Europe Genetic Testing Market
Tamanho do mercado em biliões de dólares
CAGR :
% 
 USD
4,344.02 Billion
USD
12,730.46 Billion
2021
2029
USD
4,344.02 Billion
USD
12,730.46 Billion
2021
2029
| 2022 –2029 | |
| USD 4,344.02 Billion | |
| USD 12,730.46 Billion | |
|
|
|
Europe Genetic Testing Market, By Type (Carrier Testing, Diagnostic Testing, Prenatal Testing, New Born Screening, Predictive And Presymptomatic Testing, Other Types), Technology (DNA Sequencing (NGS-Based Testing), Polymerase Chain Reaction, Microarrays, Whole Genome Sequencing, Fluorescence In Situ Hybridization (FISH), Others), Diseases (Rare Genetic Disorder, Cancer, Cystic Fibrosis, Sickle Cell Anemia, Duchenne Muscular Dystrophy, Thalassemia, Huntington’s Disease, Fragile X Syndrome, Duchenne Muscular Dystrophy, Others) End User (Hospitals, Clinics, Diagnostic Centres, Private Clinics, Laboratory Service Providers, Private Laboratories) Industry Trends and Forecast to 2029
Market Analysis and Insights
Europe genetic testing market is driven by the factors such as the high prevalence of genetic disorders, growing technological advancements in the genetic testing market which enhance its demand, as well as increasing investment in research and development, which leads to market growth. Currently, healthcare expenditure has increased across developed and emerging countries which are expected to create a competitive advantage for manufacturers to develop new and innovative genetic testing markets. However, the high cost associated with genetic testing and stringent regulatory frameworks for genetic testing.
Europe genetic testing market report provides details of market share, new developments, and product pipeline analysis, the impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, product approvals, strategic decisions, product launches, geographic expansions, and technological innovations in the market. To understand the analysis and the market scenario contact us for an analyst brief, our team will help you create a revenue impact solution to achieve your desired goal. The scalability and business expansion of the retail units in the developing countries of various region and partnership with suppliers for safe distribution of machine and drugs products are the major drivers which propelled the demand of the market in the forecast period.
Market Definition
Genetic testing is a kind of medical test that identifies changes in genes, chromosomes, or proteins. The outcome of a genetic test can confirm or rule out a suspected genetic condition or aid in determining a person’s chance of developing or passing on a genetic disorder. Over 77,000 genetic tests are currently in use, and others are being developed.
The increasing innovations and technologies and rising number of players in the market, and novel product launches are also propelling the growth of the Europe genetic testing market.
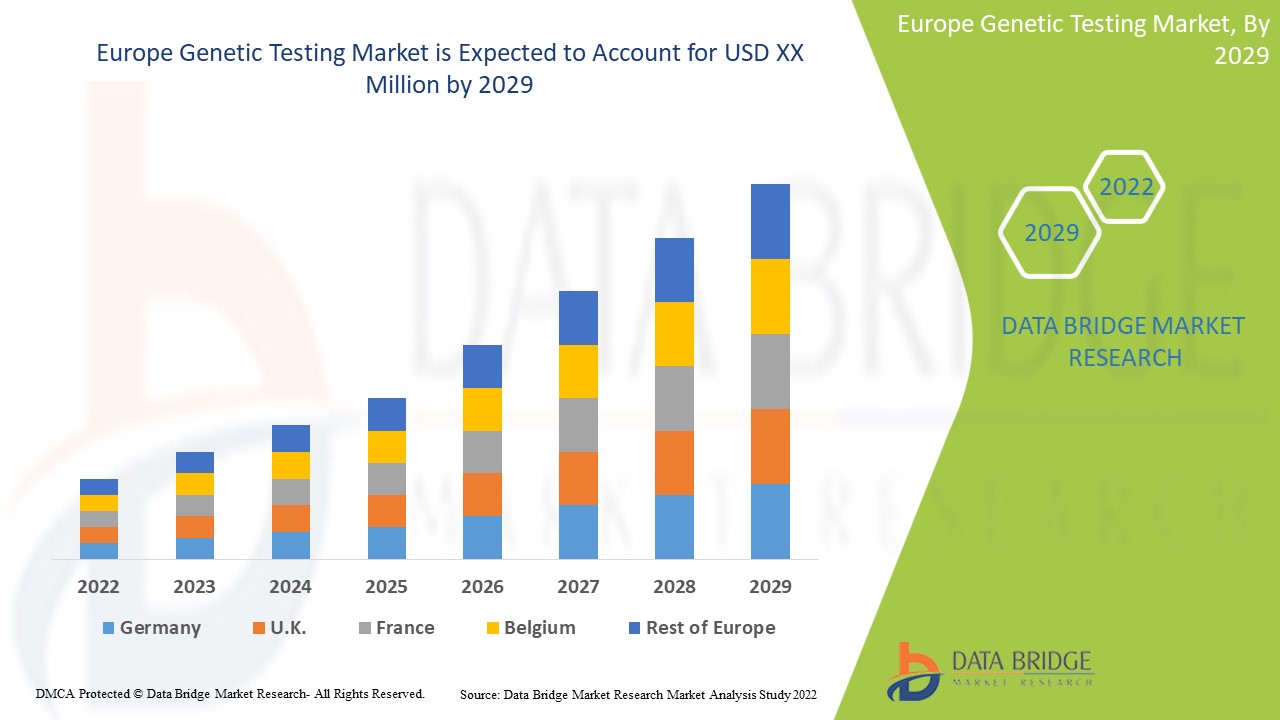
Europe genetic testing market is expected to gain market growth in the forecast period of 2022 to 2029. Data Bridge Market Research analyses that the market is growing with a CAGR of 14.6% in the forecast period of 2022 to 2029 and is expected to reach USD 12,730.46 million by 2029 from USD 4,344.02 million in 2021.
|
Report Metric |
Details |
|
Forecast Period |
2022 to 2029 |
|
Base Year |
2021 |
|
Historic Years |
2020 |
|
Quantitative Units |
Revenue in USD Million |
|
Segments Covered |
By Type (Carrier Testing, Diagnostic Testing, Prenatal Testing, New Born Screening, Predictive And Presymptomatic Testing, Other Types), Technology (DNA Sequencing (NGS-Based Testing), Polymerase Chain Reaction, Microarrays, Whole Genome Sequencing, Fluorescence In Situ Hybridization (FISH), Others), Diseases (Rare Genetic Disorder, Cancer, Cystic Fibrosis, Sickle Cell Anemia, Duchenne Muscular Dystrophy, Thalassemia, Huntington’s Disease, Fragile X Syndrome, Duchenne Muscular Dystrophy, Others) End User (Hospitals, Clinics, Diagnostic Centres, Private Clinics, Laboratory Service Providers, Private Laboratories) |
|
Countries Covered |
Germany, France, U.K. Italy, Spain, Russia, Turkey, Belgium, Netherlands, Switzerland, Rest Of Europe |
|
Market Players Covered |
Thermo Fisher Scientific Inc., Bio-Rad Laboratories, Inc., PerkinElmer Inc., Illumina, Inc., QIAGEN, F. Hoffmann-La Roche Ltd., Myriad Genetics, Inc., Abbott, Eurofins Scientific, Biocartis, Cepheid ( A subsidiary of Danaher), PacBio, BioReference, Natera, Inc. among others |
Europe Genetic Testing Market Dynamics
Drivers
- Growing prevalence of genetic disorders
For instance,
- As per the publication of January 2019, entitled “European Breast Cancer Council manifesto 2018: Genetic risk prediction testing in breast cancer”, this has been indicated that BRCA1/2 tests are widely spread across U.K. and other European regions indicated for cancer testing.
- Organized genetic testing programs are provided in European Union which ensures that high-quality screening services are provided to population.
Therefore, this is raising the demand for genetic testing market.
- Increase In The Adoption Of Next Generation Sequencing
As genomics-focused pharmacology continues to play a greater role in the treatment of various chronic diseases, especially cancer, next-generation sequencing (NGS) is evolving as a powerful tool for providing a deeper and more precise insight into the molecular underpinnings of individual tumors and specific receptors.
NGS offers advantages in accuracy, sensitivity, and speed compared to traditional methods that have the potential to make a significant impact on the field of oncology. Because NGS can assess multiple genes in a single assay, the need to order multiple tests to identify the causative mutation is eliminated.
For instance,
- NGS has been also explored for comprehensive profiling of pharmacogenetics with relevance to drug Pharmacokinetics and Pharmacodynamics, initial reports of 2017 suggest that this technology may represent a reliable and efficient tool to discover both common and rare genetic variations in these genes.
Thus, this is expected to act as a driver for the growth of the genetic testing market.
Opportunities
-
Increasing Research And Development
For instance,
-
As per the publication of 2020, entitled “Availability and funding of clinical genomic sequencing globally”, this has been indicated that maximally many European countries other than United Kingdom have increasing availability of genetic tests for promoting the growth of NGS (next generation sequencing) and genomic medicine.
-
Rising Disposable Income
The expense of money used by a country on its healthcare and its growth rate over time is inclined by a wide variety of economic and social factors including the financing arrangements and structure of the organization of the health system. In particular, there is a strong association between the complete income level of a country and how much the population of that country spends on health care.
Also, the strategic initiatives taken by key market players will provide structural integrity and future opportunities for the genetic testing market in the forecast period of 2022-2029.
Restraints/Challenges
- High Cost Of Genetic Testing
Genetic testing can be expensive and may not be covered by some health insurance plans. The numerous genetic tests differ in costs as per the targeted disease for which it is tested.
According to Breastcancer.org, the cost of genetic testing for cancer can vary greatly and can be between $300 and $5,000. How much genetic testing costs can depend on the type of test as well as how complex it is
Genetic testing can cost anywhere from $100 to more than $2,000, depending on the test's nature and complexity. If more than one test is required or numerous family members must be tested to acquire a significant result, the expense rises. The cost of neonatal screening varies by state.
Covid-19 Impact on the Europe Genetic Testing Market
The COVID-19 has positively affected the market as many genetic and serological tests were conducted for COVID-19 which raises the demand for genetic testing during this period.
Recent Development
- In December 2021, Thermo Fisher Scientific Inc., announced that it has completed its acquisition of PPD, Inc., a leading global provider of clinical research services to the biopharma and biotech industry, for $17.4 billion. This acquisition helped to generate more revenue and boosted the market growth.
Europe Genetic Testing Market Scope
Europe genetic testing market is segmented into type, technology, diseases and end user. The growth amongst these segments will help you analyse meagre growth segments in the industries and provide the users with a valuable market overview and market insights to make strategic decisions to identify core market applications.
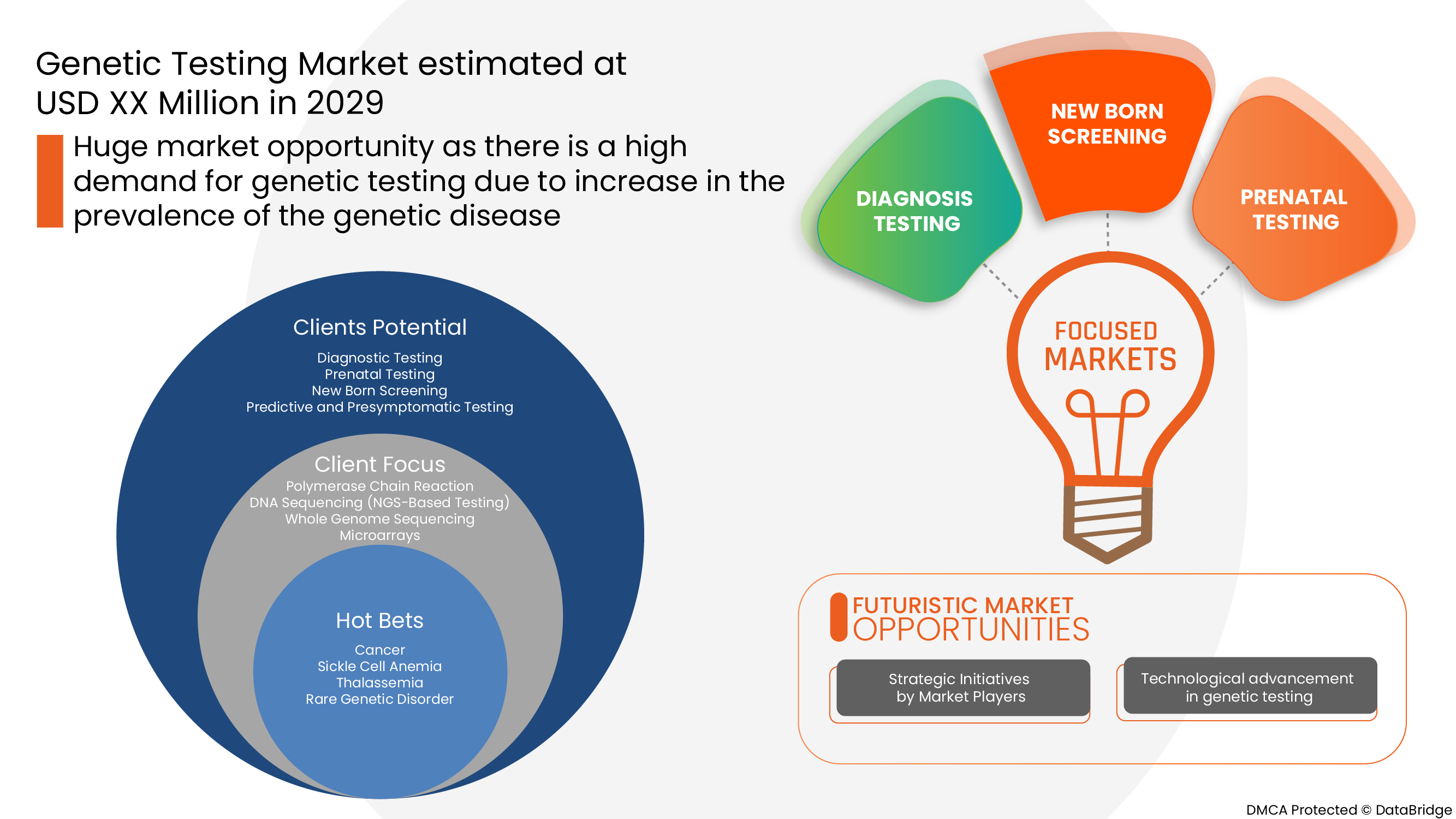
Type
- Diagnostic Testing
- Prenatal Testing
- New Born Screening
- Predictive and Presymptomatic Testing
- Carrier Testing
- Other Types
On the basis of type, the Europe genetic testing market is segmented into diagnostic testing, prenatal testing, new born screening, predictive and presymptomatic testing, carrier testing and other types.
Technology
- Polymerase Chain Reaction
- DNA Sequencing (NGS-Based Testing)
- Whole Genome Sequencing
- Microarrays
- Fluorescence In Situ Hybridization (FISH)
- Others
On the basis of technology, the Europe genetic testing market is segmented into DNA sequencing (NGS-based testing), polymerase chain reaction, microarrays, whole genome sequencing, fluorescence in situ hybridization (FISH), others.
Diseases
- Cancer
- Sickle Cell Anemia
- Thalassemia
- Rare Genetic Disorder
- Fragile X Syndrome
- Duchenne Muscular Dystrophy
- Huntington's Disease
- Cystic Fibrosis
- Others
On the basis of diseases, the Europe genetic testing market is segmented into rare genetic disorder, cancer, cystic fibrosis, sickle cell anemia, duchenne muscular dystrophy, thalassemia, huntington’s disease, fragile X syndrome, other.
End User
- Hospitals
- Clinics
- Diagnostic Centers
- Private Clinics
- Laboratory Service Providers
- Private Laboratories
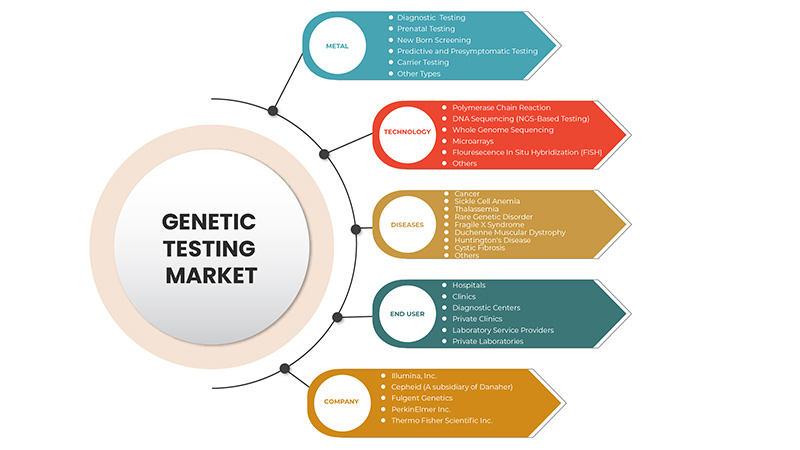
On the basis of end users, the Europe genetic testing market is segmented into hospitals, clinics, diagnostic centers, private clinics, laboratory service providers, private laboratories.
Genetic Testing Market Regional Analysis/Insights
The genetic testing market is analysed and market size insights and trends are provided by country, type, technology, diseases and end user as referenced above.
Germany are the major dominating countries in the market due to the growing prevalence of genetic disorders amongst the population in these countries. And they are dominating the genetic testing market in terms of market share and market revenue and will continue to flourish its dominance during the forecast period. This is due to genetic defects and chromosomal aberrations in the population in the regions and rapid research development is boosting the market.
The country section of the report also provides individual market impacting factors and changes in regulations in the market that impact the current and future trends of the market. Data points, such as new and replacement sales, country demographics, disease epidemiology, and import-export tariffs, are some of the major pointers used to forecast the market scenario for individual countries. In addition, the presence and availability of global brands and their challenges faced due to high competition from local and domestic brands, and impact of sales channels are considered while providing forecast analysis of the country data.
Competitive Landscape and Genetic Testing Market Share Analysis
The genetic testing market competitive landscape provides details about the competitors. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, and application dominance. The above data points provided are only related to the companies' focus on the genetic testing market.
Some of the major players operating in the genetic testing market are Thermo Fisher Scientific Inc., Illumina, Inc., QIAGEN, F. Hoffmann-La Roche Ltd., Myriad Genetics, Inc., Abbott, Eurofins Scientific, Biocartis, Cepheid ( A subsidiary of Danaher), PacBio, BioReference, Natera, Inc. among others
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The market data is analysed and estimated using market statistical and coherent models. In addition, market share analysis and key trend analysis are the major success factors in the market report. The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market, and primary (industry expert) validation. Apart from this, data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Company Market Share Analysis, Standards of Measurement, Global vs. Regional and Vendor Share Analysis. Please request analyst call in case of further inquiry.
SKU-
Obtenha acesso online ao relatório sobre a primeira nuvem de inteligência de mercado do mundo
- Painel interativo de análise de dados
- Painel de análise da empresa para oportunidades de elevado potencial de crescimento
- Acesso de analista de pesquisa para personalização e customização. consultas
- Análise da concorrência com painel interativo
- Últimas notícias, atualizações e atualizações Análise de tendências
- Aproveite o poder da análise de benchmark para um rastreio abrangente da concorrência
Índice
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF THE EUROPE GENETIC TESTING MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 TYPE LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 MARKET APPLICATION COVERAGE GRID
2.11 VENDOR SHARE ANALYSIS
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL ANALYSIS
4.2 PORTER’S FIVE FORCES
4.3 STRATEGIC INITIATIVES:
4.4 CONCLUSION:
4.5 INDUSTRY INSIGHTS
4.5.1 CANCER GENETICS RISK ASSESSMENT AND COUNSELING
4.5.2 GENETIC TESTS PRICING
4.5.3 KEY INSIGHTS
5 EPIDERMIOLOGY
6 EUROPE GENETIC TESTING MARKET: REGULATIONS
7 MARKET OVERVIEW
7.1 DRIVERS
7.1.1 GROWING PREVALENCE OF GENETIC DISORDERS
7.1.2 INCREASE IN THE ADOPTION OF NEXT GENERATION SEQUENCING
7.1.3 WIDE PRODUCT PORTFOLIO OFFERED BY A MAJOR PLAYER
7.1.4 INCREASE TREND TOWARD PERSONALIZED MEDICATION
7.2 RESTRAINTS
7.2.1 HIGH COST OF GENETIC TESTING
7.2.2 CYBER SECURITY CONCERNS IN GENOMICS
7.3 OPPORTUNITIES
7.3.1 STRATEGIC INITIATIVES BY THE KEY MARKET PLAYER
7.3.2 TECHNOLOGICAL ADVANCEMENT
7.3.3 INCREASING RESEARCH AND DEVELOPMENT
7.3.4 RISING DISPOSABLE INCOME
7.4 CHALLENGES
7.4.1 LACK OF SKILLED PROFESSIONALS TO PERFORM GENETIC TESTING
7.4.2 STRINGENT REGULATION POLICY
8 EUROPE GENETIC TESTING MARKET, BY TYPE
8.1 OVERVIEW
8.2 DIAGNOSTIC TESTING
8.3 PRENATAL TESTING
8.3.1 NON-INVASIVE SCREENING
8.3.1.1 BY SCREENING METHOD
8.3.1.1.1 WHOLE GENOME SEQUENCING
8.3.1.1.2 COUNTING OF CFDNA FRAGMENTS
8.3.1.1.3 OTHERS
8.3.1.2 BY CONDITION
8.3.1.2.1 TRISOMY 21
8.3.1.2.2 KLINEFELTER SYNDROME
8.3.1.2.3 JACOBS SYNDROME
8.3.1.2.4 CYSTIC FIBROSIS
8.3.1.2.5 TURNER SYNDROME
8.3.1.2.6 TRISOMY 18
8.3.1.2.7 HEMOPHILIA
8.3.1.2.8 TRISOMY 13
8.3.1.2.9 MICRODELETION SYNDROME
8.3.1.2.10 FETAL GENDER
8.3.1.2.11 OTHERS
8.3.1.3 BY SCREENING TYPE
8.3.1.3.1 CARRIER SEQUENCING
8.3.1.3.2 SEQUENTIAL SEQUENCING
8.3.2 MATERNAL SERUM QUAD SCREENING
8.4 NEW BORN SCREENING
8.4.1.1 SICKLE CELL DISEASE
8.4.1.2 CONGENITAL HYPOTHYROIDISM
8.4.1.3 PHENYLKETONURIA (PKU)
8.4.1.4 GALACTOSEMIA
8.4.1.5 MAPLE SYRUP URINE DISEASE
8.4.1.6 OTHERS
8.5 PREDICTIVE AND PRESYMPTOMATIC TESTING
8.6 CARRIER TESTING
8.6.1 BY TEST TYPE
8.6.1.1 MOLECULAR SCREENING TEST
8.6.1.2 BIOCHEMICAL SCREENING TEST
8.6.2 BY TYPE
8.6.2.1 EXPANDED CARRIER SCREENING
8.6.2.1.1 PREDESIGNED PANEL TESTING
8.6.2.1.2 CUSTOM-MADE PANEL TESTING
8.6.2.2 TARGETED DISEASE CARRIER SCREENING
8.6.2.2.1 BY MEDICAL CONDITION
8.6.2.2.2 HEMATOLOGICAL CONDITIONS
8.6.2.2.3 PULMONARY CONDITIONS
8.6.2.2.4 NEUROLOGICAL CONDITIONS
8.6.2.2.5 OTHER CONDITIONS
8.7 OTHER TYPES
9 EUROPE GENETIC TESTING MARKET, BY TECHNOLOGY
9.1 OVERVIEW
9.2 POLYMERASE CHAIN REACTION
9.2.1 REAL-TIME PCR (QPCR)
9.2.2 DIGITAL PCR (DPCR)
9.2.3 REVERSE TRANSCRIPTION PCR (RT-PCR)
9.2.4 HOT-START PCR
9.2.5 MULTIPLEX PCR
9.2.6 OTHER PCR
9.3 DNA SEQUENCING (NGS-BASED TESTING)
9.3.1 NEXT GENERATION SEQUENCING (NGS)
9.3.2 SANGER SEQUENCING (SINGLE GENE)
9.3.3 OTHER
9.4 WHOLE GENOME SEQUENCING
9.5 MICROARRAYS
9.5.1 DNA MICROARRAYS
9.5.2 PROTEIN MICROARRAYS
9.5.3 OTHER MICROARRAYS
9.6 FLUORESCENCE IN SITU HYBRIDIZATION (FISH)
9.7 OTHERS
10 EUROPE GENETIC TESTING MARKET, BY DISEASES
10.1 OVERVIEW
10.2 CANCER
10.2.1 BREAST
10.2.2 COLON
10.2.3 LUNG
10.2.4 PROSTATE
10.2.5 OTHERS
10.3 SICKLE CELL ANEMIA
10.4 THALASSEMIA
10.5 RARE GENETIC DISORDER
10.5.1 TRISOMY 21
10.5.2 MONOSOMY X
10.5.3 TRISOMY 13
10.5.4 MICRODELETION SYNDROME
10.5.5 TRISOMY 18
10.5.6 OTHERS
10.6 FRAGILE X SYNDROME
10.7 DUCHENNE MUSCULAR DYSTROPHY
10.8 HUNTINGTON'S DISEASE
10.9 CYSTIC FIBROSIS
10.1 OTHERS
11 EUROPE GENETIC TESTING MARKET, BY END USER
11.1 OVERVIEW
11.2 HOSPITALS
11.3 CLINICS
11.4 DIAGNOSTIC CENTERS
11.5 PRIVATE CLINICS
11.6 LABORATORY SERVICE PROVIDERS
11.7 PRIVATE LABORATORIES
12 EUROPE GENETIC TESTING MARKET, BY REGION
12.1 EUROPE
12.1.1 GERMANY
12.1.2 FRANCE
12.1.3 U.K.
12.1.4 RUSSIA
12.1.5 ITALY
12.1.6 SPAIN
12.1.7 TURKEY
12.1.8 NETHERLANDS
12.1.9 SWITZERLAND
12.1.10 BELGIUM
12.1.11 REST OF EUROPE
13 EUROPE GENETIC TESTING MARKET, COMPANY LANDSCAPE
13.1 COMPANY SHARE ANALYSIS: EUROPE
14 WOT ANALYSIS
15 COMPANY PROFILE
15.1 ILLUMINA, INC.
15.1.1 COMPANY SNAPSHOT
15.1.2 COMPANY SHARE ANALYSIS
15.1.3 PRODUCT PORTFOLIO
15.1.4 RECENT DEVELOPMENT
15.1.4.1 ACQUISITION
15.1.4.2 COLLABORATION
15.2 CEPHEID
15.2.1 COMPANY SNAPSHOT
15.2.2 REVENUE ANALYSIS
15.2.3 COMPANY SHARE ANALYSIS
15.2.4 PRODUCT PORTFOLIO
15.2.5 RECENT DEVELOPMENT
15.2.5.1 BUSINEES EXPANSION
15.3 FULGENT GENETICS
15.3.1 COMPANY SNAPSHOT
15.3.2 REVENUE ANALYSIS
15.3.3 COMPANY SHARE ANALYSIS
15.3.4 PRODUCT PORTFOLIO
15.3.5 RECENT DEVELOPMENT
15.3.5.1 ACQUISITION
15.4 PERKINELMER INC.
15.4.1 COMPANY SNAPSHOT
15.4.2 REVENUE ANALYSIS
15.4.3 COMPANY SHARE ANALYSIS
15.4.4 PRODUCT PORTFOLIO
15.4.5 RECENT DEVELOPMENT
15.4.5.1 PRODUCT LAUNCH
15.5 THERMO FISHER SCIENTIFIC INC.
15.5.1 COMPANY SNAPSHOT
15.5.2 REVENUE ANALYSIS
15.5.3 COMPANY SHARE ANALYSIS
15.5.4 PRODUCT PORTFOLIO
15.5.5 RECENT DEVELOPMENT
15.5.5.1 COLLABORATION
15.6 ABBOTT
15.6.1 COMPANY SNAPSHOT
15.6.2 REVENUE ANALYSIS
15.6.3 PRODUCT PORTFOLIO
15.6.4 RECENT DEVELOPMENT
15.7 BIOCARTIS
15.7.1 COMPANY SNAPSHOT
15.7.2 REVENUE ANALYSIS
15.7.3 PRODUCT PORTFOLIO
15.7.4 RECENT DEVELOPMENT
15.7.4.1 PARTNERSHIP
15.7.4.2 AGREEMENT
15.8 BIO-HELIX
15.8.1 COMPANY SNAPSHOT
15.8.2 PRODUCT PORTFOLIO
15.8.3 RECENT DEVELOPMENT
15.9 BIO-RAD LABORATORIES, INC.
15.9.1 COMPANY SNAPSHOT
15.9.2 REVENUE ANALYSIS
15.9.3 PRODUCT PORTFOLIO
15.9.4 RECENT DEVELOPMENTS
15.9.4.1 ACQUISITION
15.9.4.2 PARTNERSHIP
15.1 BIOREFERENCE
15.10.1 COMPANY SNAPSHOT
15.10.2 REVENUE ANALYSIS
15.10.3 PRODUCT PORTFOLIO
15.10.4 RECENT DEVELOPMENT
15.10.4.1 ACQUISITION
15.11 ELITECHGROUP
15.11.1 COMPANY SNAPSHOT
15.11.2 PRODUCT PORTFOLIO
15.11.3 RECENT DEVELOPMENTS
15.11.3.1 PRODUCT LAUNCH
15.11.3.2 BUSINESS EXPANSION
15.12 EUROFINS SCIENTIFIC
15.12.1 COMPANY SNAPSHOT
15.12.2 REVENUE ANALYSIS
15.12.3 PRODUCT PORTFOLIO
15.12.4 RECENT DEVELOPMENT
15.12.4.1 PRODUCT LAUNCH
15.13 EUGENE LABS
15.13.1 COMPANY SNAPSHOT
15.13.2 PRODUCT PORTFOLIO
15.13.3 RECENT DEVELOPMENT
15.14 F. HOFFMANN-LA ROCHE LTD)
15.14.1 COMPANY SNAPSHOT
15.14.2 REVENUE ANALYSIS (PARENT COMPANY)
15.14.3 PRODUCT PORTFOLIO
15.14.4 RECENT DEVELOPMENTS
15.14.4.1 PRODUCT LAUNCH
15.15 GENES2ME
15.15.1 COMPANY SNAPSHOT
15.15.2 PRODUCT PORTFOLIO
15.15.3 RECENT DEVELOPMENT
15.16 INVITAE CORPORATION
15.16.1 COMPANY SNAPSHOT
15.16.2 REVENUE ANALYSIS
15.16.3 PRODUCT PORTFOLIO
15.16.4 RECENT DEVELOPMENTS
15.17 MAPMYGENOME
15.17.1 COMPANY SNAPSHOT
15.17.2 PRODUCT PORTFOLIO
15.17.3 RECENT DEVELOPMENT
15.18 MEDGENOME
15.18.1 COMPANY SNAPSHOT
15.18.2 PRODUCT PORTFOLIO
15.18.3 RECENT DEVELOPMENT
15.19 MYRIAD GENETICS
15.19.1 COMPANY SNAPSHOT
15.19.2 REVENUE ANALYSIS
15.19.3 PRODUCT PORTFOLIO
15.19.4 RECENT DEVELOPMENT
15.2 NATERA, INC.
15.20.1 COMPANY SNAPSHOT
15.20.2 PRODUCT PORTFOLIO
15.20.3 RECENT DEVELOPMENT
15.20.3.1 PARTNERSHIP
15.21 OTOGENRTICS
15.21.1 COMPANY SNAPSHOT
15.21.2 PRODUCT PORTFOLIO
15.21.3 RECENT DEVELOPMENT
15.22 PACBIO
15.22.1 COMPANY SNAPSHOT
15.22.2 REVENUE ANALYSIS
15.22.3 PRODUCT PORTFOLIO
15.22.4 RECENT DEVELOPMENT
15.23 QIAGEN
15.23.1 COMPANY SNAPSHOT
15.23.2 PRODUCT PORTFOLIO
15.23.3 RECENT DEVELOPMENTS
15.23.3.1 PARTNERSHIP
15.23.3.2 PRODUCT LAUNCH
15.24 SEMA4 OPCO, INC.
15.24.1 COMPANY SNAPSHOT
15.24.2 REVENUE ANALYSIS
15.24.3 PRODUCT PORTFOLIO
15.24.4 RECENT DEVELOPMENT
15.25 SORENSON GENOMICS
15.25.1 COMPANY SNAPSHOT
15.25.2 PRODUCT PORTFOLIO
15.25.3 RECENT DEVELOPMENT
16 QUESTIONNAIRE
17 RELATED REPORTS
Lista de Tabela
TABLE 1 EUROPE GENETIC TESTING MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 2 EUROPE GENETIC TESTING MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 3 EUROPE GENETIC TESTING MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 4 EUROPE DIAGNOSTIC TESTING IN GENETIC TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 5 EUROPE PRENATAL TESTING IN GENETIC TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 6 EUROPE PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 7 EUROPE PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 8 EUROPE PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 9 EUROPE NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2020-2029 (USD MILLION)
TABLE 10 EUROPE NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2020-2029 (UNITS)
TABLE 11 EUROPE NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2020-2029 (ASP)
TABLE 12 EUROPE NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY CONDITION, 2020-2029 (USD MILLION)
TABLE 13 EUROPE NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2020-2029 (USD MILLION)
TABLE 14 EUROPE NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2020-2029 (UNITS)
TABLE 15 EUROPE NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2020-2029 (ASP)
TABLE 16 EUROPE NEW BORN SCREENING IN GENETIC TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 17 EUROPE NEW-BORN SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 18 EUROPE PREDICTIVE AND PRESYMPTOMATIC TESTING IN GENETIC TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 19 EUROPE CARRIER TESTING IN GENETIC TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 20 EUROPE CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 21 EUROPE CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 22 EUROPE CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2020-2029 (ASP)
TABLE 23 EUROPE CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 24 EUROPE CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 25 EUROPE CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 26 EUROPE EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 27 EUROPE EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 28 EUROPE EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 29 EUROPE CARRIER TESTING IN GENETIC TESTING MARKET, BY MEDICAL CONDITION, 2020-2029 (USD MILLION)
TABLE 30 EUROPE OTHER TYPES IN GENETIC TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 31 EUROPE GENETIC TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 32 EUROPE POLYMERASE CHAIN REACTION IN GENETIC TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 33 EUROPE POLYMERASE CHAIN REACTION IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 34 EUROPE DNA SEQUENCING (NGS-BASED TESTING) IN GENETIC TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 35 EUROPE DNA SEQUENCING (NGS-BASED TESTING) IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 36 EUROPE WHOLE GENOME SEQUENCING IN GENETIC TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 37 EUROPE MICROARRAYS IN GENETIC TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 38 EUROPE MICROARRAYS IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 39 EUROPE FLUORESCENCE IN SITU HYBRIDIZATION (FISH) IN GENETIC TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 40 EUROPE OTHERS IN GENETIC TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 41 EUROPE GENETIC TESTING MARKET, BY DISEASES, 2020-2029 (USD MILLION)
TABLE 42 EUROPE CANCER IN GENETIC TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 43 EUROPE CANCER IN GENETIC TESTING MARKET, BY DISEASES, 2020-2029 (USD MILLION)
TABLE 44 EUROPE SICKLE CELL ANEMIA IN GENETIC TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 45 EUROPE THALASSEMIA IN GENETIC TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 46 EUROPE RARE GENETIC DISORDER IN GENETIC TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 47 EUROPE RARE GENETIC DISORDER IN GENETIC TESTING MARKET, BY DISEASES, 2020-2029 (USD MILLION)
TABLE 48 EUROPE FRAGILE X SYNDROME IN GENETIC TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 49 EUROPE DUCHENNE MUSCULAR DYSTROPHY IN GENETIC TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 50 EUROPE HUNTINGTON'S DISEASE IN GENETIC TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 51 EUROPE CYSTIC FIBROSIS IN GENETIC TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 52 EUROPE OTHERS IN GENETIC TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 53 EUROPE GENETIC TESTING MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 54 EUROPE HOSPITALS IN GENETIC TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 55 EUROPE CLINICS IN GENETIC TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 56 EUROPE DIAGNOSTIC CENTERS IN GENETIC TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 57 EUROPE PRIVATE CLINICS IN GENETIC TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 58 EUROPE LABORATORY SERVICE PROVIDERS IN GENETIC TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 59 EUROPE PRIVATE LABORATORIES IN GENETIC TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 60 EUROPE GENETIC TESTING MARKET, BY COUNTRY, 2020-2029 (USD MILLION)
TABLE 61 EUROPE GENETIC TESTING MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 62 EUROPE GENETIC TESTING MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 63 EUROPE GENETIC TESTING MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 64 EUROPE PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 65 EUROPE PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 66 EUROPE PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 67 EUROPE NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2020-2029 (USD MILLION)
TABLE 68 EUROPE NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2020-2029 (UNITS)
TABLE 69 EUROPE NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2020-2029 (ASP)
TABLE 70 EUROPE NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY CONDITION, 2020-2029 (USD MILLION)
TABLE 71 EUROPE NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2020-2029 (USD MILLION)
TABLE 72 EUROPE NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2020-2029 (UNITS)
TABLE 73 EUROPE NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2020-2029 (ASP)
TABLE 74 EUROPE NEW-BORN SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 75 EUROPE CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 76 EUROPE CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 77 EUROPE CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2020-2029 (ASP)
TABLE 78 EUROPE CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 79 EUROPE CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 80 EUROPE CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 81 EUROPE EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 82 EUROPE EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 83 EUROPE EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 84 EUROPE CARRIER TESTING IN GENETIC TESTING MARKET, BY MEDICAL CONDITION, 2020-2029 (USD MILLION)
TABLE 85 EUROPE GENETIC TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 86 EUROPE POLYMERASE CHAIN REACTION IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 87 EUROPE DNA SEQUENCING IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 88 EUROPE MICROARRAYS IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 89 EUROPE GENETIC TESTING MARKET, BY DISEASES, 2020-2029 (USD MILLION)
TABLE 90 EUROPE RARE GENETIC DISORDER IN GENETIC TESTING MARKET, BY DISEASES, 2020-2029 (USD MILLION)
TABLE 91 EUROPE CANCER IN GENETIC TESTING MARKET, BY DISEASES, 2020-2029 (USD MILLION)
TABLE 92 EUROPE GENETIC TESTING MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 93 GERMANY GENETIC TESTING MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 94 GERMANY GENETIC TESTING MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 95 GERMANY GENETIC TESTING MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 96 GERMANY PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 97 GERMANY PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 98 GERMANY PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 99 GERMANY NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2020-2029 (USD MILLION)
TABLE 100 GERMANY NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2020-2029 (UNITS)
TABLE 101 GERMANY NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2020-2029 (ASP)
TABLE 102 GERMANY NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY CONDITION, 2020-2029 (USD MILLION)
TABLE 103 GERMANY NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2020-2029 (USD MILLION)
TABLE 104 GERMANY NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2020-2029 (UNITS)
TABLE 105 GERMANY NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2020-2029 (ASP)
TABLE 106 GERMANY NEW-BORN SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 107 GERMANY CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 108 GERMANY CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 109 GERMANY CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2020-2029 (ASP)
TABLE 110 GERMANY CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 111 GERMANY CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 112 GERMANY CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 113 GERMANY EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 114 GERMANY EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 115 GERMANY EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 116 GERMANY CARRIER TESTING IN GENETIC TESTING MARKET, BY MEDICAL CONDITION, 2020-2029 (USD MILLION)
TABLE 117 GERMANY GENETIC TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 118 GERMANY POLYMERASE CHAIN REACTION IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 119 GERMANY DNA SEQUENCING IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 120 GERMANY MICROARRAYS IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 121 GERMANY GENETIC TESTING MARKET, BY DISEASES, 2020-2029 (USD MILLION)
TABLE 122 GERMANY RARE GENETIC DISORDER IN GENETIC TESTING MARKET, BY DISEASES, 2020-2029 (USD MILLION)
TABLE 123 GERMANY CANCER IN GENETIC TESTING MARKET, BY DISEASES, 2020-2029 (USD MILLION)
TABLE 124 GERMANY GENETIC TESTING MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 125 FRANCE GENETIC TESTING MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 126 FRANCE GENETIC TESTING MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 127 FRANCE GENETIC TESTING MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 128 FRANCE PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 129 FRANCE PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 130 FRANCE PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 131 FRANCE NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2020-2029 (USD MILLION)
TABLE 132 FRANCE NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2020-2029 (UNITS)
TABLE 133 FRANCE NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2020-2029 (ASP)
TABLE 134 FRANCE NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY CONDITION, 2020-2029 (USD MILLION)
TABLE 135 FRANCE NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2020-2029 (USD MILLION)
TABLE 136 FRANCE NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2020-2029 (UNITS)
TABLE 137 FRANCE NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2020-2029 (ASP)
TABLE 138 FRANCE NEW-BORN SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 139 FRANCE CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 140 FRANCE CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 141 FRANCE CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2020-2029 (ASP)
TABLE 142 FRANCE CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 143 FRANCE CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 144 FRANCE CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 145 FRANCE EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 146 FRANCE EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 147 FRANCE EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 148 FRANCE CARRIER TESTING IN GENETIC TESTING MARKET, BY MEDICAL CONDITION, 2020-2029 (USD MILLION)
TABLE 149 FRANCE GENETIC TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 150 FRANCE POLYMERASE CHAIN REACTION IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 151 FRANCE DNA SEQUENCING IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 152 FRANCE MICROARRAYS IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 153 FRANCE GENETIC TESTING MARKET, BY DISEASES, 2020-2029 (USD MILLION)
TABLE 154 FRANCE RARE GENETIC DISORDER IN GENETIC TESTING MARKET, BY DISEASES, 2020-2029 (USD MILLION)
TABLE 155 FRANCE CANCER IN GENETIC TESTING MARKET, BY DISEASES, 2020-2029 (USD MILLION)
TABLE 156 FRANCE GENETIC TESTING MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 157 U.K. GENETIC TESTING MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 158 U.K. GENETIC TESTING MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 159 U.K. GENETIC TESTING MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 160 U.K. PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 161 U.K. PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 162 U.K. PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 163 U.K. NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2020-2029 (USD MILLION)
TABLE 164 U.K. NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2020-2029 (UNITS)
TABLE 165 U.K. NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2020-2029 (ASP)
TABLE 166 U.K. NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY CONDITION, 2020-2029 (USD MILLION)
TABLE 167 U.K. NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2020-2029 (USD MILLION)
TABLE 168 U.K. NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2020-2029 (UNITS)
TABLE 169 U.K. NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2020-2029 (ASP)
TABLE 170 U.K. NEW-BORN SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 171 U.K. CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 172 U.K. CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 173 U.K. CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2020-2029 (ASP)
TABLE 174 U.K. CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 175 U.K. CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 176 U.K. CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 177 U.K. EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 178 U.K. EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 179 U.K. EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 180 U.K. CARRIER TESTING IN GENETIC TESTING MARKET, BY MEDICAL CONDITION, 2020-2029 (USD MILLION)
TABLE 181 U.K. GENETIC TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 182 U.K. POLYMERASE CHAIN REACTION IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 183 U.K. DNA SEQUENCING IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 184 U.K. MICROARRAYS IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 185 U.K. GENETIC TESTING MARKET, BY DISEASES, 2020-2029 (USD MILLION)
TABLE 186 U.K. RARE GENETIC DISORDER IN GENETIC TESTING MARKET, BY DISEASES, 2020-2029 (USD MILLION)
TABLE 187 U.K. CANCER IN GENETIC TESTING MARKET, BY DISEASES, 2020-2029 (USD MILLION)
TABLE 188 U.K. GENETIC TESTING MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 189 RUSSIA GENETIC TESTING MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 190 RUSSIA GENETIC TESTING MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 191 RUSSIA GENETIC TESTING MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 192 RUSSIA PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 193 RUSSIA PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 194 RUSSIA PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 195 RUSSIA NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2020-2029 (USD MILLION)
TABLE 196 RUSSIA NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2020-2029 (UNITS)
TABLE 197 RUSSIA NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2020-2029 (ASP)
TABLE 198 RUSSIA NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY CONDITION, 2020-2029 (USD MILLION)
TABLE 199 RUSSIA NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2020-2029 (USD MILLION)
TABLE 200 RUSSIA NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2020-2029 (UNITS)
TABLE 201 RUSSIA NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2020-2029 (ASP)
TABLE 202 RUSSIA NEW-BORN SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 203 RUSSIA CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 204 RUSSIA CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 205 RUSSIA CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2020-2029 (ASP)
TABLE 206 RUSSIA CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 207 RUSSIA CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 208 RUSSIA CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 209 RUSSIA EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 210 RUSSIA EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 211 RUSSIA EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 212 RUSSIA CARRIER TESTING IN GENETIC TESTING MARKET, BY MEDICAL CONDITION, 2020-2029 (USD MILLION)
TABLE 213 RUSSIA GENETIC TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 214 RUSSIA POLYMERASE CHAIN REACTION IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 215 RUSSIA DNA SEQUENCING IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 216 RUSSIA MICROARRAYS IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 217 RUSSIA GENETIC TESTING MARKET, BY DISEASES, 2020-2029 (USD MILLION)
TABLE 218 RUSSIA RARE GENETIC DISORDER IN GENETIC TESTING MARKET, BY DISEASES, 2020-2029 (USD MILLION)
TABLE 219 RUSSIA CANCER IN GENETIC TESTING MARKET, BY DISEASES, 2020-2029 (USD MILLION)
TABLE 220 RUSSIA GENETIC TESTING MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 221 ITALY GENETIC TESTING MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 222 ITALY GENETIC TESTING MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 223 ITALY GENETIC TESTING MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 224 ITALY PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 225 ITALY PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 226 ITALY PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 227 ITALY NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2020-2029 (USD MILLION)
TABLE 228 ITALY NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2020-2029 (UNITS)
TABLE 229 ITALY NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2020-2029 (ASP)
TABLE 230 ITALY NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY CONDITION, 2020-2029 (USD MILLION)
TABLE 231 ITALY NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2020-2029 (USD MILLION)
TABLE 232 ITALY NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2020-2029 (UNITS)
TABLE 233 ITALY NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2020-2029 (ASP)
TABLE 234 ITALY NEW-BORN SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 235 ITALY CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 236 ITALY CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 237 ITALY CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2020-2029 (ASP)
TABLE 238 ITALY CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 239 ITALY CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 240 ITALY CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 241 ITALY EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 242 ITALY EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 243 ITALY EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 244 ITALY CARRIER TESTING IN GENETIC TESTING MARKET, BY MEDICAL CONDITION, 2020-2029 (USD MILLION)
TABLE 245 ITALY GENETIC TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 246 ITALY POLYMERASE CHAIN REACTION IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 247 ITALY DNA SEQUENCING IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 248 ITALY MICROARRAYS IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 249 ITALY GENETIC TESTING MARKET, BY DISEASES, 2020-2029 (USD MILLION)
TABLE 250 ITALY RARE GENETIC DISORDER IN GENETIC TESTING MARKET, BY DISEASES, 2020-2029 (USD MILLION)
TABLE 251 ITALY CANCER IN GENETIC TESTING MARKET, BY DISEASES, 2020-2029 (USD MILLION)
TABLE 252 ITALY GENETIC TESTING MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 253 SPAIN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 254 SPAIN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 255 SPAIN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 256 SPAIN PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 257 SPAIN PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 258 SPAIN PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 259 SPAIN NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2020-2029 (USD MILLION)
TABLE 260 SPAIN NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2020-2029 (UNITS)
TABLE 261 SPAIN NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2020-2029 (ASP)
TABLE 262 SPAIN NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY CONDITION, 2020-2029 (USD MILLION)
TABLE 263 SPAIN NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2020-2029 (USD MILLION)
TABLE 264 SPAIN NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2020-2029 (UNITS)
TABLE 265 SPAIN NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2020-2029 (ASP)
TABLE 266 SPAIN NEW-BORN SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 267 SPAIN CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 268 SPAIN CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 269 SPAIN CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2020-2029 (ASP)
TABLE 270 SPAIN CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 271 SPAIN CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 272 SPAIN CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 273 SPAIN EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 274 SPAIN EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 275 SPAIN EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 276 SPAIN CARRIER TESTING IN GENETIC TESTING MARKET, BY MEDICAL CONDITION, 2020-2029 (USD MILLION)
TABLE 277 SPAIN GENETIC TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 278 SPAIN POLYMERASE CHAIN REACTION IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 279 SPAIN DNA SEQUENCING IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 280 SPAIN MICROARRAYS IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 281 SPAIN GENETIC TESTING MARKET, BY DISEASES, 2020-2029 (USD MILLION)
TABLE 282 SPAIN RARE GENETIC DISORDER IN GENETIC TESTING MARKET, BY DISEASES, 2020-2029 (USD MILLION)
TABLE 283 SPAIN CANCER IN GENETIC TESTING MARKET, BY DISEASES, 2020-2029 (USD MILLION)
TABLE 284 SPAIN GENETIC TESTING MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 285 TURKEY GENETIC TESTING MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 286 TURKEY GENETIC TESTING MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 287 TURKEY GENETIC TESTING MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 288 TURKEY PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 289 TURKEY PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 290 TURKEY PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 291 TURKEY NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2020-2029 (USD MILLION)
TABLE 292 TURKEY NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2020-2029 (UNITS)
TABLE 293 TURKEY NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2020-2029 (ASP)
TABLE 294 TURKEY NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY CONDITION, 2020-2029 (USD MILLION)
TABLE 295 TURKEY NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2020-2029 (USD MILLION)
TABLE 296 TURKEY NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2020-2029 (UNITS)
TABLE 297 TURKEY NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2020-2029 (ASP)
TABLE 298 TURKEY NEW-BORN SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 299 TURKEY CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 300 TURKEY CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 301 TURKEY CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2020-2029 (ASP)
TABLE 302 TURKEY CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 303 TURKEY CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 304 TURKEY CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 305 TURKEY EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 306 TURKEY EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 307 TURKEY EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 308 TURKEY CARRIER TESTING IN GENETIC TESTING MARKET, BY MEDICAL CONDITION, 2020-2029 (USD MILLION)
TABLE 309 TURKEY GENETIC TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 310 TURKEY POLYMERASE CHAIN REACTION IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 311 TURKEY DNA SEQUENCING IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 312 TURKEY MICROARRAYS IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 313 TURKEY GENETIC TESTING MARKET, BY DISEASES, 2020-2029 (USD MILLION)
TABLE 314 TURKEY RARE GENETIC DISORDER IN GENETIC TESTING MARKET, BY DISEASES, 2020-2029 (USD MILLION)
TABLE 315 TURKEY CANCER IN GENETIC TESTING MARKET, BY DISEASES, 2020-2029 (USD MILLION)
TABLE 316 TURKEY GENETIC TESTING MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 317 NETHERLANDS GENETIC TESTING MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 318 NETHERLANDS GENETIC TESTING MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 319 NETHERLANDS GENETIC TESTING MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 320 NETHERLANDS PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 321 NETHERLANDS PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 322 NETHERLANDS PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 323 NETHERLANDS NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2020-2029 (USD MILLION)
TABLE 324 NETHERLANDS NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2020-2029 (UNITS)
TABLE 325 NETHERLANDS NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2020-2029 (ASP)
TABLE 326 NETHERLANDS NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY CONDITION, 2020-2029 (USD MILLION)
TABLE 327 NETHERLANDS NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2020-2029 (USD MILLION)
TABLE 328 NETHERLANDS NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2020-2029 (UNITS)
TABLE 329 NETHERLANDS NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2020-2029 (ASP)
TABLE 330 NETHERLANDS NEW-BORN SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 331 NETHERLANDS CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 332 NETHERLANDS CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 333 NETHERLANDS CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2020-2029 (ASP)
TABLE 334 NETHERLANDS CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 335 NETHERLANDS CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 336 NETHERLANDS CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 337 NETHERLANDS EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 338 NETHERLANDS EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 339 NETHERLANDS EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 340 NETHERLANDS CARRIER TESTING IN GENETIC TESTING MARKET, BY MEDICAL CONDITION, 2020-2029 (USD MILLION)
TABLE 341 NETHERLANDS GENETIC TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 342 NETHERLANDS POLYMERASE CHAIN REACTION IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 343 NETHERLANDS DNA SEQUENCING IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 344 NETHERLANDS MICROARRAYS IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 345 NETHERLANDS GENETIC TESTING MARKET, BY DISEASES, 2020-2029 (USD MILLION)
TABLE 346 NETHERLANDS RARE GENETIC DISORDER IN GENETIC TESTING MARKET, BY DISEASES, 2020-2029 (USD MILLION)
TABLE 347 NETHERLANDS CANCER IN GENETIC TESTING MARKET, BY DISEASES, 2020-2029 (USD MILLION)
TABLE 348 NETHERLANDS GENETIC TESTING MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 349 SWITZERLAND GENETIC TESTING MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 350 SWITZERLAND GENETIC TESTING MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 351 SWITZERLAND GENETIC TESTING MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 352 SWITZERLAND PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 353 SWITZERLAND PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 354 SWITZERLAND PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 355 SWITZERLAND NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2020-2029 (USD MILLION)
TABLE 356 SWITZERLAND NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2020-2029 (UNITS)
TABLE 357 SWITZERLAND NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2020-2029 (ASP)
TABLE 358 SWITZERLAND NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY CONDITION, 2020-2029 (USD MILLION)
TABLE 359 SWITZERLAND NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2020-2029 (USD MILLION)
TABLE 360 SWITZERLAND NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2020-2029 (UNITS)
TABLE 361 SWITZERLAND NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2020-2029 (ASP)
TABLE 362 SWITZERLAND NEW-BORN SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 363 SWITZERLAND CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 364 SWITZERLAND CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 365 SWITZERLAND CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2020-2029 (ASP)
TABLE 366 SWITZERLAND CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 367 SWITZERLAND CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 368 SWITZERLAND CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 369 SWITZERLAND EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 370 SWITZERLAND EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 371 SWITZERLAND EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 372 SWITZERLAND CARRIER TESTING IN GENETIC TESTING MARKET, BY MEDICAL CONDITION, 2020-2029 (USD MILLION)
TABLE 373 SWITZERLAND GENETIC TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 374 SWITZERLAND POLYMERASE CHAIN REACTION IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 375 SWITZERLAND DNA SEQUENCING IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 376 SWITZERLAND MICROARRAYS IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 377 SWITZERLAND GENETIC TESTING MARKET, BY DISEASES, 2020-2029 (USD MILLION)
TABLE 378 SWITZERLAND RARE GENETIC DISORDER IN GENETIC TESTING MARKET, BY DISEASES, 2020-2029 (USD MILLION)
TABLE 379 SWITZERLAND CANCER IN GENETIC TESTING MARKET, BY DISEASES, 2020-2029 (USD MILLION)
TABLE 380 SWITZERLAND GENETIC TESTING MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 381 BELGIUM GENETIC TESTING MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 382 BELGIUM GENETIC TESTING MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 383 BELGIUM GENETIC TESTING MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 384 BELGIUM PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 385 BELGIUM PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 386 BELGIUM PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 387 BELGIUM NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2020-2029 (USD MILLION)
TABLE 388 BELGIUM NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2020-2029 (UNITS)
TABLE 389 BELGIUM NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2020-2029 (ASP)
TABLE 390 BELGIUM NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY CONDITION, 2020-2029 (USD MILLION)
TABLE 391 BELGIUM NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2020-2029 (USD MILLION)
TABLE 392 BELGIUM NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2020-2029 (UNITS)
TABLE 393 BELGIUM NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2020-2029 (ASP)
TABLE 394 BELGIUM NEW-BORN SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 395 BELGIUM CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 396 BELGIUM CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 397 BELGIUM CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2020-2029 (ASP)
TABLE 398 BELGIUM CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 399 BELGIUM CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 400 BELGIUM CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 401 BELGIUM EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 402 BELGIUM EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 403 BELGIUM EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 404 BELGIUM CARRIER TESTING IN GENETIC TESTING MARKET, BY MEDICAL CONDITION, 2020-2029 (USD MILLION)
TABLE 405 BELGIUM GENETIC TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 406 BELGIUM POLYMERASE CHAIN REACTION IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 407 BELGIUM DNA SEQUENCING IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 408 BELGIUM MICROARRAYS IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 409 BELGIUM GENETIC TESTING MARKET, BY DISEASES, 2020-2029 (USD MILLION)
TABLE 410 BELGIUM RARE GENETIC DISORDER IN GENETIC TESTING MARKET, BY DISEASES, 2020-2029 (USD MILLION)
TABLE 411 BELGIUM CANCER IN GENETIC TESTING MARKET, BY DISEASES, 2020-2029 (USD MILLION)
TABLE 412 BELGIUM GENETIC TESTING MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 413 REST OF EUROPE GENETIC TESTING MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 414 REST OF EUROPE GENETIC TESTING MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 415 REST OF EUROPE GENETIC TESTING MARKET, BY TYPE, 2020-2029 (ASP)
Lista de Figura
FIGURE 1 EUROPE GENETIC TESTING MARKET: SEGMENTATION
FIGURE 2 EUROPE GENETIC TESTING MARKET: DATA TRIANGULATION
FIGURE 3 EUROPE GENETIC TESTING MARKET: DROC ANALYSIS
FIGURE 4 EUROPE GENETIC TESTING MARKET: EUROPE VS REGIONAL MARKET ANALYSIS
FIGURE 5 EUROPE GENETIC TESTING MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 EUROPE GENETIC TESTING MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 EUROPE GENETIC TESTING MARKET: DBMR MARKET POSITION GRID
FIGURE 8 EUROPE GENETIC TESTING MARKET: APPLICATION COVERAGE GRID
FIGURE 9 EUROPE GENETIC TESTING MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 EUROPE GENETIC TESTING MARKET: SEGMENTATION
FIGURE 11 INCREASING PREVALENCE OF LYMPHEDEMA AND RISING HEALTHCARE EXPENDITURE ARE EXPECTED TO DRIVE THE EUROPE GENETIC TESTING MARKET IN THE FORECAST PERIOD OF 2022 TO 2029
FIGURE 12 DIAGNOSTIC TESTING SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE EUROPE GENETIC TESTING MARKET IN 2022 & 2029
FIGURE 13 NORTH AMERICA IS EXPECTED TO DOMINATE THE EUROPE GENETIC TESTING MARKET AND ASIA-PACIFIC EXPECTED TO GROW WITH THE HIGHEST CAGR IN THE FORECAST PERIOD OF 2022 TO 2029
FIGURE 14 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF EUROPE GENETIC TESTING MARKET
FIGURE 15 EUROPE GENETIC TESTING MARKET: BY TYPE, 2021
FIGURE 16 EUROPE GENETIC TESTING MARKET: BY TYPE, 2022-2029 (USD MILLION)
FIGURE 17 EUROPE GENETIC TESTING MARKET: BY TYPE, CAGR (2022-2029)
FIGURE 18 EUROPE GENETIC TESTING MARKET: BY TYPE, LIFELINE CURVE
FIGURE 19 EUROPE GENETIC TESTING MARKET: BY TECHNOLOGY, 2021
FIGURE 20 EUROPE GENETIC TESTING MARKET: BY TECHNOLOGY, 2022-2029 (USD MILLION)
FIGURE 21 EUROPE GENETIC TESTING MARKET: BY TECHNOLOGY, CAGR (2022-2029)
FIGURE 22 EUROPE GENETIC TESTING MARKET: BY TECHNOLOGY, LIFELINE CURVE
FIGURE 23 EUROPE GENETIC TESTING MARKET: BY DISEASES, 2021
FIGURE 24 EUROPE GENETIC TESTING MARKET: BY DISEASES, 2022-2029 (USD MILLION)
FIGURE 25 EUROPE GENETIC TESTING MARKET: BY DISEASES, CAGR (2022-2029)
FIGURE 26 EUROPE GENETIC TESTING MARKET: BY DISEASES, LIFELINE CURVE
FIGURE 27 EUROPE GENETIC TESTING MARKET: BY END USER, 2021
FIGURE 28 EUROPE GENETIC TESTING MARKET: BY END USER, 2022-2029 (USD MILLION)
FIGURE 29 EUROPE GENETIC TESTING MARKET: BY END USER, CAGR (2022-2029)
FIGURE 30 EUROPE GENETIC TESTING MARKET: BY END USER, LIFELINE CURVE
FIGURE 31 EUROPE GENETIC TESTING MARKET: SNAPSHOT (2021)
FIGURE 32 EUROPE GENETIC TESTING MARKET: BY COUNTRY (2021)
FIGURE 33 EUROPE GENETIC TESTING MARKET: BY COUNTRY (2022 & 2029)
FIGURE 34 EUROPE GENETIC TESTING MARKET: BY COUNTRY (2021 & 2029)
FIGURE 35 EUROPE GENETIC TESTING MARKET: BY TYPE (2022-2029)
FIGURE 36 EUROPE GENETIC TESTING MARKET: COMPANY SHARE 2021 (%)

Metodologia de Investigação
A recolha de dados e a análise do ano base são feitas através de módulos de recolha de dados com amostras grandes. A etapa inclui a obtenção de informações de mercado ou dados relacionados através de diversas fontes e estratégias. Inclui examinar e planear antecipadamente todos os dados adquiridos no passado. Da mesma forma, envolve o exame de inconsistências de informação observadas em diferentes fontes de informação. Os dados de mercado são analisados e estimados utilizando modelos estatísticos e coerentes de mercado. Além disso, a análise da quota de mercado e a análise das principais tendências são os principais fatores de sucesso no relatório de mercado. Para saber mais, solicite uma chamada de analista ou abra a sua consulta.
A principal metodologia de investigação utilizada pela equipa de investigação do DBMR é a triangulação de dados que envolve a mineração de dados, a análise do impacto das variáveis de dados no mercado e a validação primária (especialista do setor). Os modelos de dados incluem grelha de posicionamento de fornecedores, análise da linha de tempo do mercado, visão geral e guia de mercado, grelha de posicionamento da empresa, análise de patentes, análise de preços, análise da quota de mercado da empresa, normas de medição, análise global versus regional e de participação dos fornecedores. Para saber mais sobre a metodologia de investigação, faça uma consulta para falar com os nossos especialistas do setor.
Personalização disponível
A Data Bridge Market Research é líder em investigação formativa avançada. Orgulhamo-nos de servir os nossos clientes novos e existentes com dados e análises que correspondem e atendem aos seus objetivos. O relatório pode ser personalizado para incluir análise de tendências de preços de marcas-alvo, compreensão do mercado para países adicionais (solicite a lista de países), dados de resultados de ensaios clínicos, revisão de literatura, mercado remodelado e análise de base de produtos . A análise de mercado dos concorrentes-alvo pode ser analisada desde análises baseadas em tecnologia até estratégias de carteira de mercado. Podemos adicionar quantos concorrentes necessitar de dados no formato e estilo de dados que procura. A nossa equipa de analistas também pode fornecer dados em tabelas dinâmicas de ficheiros Excel em bruto (livro de factos) ou pode ajudá-lo a criar apresentações a partir dos conjuntos de dados disponíveis no relatório.













