mais pequeno e o potencial para uma maior fiabilidade mecânica a longo prazo, eliminando a câmara do reservatório e as válvulas necessárias.
- Com base na durabilidade, o mercado europeu de dispositivos de assistência ventricular esquerda (DAVE) está segmentado em longo prazo, médio prazo e curto prazo. Em 2022, espera-se que o segmento de longo prazo domine para liderar o mercado devido à necessidade de continuar a apoiar as bombas cardíacas durante mais tempo, o que está a impulsionar o crescimento do mercado.
- Com base no design, o mercado europeu de dispositivos de assistência ventricular esquerda (DAVE) está segmentado em axial e centrífugo. Em 2022, prevê-se que o segmento axial domine, uma vez que funciona eficientemente a altas velocidades de rotação e com uma pré-carga reduzida.
- Com base no tipo de pulso, o mercado europeu de dispositivos de assistência ventricular esquerda (DAVE) está segmentado em não pulsátil e pulsátil. Em 2022, espera-se que o segmento da categoria não pulsátil domine o mercado porque os DAVE estão a ser utilizados mais frequentemente na terapia alvo, quaisquer efeitos adversos da pulsatilidade arterial inadequada nas estruturas periféricas podem ser grandemente ampliados com os longos períodos de implantação associados a este tipo de terapêutica.
- Com base no utilizador final, o mercado europeu de dispositivos de assistência ventricular esquerda (DAVE) está segmentado em hospitais, clínicas especializadas, laboratórios de cateterismo cardíaco e outros. Em 2022, prevê-se que o segmento do setor hospitalar domine devido a um número crescente de doentes cardiovasculares, como resultado da mudança de estilos de vida e do envelhecimento da população. Em 2020, existiam mais de 19.000 hospitais em 23 países europeus.
- Com base no canal de distribuição, o mercado europeu de dispositivos de assistência ventricular esquerda (DAVE) está segmentado em concurso direto, vendas a retalho e outros. Em 2022, prevê-se que o sector do concurso directo domine porque oferece responsabilidade e produtos de maior qualidade devido à concorrência, o que aumenta a procura por estes artigos.
Análise ao nível do país de mercado do dispositivo de assistência ventricular esquerda (DAVE)
O mercado de dispositivos de assistência ventricular esquerda (DAVE) é analisado e as informações sobre o tamanho do mercado são fornecidas pelo país, dez segmentos notáveis, tais como tipo de produto, terapia, faixa etária, indicação, geração, durabilidade, design, tipo de pulso , utilizador final e distribuição canal como acima referido.
Os países abrangidos no relatório do mercado europeu do dispositivo de assistência ventricular esquerda (DAVE) são a Alemanha, França, Reino Unido, Itália, Rússia, Espanha, Turquia, Países Baixos, Suíça, Bélgica, Irlanda, Resto da Europa.
Espera-se que o segmento adulto na Alemanha cresça com a maior taxa de crescimento no período de previsão de 2022 a 2029 devido ao aumento da prevalência de doenças cardiovasculares, incluindo insuficiência cardíaca avançada, paragem cardíaca e insuficiência cardíaca congestiva em adultos.
A secção do país do relatório também fornece fatores individuais de impacto no mercado e alterações na regulamentação do mercado interno que impactam as tendências atuais e futuras do mercado. Dados como novas vendas, vendas de reposição, demografia do país, atos regulamentares e tarifas de importação e exportação são alguns dos principais indicadores utilizados para prever o cenário de mercado para países individuais. Além disso, a presença e disponibilidade de marcas europeias e os desafios enfrentados devido à grande ou escassa concorrência de marcas locais e nacionais, o impacto dos canais de vendas são considerados, ao mesmo tempo que se fornece uma análise de previsão dos dados do país.
O crescimento das atividades estratégicas dos principais players do mercado para aumentar a consciencialização sobre o dispositivo de assistência ventricular esquerda está a impulsionar o crescimento do mercado de dispositivos de assistência ventricular esquerda (DAVE).
O mercado de dispositivos de assistência ventricular esquerda (DAVE) também fornece análises de mercado detalhadas para o crescimento de cada país num mercado específico. Além disso, fornece informações detalhadas sobre a estratégia dos intervenientes no mercado e a sua presença geográfica. Os dados estão disponíveis para o período histórico de 2011 a 2020.
Análise do panorama competitivo e da quota de mercado do dispositivo de assistência ventricular esquerda (DAVE)
O panorama competitivo do mercado de dispositivos de assistência ventricular esquerda (DAVE) fornece detalhes por concorrente. Os detalhes incluídos são a visão geral da empresa, finanças da empresa, receitas geradas, potencial de mercado, investimento em investigação e desenvolvimento, novas iniciativas de mercado, localizações e instalações de produção, pontos fortes e fracos da empresa, lançamento de produtos, pipelines de testes de produtos, aprovações de produtos, patentes, largura e amplitude do produto, domínio de aplicação, curva de segurança da tecnologia. Os dados acima fornecidos estão apenas relacionados com o foco da empresa relacionado com o mercado dos dispositivos de assistência ventricular esquerda (DAVE).
As principais empresas que operam no dispositivo de assistência ventricular esquerda (DAVE) são a ABIOMED, a Abbott, a Evaheart, Inc, a Saft, a Berlin Heart, a CorWave SA, a Jarvik Heart, Inc. Os analistas DBMR compreendem os pontos fortes competitivos e fornecem análises competitivas para cada concorrente em separado.
Muitos contratos e acordos são também iniciados por empresas de todo o mundo que também estão a acelerar o mercado dos dispositivos de assistência ventricular esquerda (DAVE).
Por exemplo,
- Em janeiro de 2021, a CorWave SA recebeu um financiamento de 40 milhões de dólares de três investidores para desenvolver uma bomba cardíaca de membrana de ondas inovadora e avançada. O financiamento recebido permitiu à empresa acelerar o desenvolvimento de produtos e reforçar a sua presença no mercado global de dispositivos de assistência ventricular esquerda (DAVE).
A colaboração, o lançamento de produtos, a expansão do negócio, a premiação e o reconhecimento, as joint ventures e outras estratégias do player de mercado estão a melhorar a presença da empresa no mercado dos dispositivos de assistência ventricular esquerda (DAVE), o que também oferece o benefício para o crescimento do lucro da organização.
SKU-
Obtenha acesso online ao relatório sobre a primeira nuvem de inteligência de mercado do mundo
- Painel interativo de análise de dados
- Painel de análise da empresa para oportunidades de elevado potencial de crescimento
- Acesso de analista de pesquisa para personalização e customização. consultas
- Análise da concorrência com painel interativo
- Últimas notícias, atualizações e atualizações Análise de tendências
- Aproveite o poder da análise de benchmark para um rastreio abrangente da concorrência
Índice
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 PRODUCT TYPE LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 MARKET END USER COVERAGE GRID
2.11 VENDOR SHARE ANALYSIS
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL
4.2 PORTER'S
4.3 INDUSTRIAL INSIGHTS:
5 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: REGULATIONS
5.1 REGULATION IN U.S.
5.1.1 GUIDELINES-
5.2 REGULATION IN EUROPE
5.2.1 GUIDELINES FOR THE MANUFACTURERS:
5.3 REGULATION IN CANADA
5.3.1 GUIDELINES FOR THE MANUFACTURERS:
5.4 REGULATION IN MEXICO
5.4.1 GUIDELINES FOR THE MANUFACTURERS:
6 MARKET OVERVIEW
6.1 DRIVERS
6.1.1 INCREASING GERIATRIC POPULATION ALONG WITH RISING PREVALENCE OF CARDIAC DISEASES
6.1.2 PRESENCE OF FAVOURABLE REIMBURSEMENT POLICIES
6.1.3 CHANGING LIFESTYLE TRIGGERS THE DEVELOPMENT OF CARDIOVASCULAR DISEASES
6.2 RESTRAINTS
6.2.1 HIGH COST OF LVAD IMPLANTATION AND TREATMENT
6.2.2 COMPLICATIONS AND RISKS ASSOCIATED WITH LVAD
6.3 OPPORTUNITIES
6.3.1 INCREASE IN HEALTHCARE EXPENDITURE
6.3.2 INCREASE IN MINIMALLY INVASIVE PROCEDURE
6.3.3 INCREASING SHORTAGE OF ORGAN DONORS
6.3.4 TECHNOLOGICAL ADVANCEMENTS IN LEFT VENTRICULAR ASSIST DEVICES
6.4 CHALLENGES
6.4.1 ONGOING COVID-19
6.4.2 INCREASE IN PRODUCT RECALL
7 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE
7.1 OVERVIEW
7.2 HEART PUMP
7.3 CONTROLLER
7.4 BATTERIES
7.4.1 RECHARGEABLE
7.4.2 NON-RECHARGEABLE
7.5 WIRES
8 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY
8.1 OVERVIEW
8.2 BRIDGE-TO-TRANSPLANT (BTT) THERAPY
8.2.1 HEART PUMP
8.2.2 CONTROLLER
8.2.3 BATTERIES
8.2.4 WIRES
8.3 DESTINATION THERAPY
8.3.1 HEART PUMP
8.3.2 CONTROLLER
8.3.3 BATTERIES
8.3.4 WIRES
8.4 BRIDGE-TO-CANDIDACY (BTC) THERAPY
8.4.1 HEART PUMP
8.4.2 CONTROLLER
8.4.3 BATTERIES
8.4.4 WIRES
8.5 BRIDGE-TO-RECOVERY (BTR) THERAPY
8.5.1 HEART PUMP
8.5.2 CONTROLLER
8.5.3 BATTERIES
8.5.4 WIRES
9 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP
9.1 OVERVIEW
9.2 ADULT
9.2.1 19-39 YEARS
9.2.2 40-59 YEARS
9.2.3 60-79 YEARS
9.2.4 ABOVE 80 YEARS
9.3 PEDIATRIC
10 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY GENERATION
10.1 OVERVIEW
10.2 SECOND GENERATION DEVICES
10.3 THIRD GENERATION DEVICES
10.4 FIRST GENERATION DEVICES
11 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DESIGN
11.1 OVERVIEW
11.2 AXIAL
11.3 CENTRIFUGAL
12 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION
12.1 OVERVIEW
12.2 CONGESTIVE HEART FAILURE
12.2.1 HEART PUMP
12.2.2 CONTROLLER
12.2.3 BATTERIES
12.2.4 WIRES
12.3 CONGENITAL HEART DISEASE
12.3.1 HEART PUMP
12.3.2 CONTROLLER
12.3.3 BATTERIES
12.3.4 WIRES
12.4 MYOCARDITIS
12.4.1 HEART PUMP
12.4.2 CONTROLLER
12.4.3 BATTERIES
12.4.4 WIRES
12.5 CARDIAC ARREST
12.5.1 HEART PUMP
12.5.2 CONTROLLER
12.5.3 BATTERIES
12.5.4 WIRES
12.6 FAMILIAL ARRHYTHMIAS AND ARRHYTHMIC CARDIOMYOPATHIES
12.6.1 HEART PUMP
12.6.2 CONTROLLER
12.6.3 BATTERIES
12.6.4 WIRES
12.7 ADVANCED HEART FAILURE
12.7.1 HEART PUMP
12.7.2 CONTROLLER
12.7.3 BATTERIES
12.7.4 WIRES
12.8 OTHERS
13 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET, BY DURABILITY
13.1 OVERVIEW
13.2 LONG-TERM
13.3 INTERMEDIATE-TERM
13.4 SHORT-TERM
14 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET, BY PULSE TYPE
14.1 OVERVIEW
14.2 NONPULSATILE
14.3 PULSATILE
15 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET, BY END USER
15.1 OVERVIEW
15.2 HOSPITAL
15.3 CARDIAC CATH LABORATORIES
15.4 SPECIALTY CLINICS
15.5 OTHERS
16 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET, BY DISTRIBUTION CHANNEL
16.1 OVERVIEW
16.2 DIRECT TENDER
16.3 RETAIL SALES
16.4 OTHERS
17 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION
17.1 EUROPE
17.1.1 GERMANY
17.1.2 FRANCE
17.1.3 U.K.
17.1.4 ITALY
17.1.5 RUSSIA
17.1.6 SPAIN
17.1.7 TURKEY
17.1.8 NETHERLANDS
17.1.9 SWITZERLAND
17.1.10 BELGIUM
17.1.11 IRELAND
17.1.12 REST OF EUROPE
18 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: COMPANY LANDSCAPE
18.1 COMPANY SHARE ANALYSIS: EUROPE
19 SWOT ANALYSIS
20 COMPANY PROFILE
20.1 ABIOMED
20.1.1 COMPANY SNAPSHOT
20.1.2 REVENUE ANALYSIS
20.1.3 COMPANY SHARE ANALYSIS
20.1.4 PRODUCT PORTFOLIO
20.1.5 RECENT DEVELOPMENTS
20.1.5.1 FDA APPROVAL
20.1.5.2 FDA APPROVAL
20.2 ABBOTT
20.2.1 COMPANY SNAPSHOT
20.2.2 REVENUE ANALYSIS
20.2.3 COMPANY SHARE ANALYSIS
20.2.4 PRODUCT PORTFOLIO
20.2.5 RECENT DEVELOPMENTS
20.2.5.1 SUPPLY INCREMENT
20.2.5.2 BREAK THROUGH DEVICE DESIGNATION
20.3 BERLIN HEART
20.3.1 COMPANY SNAPSHOT
20.3.2 COMPANY SHARE ANALYSIS
20.3.3 PRODUCT PORTFOLIO
20.3.4 RECENT DEVELOPMENT
20.3.4.1 PRODUCT APPROVAL
20.4 SAFT (A SUBSIDIARY OF TOTALENERGIES)
20.4.1 COMPANY SNAPSHOT
20.4.2 REVENUE ANALYSIS
20.4.3 COMPANY SHARE ANALYSIS
20.4.4 PRODUCT PORTFOLIO
20.4.5 RECENT DEVELOPMENT
20.4.5.1 PARTNERSHIP
20.5 JARVIK HEART, INC.
20.5.1 COMPANY SNAPSHOT
20.5.2 COMPANY SHARE ANALYSIS
20.5.3 PRODUCT PORTFOLIO
20.5.4 RECENT DEVELOPMENT
20.5.4.1 FDA APPROVAL
20.6 CORWAVE SA
20.6.1 COMPANY SNAPSHOT
20.6.2 PRODUCT PORTFOLIO
20.6.3 RECENT DEVELOPMENT
20.6.3.1 EXPANSION
20.7 EVAHEART, INC
20.7.1 COMPANY SNAPSHOT
20.7.2 PRODUCT PORTFOLIO
20.7.3 RECENT DEVELOPMENT
20.7.3.1 PRODUCT TRIAL
21 QUESTIONNAIRE
22 RELATED REPORTS
Lista de Tabela
TABLE 1 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 2 EUROPE HEART PUMP IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 3 EUROPE CONTROLLER IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 4 EUROPE BATTERIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 5 EUROPE BATTERIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 6 EUROPE WIRES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 7 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 8 EUROPE BRIDGE-TO-TRANSPLANT (BTT) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 9 EUROPE BRIDGE-TO-TRANSPLANT (BTT) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 10 EUROPE DESTINATION THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 11 EUROPE DESTINATION THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 12 EUROPE BRIDGE-TO-CANDIDACY (BTC) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 13 EUROPE BRIDGE-TO-CANDIDACY (BTC) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 14 EUROPE BRIDGE-TO-RECOVERY (BTR) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 15 EUROPE BRIDGE-TO-RECOVERY (BTR) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 16 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 17 EUROPE ADULT IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 18 EUROPE ADULT IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 19 EUROPE PEDIATRIC IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 20 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY GENERATION, 2020-2029 (USD MILLION)
TABLE 21 EUROPE SECOND GENERATION DEVICES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 22 EUROPE THIRD GENERATION DEVICES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 23 EUROPE FIRST GENERATION DEVICES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 24 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DESIGN, 2020-2029 (USD MILLION)
TABLE 25 EUROPE AXIAL IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 26 EUROPE CENTRIFUGAL IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 27 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 28 EUROPE CONGESTIVE HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 29 EUROPE CONGESTIVE HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 30 EUROPE CONGENITAL HEART DISEASE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 31 EUROPE CONGENITAL HEART DISEASE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 32 EUROPE MYOCARDITIS IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 33 EUROPE MYOCARDITIS IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 34 EUROPE CARDIAC ARREST IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 35 EUROPE CARDIAC ARREST IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 36 EUROPE FAMILIAL ARRHYTHYMIAS AND ARRHYTHMIC CARDIOMYOPATHIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 37 EUROPE FAMILIAL ARRHYTHMIAS AND ARRHYTHMIC CARDIOMYOPATHIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 38 EUROPE ADVANCED HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 39 EUROPE ADVANCED HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 40 EUROPE OTHERS IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 41 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DURABILITY, 2020-2029 (USD MILLION)
TABLE 42 EUROPE LONG TERM IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 43 EUROPE INTERMEDIATE-TERM IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 44 EUROPE SHORT-TERM IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION))
TABLE 45 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PULSE TYPE, 2020-2029 (USD MILLION)
TABLE 46 EUROPE NONPULSATILE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 47 EUROPE PULSATILE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 48 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 49 EUROPE HOSPITALS IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 50 EUROPE CARDIAC CATH LABORATORIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 51 EUROPE SPECIALTY CLINICS IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 52 EUROPE OTHERS IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 53 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 54 EUROPE DIRECT TENDER IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 55 EUROPE RETAIL SALES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 56 EUROPE OTHERS IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 57 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY COUNTRY, 2022-2029 (USD MILLION)
TABLE 58 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 59 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 60 EUROPE BATTERIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 61 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 62 EUROPE BRIDGE-TO-TRANSPLANT (BTT) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 63 EUROPE DESTINATION THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 64 EUROPE BRIDGE-TO-CANDIDACY (BTC) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 65 EUROPE BRIDGE-TO-RECOVERY (BTR) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 66 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 67 EUROPE ADULT IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 68 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 69 EUROPE CONGESTIVE HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 70 EUROPE CONGENITAL HEART DISEASE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 71 EUROPE MYOCARDITIS IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 72 EUROPE CARDIAC ARREST IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 73 EUROPE FAMILIAL ARRHYTHMIAS AND ARRHYTHMIC CARDIOMYOPATHIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 74 EUROPE ADVANCED HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 75 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY GENERATION, 2020-2029 (USD MILLION)
TABLE 76 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DURABILITY, 2020-2029 (USD MILLION)
TABLE 77 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DESIGN, 2020-2029 (USD MILLION)
TABLE 78 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PULSE TYPE, 2020-2029 (USD MILLION)
TABLE 79 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 80 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 81 GERMANY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 82 GERMANY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 83 GERMANY BATTERIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 84 GERMANY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 85 GERMANY BRIDGE-TO-TRANSPLANT (BTT) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 86 GERMANY DESTINATION THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 87 GERMANY BRIDGE-TO-CANDIDACY (BTC) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 88 GERMANY BRIDGE-TO-RECOVERY (BTR) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 89 GERMANY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 90 GERMANY ADULT IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 91 GERMANY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 92 GERMANY CONGESTIVE HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 93 GERMANY CONGENITAL HEART DISEASE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 94 GERMANY MYOCARDITIS IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 95 GERMANY CARDIAC ARREST IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 96 GERMANY FAMILIAL ARRHYTHMIAS AND ARRHYTHMIC CARDIOMYOPATHIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 97 GERMANY ADVANCED HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 98 GERMANY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY GENERATION, 2020-2029 (USD MILLION)
TABLE 99 GERMANY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DURABILITY, 2020-2029 (USD MILLION)
TABLE 100 GERMANY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DESIGN, 2020-2029 (USD MILLION)
TABLE 101 GERMANY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PULSE TYPE, 2020-2029 (USD MILLION)
TABLE 102 GERMANY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 103 GERMANY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 104 FRANCE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 105 FRANCE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 106 FRANCE BATTERIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 107 FRANCE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 108 FRANCE BRIDGE-TO-TRANSPLANT (BTT) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 109 FRANCE DESTINATION THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 110 FRANCE BRIDGE-TO-CANDIDACY (BTC) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 111 FRANCE BRIDGE-TO-RECOVERY (BTR) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 112 FRANCE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 113 FRANCE ADULT IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 114 FRANCE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 115 FRANCE CONGESTIVE HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 116 FRANCE CONGENITAL HEART DISEASE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 117 FRANCE MYOCARDITIS IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 118 FRANCE CARDIAC ARREST IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 119 FRANCE FAMILIAL ARRHYTHMIAS AND ARRHYTHMIC CARDIOMYOPATHIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 120 FRANCE ADVANCED HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 121 FRANCE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY GENERATION, 2020-2029 (USD MILLION)
TABLE 122 FRANCE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DURABILITY, 2020-2029 (USD MILLION)
TABLE 123 FRANCE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DESIGN, 2020-2029 (USD MILLION)
TABLE 124 FRANCE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PULSE TYPE, 2020-2029 (USD MILLION)
TABLE 125 FRANCE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 126 FRANCE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 127 U.K. LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 128 U.K. LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 129 U.K. BATTERIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 130 U.K. LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 131 U.K. BRIDGE-TO-TRANSPLANT (BTT) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 132 U.K. DESTINATION THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 133 U.K. BRIDGE-TO-CANDIDACY (BTC) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 134 U.K. BRIDGE-TO-RECOVERY (BTR) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 135 U.K. LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 136 U.K. ADULT IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 137 U.K. LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 138 U.K. CONGESTIVE HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 139 U.K. CONGENITAL HEART DISEASE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 140 U.K. MYOCARDITIS IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 141 U.K. CARDIAC ARREST IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 142 U.K. FAMILIAL ARRHYTHMIAS AND ARRHYTHMIC CARDIOMYOPATHIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 143 U.K. ADVANCED HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 144 U.K. LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY GENERATION, 2020-2029 (USD MILLION)
TABLE 145 U.K. LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DURABILITY, 2020-2029 (USD MILLION)
TABLE 146 U.K. LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DESIGN, 2020-2029 (USD MILLION)
TABLE 147 U.K. LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PULSE TYPE, 2020-2029 (USD MILLION)
TABLE 148 U.K. LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 149 U.K. LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 150 ITALY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 151 ITALY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 152 ITALY BATTERIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 153 ITALY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 154 ITALY BRIDGE-TO-TRANSPLANT (BTT) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 155 ITALY DESTINATION THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 156 ITALY BRIDGE-TO-CANDIDACY (BTC) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 157 ITALY BRIDGE-TO-RECOVERY (BTR) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 158 ITALY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 159 ITALY ADULT IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 160 ITALY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 161 ITALY CONGESTIVE HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 162 ITALY CONGENITAL HEART DISEASE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 163 ITALY MYOCARDITIS IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 164 ITALY CARDIAC ARREST IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 165 ITALY FAMILIAL ARRHYTHMIAS AND ARRHYTHMIC CARDIOMYOPATHIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 166 ITALY ADVANCED HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 167 ITALY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY GENERATION, 2020-2029 (USD MILLION)
TABLE 168 ITALY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DURABILITY, 2020-2029 (USD MILLION)
TABLE 169 ITALY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DESIGN, 2020-2029 (USD MILLION)
TABLE 170 ITALY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PULSE TYPE, 2020-2029 (USD MILLION)
TABLE 171 ITALY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 172 ITALY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 173 RUSSIA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 174 RUSSIA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 175 RUSSIA BATTERIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 176 RUSSIA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 177 RUSSIA BRIDGE-TO-TRANSPLANT (BTT) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 178 RUSSIA DESTINATION THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 179 RUSSIA BRIDGE-TO-CANDIDACY (BTC) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 180 RUSSIA BRIDGE-TO-RECOVERY (BTR) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 181 RUSSIA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 182 RUSSIA ADULT IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 183 RUSSIA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 184 RUSSIA CONGESTIVE HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 185 RUSSIA CONGENITAL HEART DISEASE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 186 RUSSIA MYOCARDITIS IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 187 RUSSIA CARDIAC ARREST IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 188 RUSSIA FAMILIAL ARRHYTHMIAS AND ARRHYTHMIC CARDIOMYOPATHIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 189 RUSSIA ADVANCED HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 190 RUSSIA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY GENERATION, 2020-2029 (USD MILLION)
TABLE 191 RUSSIA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DURABILITY, 2020-2029 (USD MILLION)
TABLE 192 RUSSIA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DESIGN, 2020-2029 (USD MILLION)
TABLE 193 RUSSIA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PULSE TYPE, 2020-2029 (USD MILLION)
TABLE 194 RUSSIA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 195 RUSSIA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 196 SPAIN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 197 SPAIN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 198 SPAIN BATTERIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 199 SPAIN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 200 SPAIN BRIDGE-TO-TRANSPLANT (BTT) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 201 SPAIN DESTINATION THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 202 SPAIN BRIDGE-TO-CANDIDACY (BTC) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 203 SPAIN BRIDGE-TO-RECOVERY (BTR) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 204 SPAIN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 205 SPAIN ADULT IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 206 SPAIN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 207 SPAIN CONGESTIVE HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 208 SPAIN CONGENITAL HEART DISEASE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 209 SPAIN MYOCARDITIS IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 210 SPAIN CARDIAC ARREST IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 211 SPAIN FAMILIAL ARRHYTHMIAS AND ARRHYTHMIC CARDIOMYOPATHIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 212 SPAIN ADVANCED HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 213 SPAIN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY GENERATION, 2020-2029 (USD MILLION)
TABLE 214 SPAIN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DURABILITY, 2020-2029 (USD MILLION)
TABLE 215 SPAIN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DESIGN, 2020-2029 (USD MILLION)
TABLE 216 SPAIN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PULSE TYPE, 2020-2029 (USD MILLION)
TABLE 217 SPAIN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 218 SPAIN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 219 TURKEY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 220 TURKEY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 221 TURKEY BATTERIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 222 TURKEY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 223 TURKEY BRIDGE-TO-TRANSPLANT (BTT) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 224 TURKEY DESTINATION THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 225 TURKEY BRIDGE-TO-CANDIDACY (BTC) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 226 TURKEY BRIDGE-TO-RECOVERY (BTR) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 227 TURKEY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 228 TURKEY ADULT IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 229 TURKEY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 230 TURKEY CONGESTIVE HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 231 TURKEY CONGENITAL HEART DISEASE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 232 TURKEY MYOCARDITIS IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 233 TURKEY CARDIAC ARREST IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 234 TURKEY FAMILIAL ARRHYTHMIAS AND ARRHYTHMIC CARDIOMYOPATHIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 235 TURKEY ADVANCED HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 236 TURKEY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY GENERATION, 2020-2029 (USD MILLION)
TABLE 237 TURKEY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DURABILITY, 2020-2029 (USD MILLION)
TABLE 238 TURKEY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DESIGN, 2020-2029 (USD MILLION)
TABLE 239 TURKEY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PULSE TYPE, 2020-2029 (USD MILLION)
TABLE 240 TURKEY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 241 TURKEY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 242 NETHERLANDS LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 243 NETHERLANDS LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 244 NETHERLANDS BATTERIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 245 NETHERLANDS LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 246 NETHERLANDS BRIDGE-TO-TRANSPLANT (BTT) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 247 NETHERLANDS DESTINATION THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 248 NETHERLANDS BRIDGE-TO-CANDIDACY (BTC) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 249 NETHERLANDS BRIDGE-TO-RECOVERY (BTR) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 250 NETHERLANDS LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 251 NETHERLANDS ADULT IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 252 NETHERLANDS LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 253 NETHERLANDS CONGESTIVE HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 254 NETHERLANDS CONGENITAL HEART DISEASE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 255 NETHERLANDS MYOCARDITIS IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 256 NETHERLANDS CARDIAC ARREST IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 257 NETHERLANDS FAMILIAL ARRHYTHMIAS AND ARRHYTHMIC CARDIOMYOPATHIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 258 NETHERLANDS ADVANCED HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 259 NETHERLANDS LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY GENERATION, 2020-2029 (USD MILLION)
TABLE 260 NETHERLANDS LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DURABILITY, 2020-2029 (USD MILLION)
TABLE 261 NETHERLANDS LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DESIGN, 2020-2029 (USD MILLION)
TABLE 262 NETHERLANDS LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PULSE TYPE, 2020-2029 (USD MILLION)
TABLE 263 NETHERLANDS LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 264 NETHERLANDS LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 265 SWITZERLAND LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 266 SWITZERLAND LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 267 SWITZERLAND BATTERIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 268 SWITZERLAND LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 269 SWITZERLAND BRIDGE-TO-TRANSPLANT (BTT) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 270 SWITZERLAND DESTINATION THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 271 SWITZERLAND BRIDGE-TO-CANDIDACY (BTC) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 272 SWITZERLAND BRIDGE-TO-RECOVERY (BTR) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 273 SWITZERLAND LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 274 SWITZERLAND ADULT IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 275 SWITZERLAND LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 276 SWITZERLAND CONGESTIVE HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 277 SWITZERLAND CONGENITAL HEART DISEASE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 278 SWITZERLAND MYOCARDITIS IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 279 SWITZERLAND CARDIAC ARREST IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 280 SWITZERLAND FAMILIAL ARRHYTHMIAS AND ARRHYTHMIC CARDIOMYOPATHIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 281 SWITZERLAND ADVANCED HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 282 SWITZERLAND LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY GENERATION, 2020-2029 (USD MILLION)
TABLE 283 SWITZERLAND LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DURABILITY, 2020-2029 (USD MILLION)
TABLE 284 SWITZERLAND LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DESIGN, 2020-2029 (USD MILLION)
TABLE 285 SWITZERLAND LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PULSE TYPE, 2020-2029 (USD MILLION)
TABLE 286 SWITZERLAND LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 287 SWITZERLAND LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 288 BELGIUM LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 289 BELGIUM LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 290 BELGIUM BATTERIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 291 BELGIUM LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 292 BELGIUM BRIDGE-TO-TRANSPLANT (BTT) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 293 BELGIUM DESTINATION THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 294 BELGIUM BRIDGE-TO-CANDIDACY (BTC) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 295 BELGIUM BRIDGE-TO-RECOVERY (BTR) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 296 BELGIUM LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 297 BELGIUM ADULT IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 298 BELGIUM LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 299 BELGIUM CONGESTIVE HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 300 BELGIUM CONGENITAL HEART DISEASE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 301 BELGIUM MYOCARDITIS IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 302 BELGIUM CARDIAC ARREST IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 303 BELGIUM FAMILIAL ARRHYTHMIAS AND ARRHYTHMIC CARDIOMYOPATHIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 304 BELGIUM ADVANCED HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 305 BELGIUM LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY GENERATION, 2020-2029 (USD MILLION)
TABLE 306 BELGIUM LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DURABILITY, 2020-2029 (USD MILLION)
TABLE 307 BELGIUM LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DESIGN, 2020-2029 (USD MILLION)
TABLE 308 BELGIUM LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PULSE TYPE, 2020-2029 (USD MILLION)
TABLE 309 BELGIUM LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 310 BELGIUM LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 311 IRELAND LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 312 IRELAND LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 313 IRELAND BATTERIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 314 IRELAND LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 315 IRELAND BRIDGE-TO-TRANSPLANT (BTT) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 316 IRELAND DESTINATION THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 317 IRELAND BRIDGE-TO-CANDIDACY (BTC) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 318 IRELAND BRIDGE-TO-RECOVERY (BTR) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 319 IRELAND LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 320 IRELAND ADULT IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 321 IRELAND LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 322 IRELAND CONGESTIVE HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 323 IRELAND CONGENITAL HEART DISEASE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 324 IRELAND MYOCARDITIS IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 325 IRELAND CARDIAC ARREST IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 326 IRELAND FAMILIAL ARRHYTHMIAS AND ARRHYTHMIC CARDIOMYOPATHIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 327 IRELAND ADVANCED HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 328 IRELAND LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY GENERATION, 2020-2029 (USD MILLION)
TABLE 329 IRELAND LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DURABILITY, 2020-2029 (USD MILLION)
TABLE 330 IRELAND LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DESIGN, 2020-2029 (USD MILLION)
TABLE 331 IRELAND LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PULSE TYPE, 2020-2029 (USD MILLION)
TABLE 332 IRELAND LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 333 IRELAND LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 334 REST OF EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
Lista de Figura
FIGURE 1 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: SEGMENTATION
FIGURE 2 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: DATA TRIANGULATION
FIGURE 3 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: DROC ANALYSIS
FIGURE 4 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: EUROPE VS REGIONAL ANALYSIS
FIGURE 5 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: DBMR MARKET POSITION GRID
FIGURE 8 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: END USER COVERAGE GRID
FIGURE 9 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET:VENDOR SHARE ANALYSIS
FIGURE 10 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: SEGMENTATION
FIGURE 11 INCREASING PREVALENCE OF CARDIOVASCULAR DISEASE AND RISING HEALTHCARE EXPENDITURE ARE EXPECTED TO DRIVE THE EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET IN THE FORECAST PERIOD OF 2022 TO 2029
FIGURE 12 HEART PUMP SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET IN 2022 & 2029
FIGURE 13 NORTH AMERICA IS EXPECTED TO DOMINATE THE EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET AND ASIA-PACIFIC TO GROW WITH THE HIGHEST CAGR IN THE FORECAST PERIOD OF 2022 TO 2029
FIGURE 14 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET
FIGURE 15 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY PRODUCT TYPE, 2021
FIGURE 16 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY PRODUCT TYPE, 2020-2029 (USD MILLION)
FIGURE 17 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY PRODUCT TYPE, CAGR (2022-2029)
FIGURE 18 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY PRODUCT TYPE, LIFELINE CURVE
FIGURE 19 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY THERAPY, 2021
FIGURE 20 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY THERAPY, 2020-2029 (USD MILLION)
FIGURE 21 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY THERAPY, CAGR (2022-2029)
FIGURE 22 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY THERAPY, LIFELINE CURVE
FIGURE 23 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY AGE GROUP, 2021
FIGURE 24 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY AGE GROUP, 2020-2029 (USD MILLION)
FIGURE 25 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY AGE GROUP, CAGR (2022-2029)
FIGURE 26 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY AGE GROUP, LIFELINE CURVE
FIGURE 27 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY GENERATION, 2021
FIGURE 28 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY GENERATION, 2020-2029 (USD MILLION)
FIGURE 29 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY GENERATION, CAGR (2022-2029)
FIGURE 30 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY GENERATION, LIFELINE CURVE
FIGURE 31 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY DESIGN, 2021
FIGURE 32 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY DESIGN, 2020-2029 (USD MILLION)
FIGURE 33 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY DESIGN, CAGR (2022-2029)
FIGURE 34 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY DESIGN, LIFELINE CURVE
FIGURE 35 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET: BY INDICATION, 2021
FIGURE 36 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET: BY INDICATION, 2020-2029 (USD MILLION)
FIGURE 37 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET: BY INDICATION, CAGR (2022-2029)
FIGURE 38 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET: BY INDICATION, LIFELINE CURVE
FIGURE 39 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET: BY DURABILITY, 2021
FIGURE 40 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET: BY DURABILITY, 2020-2029 (USD MILLION)
FIGURE 41 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET: BY DURABILITY, CAGR (2022-2029)
FIGURE 42 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET: BY DURABILITY, LIFELINE CURVE
FIGURE 43 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET: BY PULSE TYPE, 2021
FIGURE 44 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET: BY PULSE TYPE, 2020-2029 (USD MILLION)
FIGURE 45 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET: BY PULSE TYPE, CAGR (2022-2029)
FIGURE 46 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET: BY PULSE TYPE, LIFELINE CURVE
FIGURE 47 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET: BY END USER, 2021
FIGURE 48 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET: BY END USER, 2020-2029 (USD MILLION)
FIGURE 49 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET: BY END USER, CAGR (2022-2029)
FIGURE 50 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET: BY END USER, LIFELINE CURVE
FIGURE 51 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET: BY DISTRIBUTION CHANNEL, 2021
FIGURE 52 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET: BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
FIGURE 53 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET: BY DISTRIBUTION CHANNEL, CAGR (2022-2029)
FIGURE 54 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 55 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: SNAPSHOT (2021)
FIGURE 56 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY COUNTRY (2021)
FIGURE 57 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY COUNTRY (2022 & 2029)
FIGURE 58 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY COUNTRY (2021 & 2029)
FIGURE 59 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY PRODUCT TYPE (2022-2029)
FIGURE 60 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: COMPANY SHARE 2021 (%)

Metodologia de Investigação
A recolha de dados e a análise do ano base são feitas através de módulos de recolha de dados com amostras grandes. A etapa inclui a obtenção de informações de mercado ou dados relacionados através de diversas fontes e estratégias. Inclui examinar e planear antecipadamente todos os dados adquiridos no passado. Da mesma forma, envolve o exame de inconsistências de informação observadas em diferentes fontes de informação. Os dados de mercado são analisados e estimados utilizando modelos estatísticos e coerentes de mercado. Além disso, a análise da quota de mercado e a análise das principais tendências são os principais fatores de sucesso no relatório de mercado. Para saber mais, solicite uma chamada de analista ou abra a sua consulta.
A principal metodologia de investigação utilizada pela equipa de investigação do DBMR é a triangulação de dados que envolve a mineração de dados, a análise do impacto das variáveis de dados no mercado e a validação primária (especialista do setor). Os modelos de dados incluem grelha de posicionamento de fornecedores, análise da linha de tempo do mercado, visão geral e guia de mercado, grelha de posicionamento da empresa, análise de patentes, análise de preços, análise da quota de mercado da empresa, normas de medição, análise global versus regional e de participação dos fornecedores. Para saber mais sobre a metodologia de investigação, faça uma consulta para falar com os nossos especialistas do setor.
Personalização disponível
A Data Bridge Market Research é líder em investigação formativa avançada. Orgulhamo-nos de servir os nossos clientes novos e existentes com dados e análises que correspondem e atendem aos seus objetivos. O relatório pode ser personalizado para incluir análise de tendências de preços de marcas-alvo, compreensão do mercado para países adicionais (solicite a lista de países), dados de resultados de ensaios clínicos, revisão de literatura, mercado remodelado e análise de base de produtos . A análise de mercado dos concorrentes-alvo pode ser analisada desde análises baseadas em tecnologia até estratégias de carteira de mercado. Podemos adicionar quantos concorrentes necessitar de dados no formato e estilo de dados que procura. A nossa equipa de analistas também pode fornecer dados em tabelas dinâmicas de ficheiros Excel em bruto (livro de factos) ou pode ajudá-lo a criar apresentações a partir dos conjuntos de dados disponíveis no relatório.