Europe Adalimumab Market, By Indication (Rheumatoid Arthritis, Juvenile Idiopathic Arthritis, Psoriatic Arthritis, Ankylosing Spondylitis, Crohn's Disease, Hidradenitis Suppurativa, Ulcerative Colitis, Chronic Plaque Psoriasis, Non-Infectious Intermediate and Others), Type (Biologics and Biosimilars), Dosage Strength (20MG/0.4MLG, 40MG/0.8MLG and Others), Drug Type (Humira, Amgevita, Imraldi, Hyrimoz, Yuflyma, Hulio, Idacio), Population Type (Children and Adults), End User (Hospitals, Specialty Clinics, Home Healthcare and Others), Distribution Channel (Hospital Pharmacies, Retail Pharmacies, Online Pharmacies and Others) Industry Trends and Forecast to 2029.
Market Definition
Adalimumab is one of the monoclonal antibodies used to treat certain autoimmune diseases involving rheumatoid arthritis, Crohn’s diseases, among others. Adalimumab is an anti-TNF drug indicated for the treatment of inflammatory symptoms. The biologic of adalimumab is Humira and various biosimilar drugs of Humira are also available, which involves Exemptia, Hyrimoz, Cyltezo, and Hulio among others. Adalimumab works by binding to the TNF factor alpha, which reduces the chances of the inflammatory response to autoimmune diseases.
Market Analysis and Insights
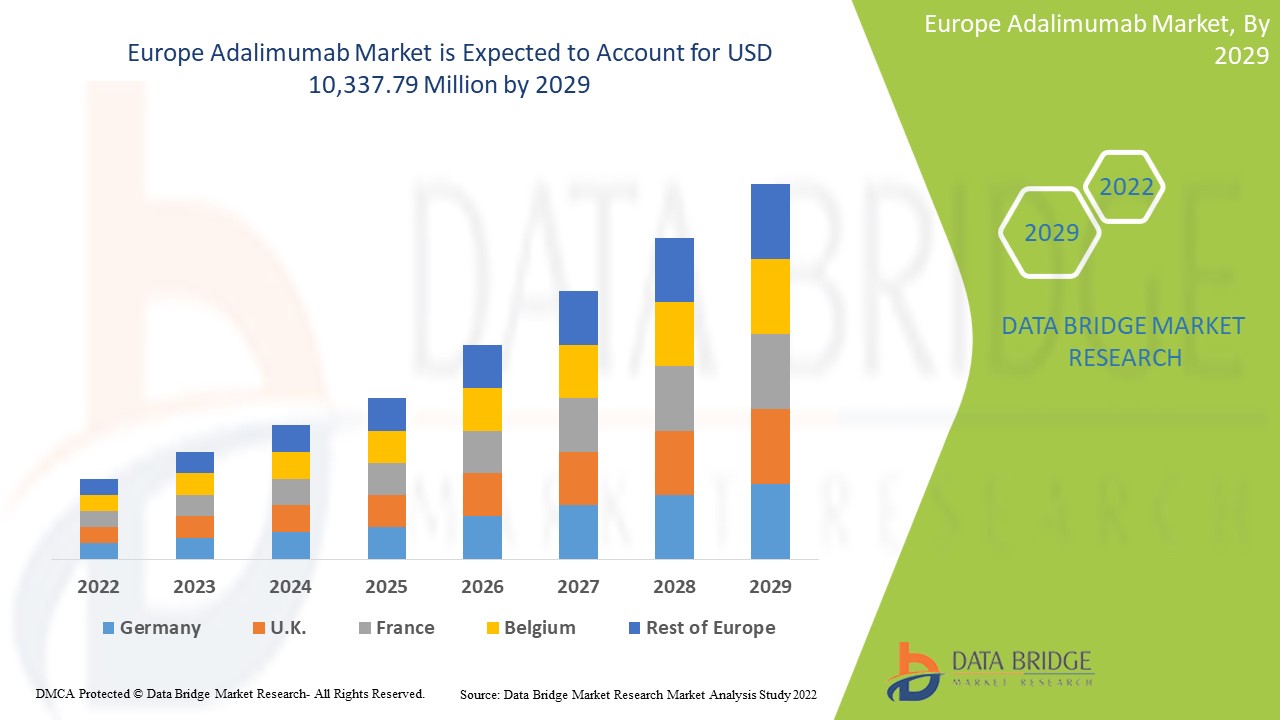
Europe adalimumab market is expected to gain market growth in the forecast period of 2022 to 2029. Data Bridge Market Research analyses that the market is growing with a CAGR of 16.2% in the forecast period of 2022 to 2029 and is expected to reach USD 10,337.79 million by 2029 from USD 3,485.94 million in 2021.
|
Report Metric |
Details |
|
Forecast Period |
2022 to 2029 |
|
Base Year |
2021 |
|
Historic Years |
2020 |
|
Quantitative Units |
Revenue in USD Million |
|
Segments Covered |
By Indication (Rheumatoid Arthritis, Juvenile Idiopathic Arthritis, Psoriatic Arthritis, Ankylosing Spondylitis, Crohn's Disease, Hidradenitis Suppurativa, Ulcerative Colitis, Chronic Plaque Psoriasis, Non-Infectious Intermediate and Others), Type (Biologics and Biosimilars), Dosage Strength (20MG/0.4MLG, 40MG/0.8MLG and Others), Drug Type (Humira, Amgevita, Imraldi, Hyrimoz, Yuflyma, Hulio, Idacio), Population Type (Children and Adults), End User (Hospitals, Specialty Clinics, Home Healthcare and Others), Distribution Channel (Hospital Pharmacies, Retail Pharmacies, Online Pharmacies and Others) |
|
Countries Covered |
Germany, France, Italy, U.K., Spain, Netherlands, Russia, Switzerland, Belgium, Turkey, Hungary, Lithuania, Austria, Ireland, Norway, Poland and Rest of Europe |
|
Market Players Covered |
The major companies which are dealing in the market are AbbVie Inc. Sandoz International GmbH, Amgen Inc., Mylan N.V. (A subsidiary of Viatris), Biogen, Celltrion Healthcare Co., Ltd., Fresenius Kabi SwissBioSim GmbH, Alvotech, Biocad, Coherus BioSciences, Shanghai Henlius Biotech, Inc., Synermore Biologics, Prestige BioPharma Ltd., and Janssen Global Services, LLC among others |
Adalimumab Market Dynamics
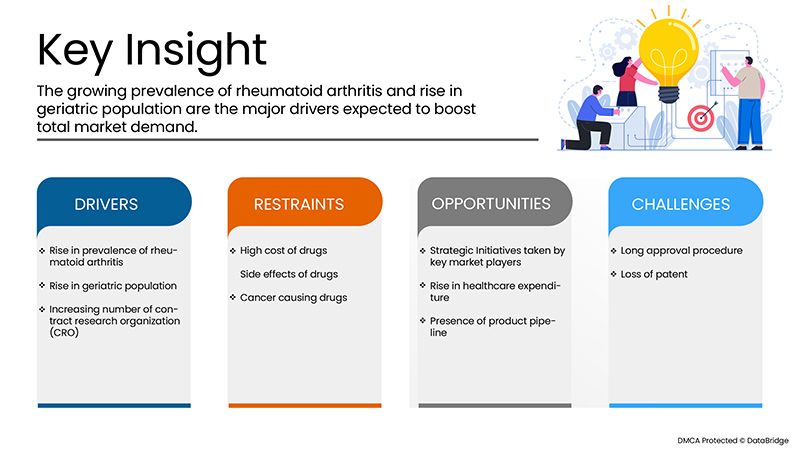
Drivers
- Increasing prevalence of rheumatoid arthritis
The prevalence of rheumatoid arthritis is increasing worldwide and this has been reported that globally, the annual incidence of RA is nearly 3 cases per 10,000 populations. Rheumatoid arthritis leads to development of inflammatory symptoms which can be treated by several drug therapies involving biological therapies among others. One of the most innovative biological therapy discovered for treatment of rheumatoid arthritis includes adalimumab, a monoclonal antibody which targets the immune cells and slows down the recruitment of immune cells that leads to decrease the inflammation at target site. The continuous rise in rheumatoid arthritis globally and in the European region has badly affected the life of sufferers and thus disease is also putting a great burden over the healthcare professionals.
- Rise in geriatric population
The geriatric population is increasing living for longer period of time with several types of chronic conditions. This has been reported that the pace of ageing population is too high and is expanding at a dramatic rate. The condition of rheumatoid arthritis is said to be associated with increase in older population. According to a study, about 703 million people were over the age of 65 years old or over in the year 2019. North America and Europe had over 200 million older age people. The study also suggested that the population has already been observed to be significantly older and rising slowly about 48%. Adult’s population suffering from rheumatoid arthritis is increasing who are largely dependent on adalimumab therapy. This thus signifies that increasing geriatric population is acting as driver for market growth.
- Increasing number of contract research organizations (CRO)
Contract research organizations provide with the support for clinical trials and other research activities conducted for drugs and medical devices. Contract Research Organisation (CRO) helps biotechnology and pharmaceutical industries for the drug development process and to reduce the overall cost of clinical trials. As various adalimumab are extensively under the clinical trials in order to fulfil the unmet demands of patients suffering from inflammatory diseases, the increasing number of contract research organizations is boosting the market growth. There is an increase in the number of contract research organizations which has simplified the process of adalimumab drugs and reduced the time needed for drug development.
Opportunities
- Strategic initiatives by market players
The demand for adalimumab has boosted in the region as well as globally, due to the growth in the rise in various chronic diseases and the rise in geriatric population. These favourable factors accomplishing enhance the need for the drugs, and to accomplish the market demand, minor and major market players are utilizing various strategies
Also, the rise in healthcare expenditure by the government in the region will provide structural integrity and future opportunities for the adalimumab market in the forecast period of 2022-2029.
Restraints/Challenges
However, the high cost associated with the drugs and some side effects seen after using drugs may hamper the growth of the market. Additionally, stringent rules and regulations will further challenge the market in the forecast period mentioned above.
This adalimumab market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on arthritis market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Post COVID-19 Impact on Adalimumab Market
The COVID-19 has not that much affected in the growth of the market. As there is rise in the prevalence of various diseases in the region such as rheumatoid arthritis, Crohn’s diseases and among others lead to rise in the development of various drugs in the market. Hence the need for the adalimumab drugs keep rising in the COVID-19 period as well.
Recent Development
- In October 2018, Sandoz, a Novartis division and the pioneer and global leader in biosimilars, today announced that the US Food and Drug Administration (FDA) approved its biosimilar, HyrimozTM (adalimumab-adaz). The FDA granted approval for the treatment of rheumatoid arthritis (RA), juvenile idiopathic arthritis (JIA) in patients four years of age and older, psoriatic arthritis (PsA), ankylosing spondylitis (AS), adult Crohn's disease (CD), ulcerative colitis (UC) and plaque psoriasis (Ps).
Europe Adalimumab Market Scope
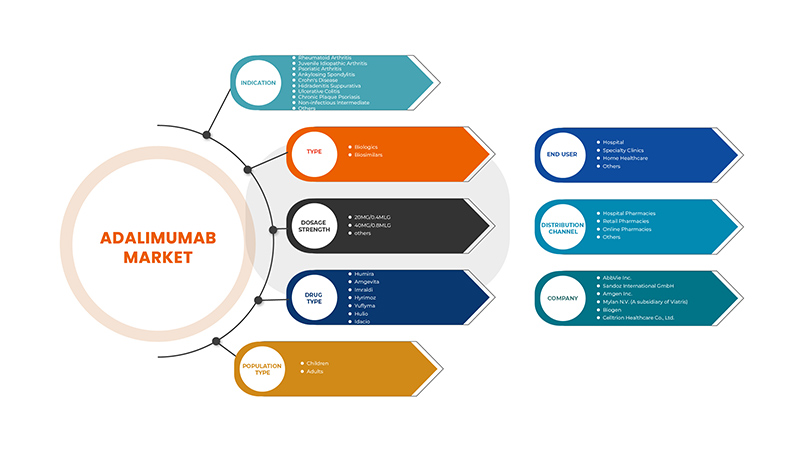
Europe adalimumab market is segmented into indication, type, dosage strength, drug type, population type, end user and distribution channel. The growth amongst these segments will help you analyse meagre growth segments in the industries and provide the users with a valuable market overview and market insights to make strategic decisions to identify core market applications.
Indication
- Rheumatoid Arthritis
- Juvenile Idiopathic Arthritis
- Psoriatic Arthritis
- Ankylosing Spondylitis
- Crohn’s Disease
- Hidradenitis Suppurativa
- Ulcerative Colitis
- Chronic Plaque Psoriasis
- Non-Infectious Intermediate
- Others
On the basis of indication, Europe adalimumab market is segmented into rheumatoid arthritis, juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn’s disease, hidradenitis suppurativa, ulcerative colitis, chronic plaque psoriasis, non-infectious intermediate and others.
Type
- Biologics
- Biosimilars
On the basis of type, Europe adalimumab market is segmented into biologics and biosimilars.
Dosage Strength
- 20MG/0.4MLG
- 40MG/0.8MLG
- Others
On the basis of dosage strength, Europe adalimumab market is segmented into 20MG/0.4MLG, 40MG/0.8MLG and others.
Drug Type
- Humira
- Amgevita
- Imraldi
- Hyrimoz
- Yuflyma
- Hulio
- Idacio
On the basis of drug type, Europe adalimumab market is segmented into Humira, Amgevita, Imraldi, Hyrimoz, Yuflyma, Hulio and Idacio.
Population Type
- Children
- Adults
On the basis of population type, Europe adalimumab market is segmented into children and adults.
End User
- Hospitals
- Specialty Clinics
- Home Healthcare
- Others
On the basis of end-users, Europe adalimumab market is segmented into hospital, specialty clinics, home healthcare and others.
Distribution Channel
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
- Others
On the basis of distribution channel, Europe adalimumab market is segmented into hospital pharmacies, retail pharmacies, online pharmacies and others.
Adalimumab Market Regional Analysis/Insights
The adalimumab market is analysed and market size insights and trends are provided by country, indication, type, dosage strength, drug type, population type, end user and distribution channel as referenced above.
Europe adalimumab market is further segmented into following countries - Germany, France, Italy, U.K., Spain, Netherlands, Russia, Switzerland, Belgium, Turkey, Hungary, Lithuania, Austria, Ireland, Norway, Poland and Rest of Europe.
Germany dominates the adalimumab market in terms of market share and market revenue and will continue to flourish its dominance during the forecast period. This is due to rise in prevalence of various chronic diseases and research and development in the drug development in the Germany region further improves this market growth.
The country section of the report also provides individual market impacting factors and changes in regulations in the market that impact the current and future trends of the market. Data points, such as new and replacement sales, country demographics, disease epidemiology, and import-export tariffs, are some of the major pointers used to forecast the market scenario for individual countries. In addition, the presence and availability of global brands and their challenges faced due to high competition from local and domestic brands, and impact of sales channels are considered while providing forecast analysis of the country data.
Competitive Landscape and Market Share Analysis
The adalimumab market competitive landscape provides details by the competitors. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, and application dominance. The above data points provided are only related to the companies' focus on adalimumab market.
Some of the major players operating in the adalimumab market are AbbVie Inc. Sandoz International GmbH, Amgen Inc., Mylan N.V. (A subsidiary of Viatris), Biogen, Celltrion Healthcare Co., Ltd., Fresenius Kabi SwissBioSim GmbH, Alvotech, Biocad, Coherus BioSciences, Shanghai Henlius Biotech, Inc., Synermore Biologics, Prestige BioPharma Ltd., Janssen Global Services, LLC among others.
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The market data is analysed and estimated using market statistical and coherent models. In addition, market share analysis and key trend analysis are the major success factors in the market report. The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market, and primary (industry expert) validation. Apart from this, data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Company Market Share Analysis, Standards of Measurement, Europe vs. Regional and Vendor Share Analysis. Please request analyst call in case of further inquiry.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analysed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Factbook) or can assist you in creating presentations from the data sets available in the report
SKU-
Obtenha acesso online ao relatório sobre a primeira nuvem de inteligência de mercado do mundo
- Painel interativo de análise de dados
- Painel de análise da empresa para oportunidades de elevado potencial de crescimento
- Acesso de analista de pesquisa para personalização e customização. consultas
- Análise da concorrência com painel interativo
- Últimas notícias, atualizações e atualizações Análise de tendências
- Aproveite o poder da análise de benchmark para um rastreio abrangente da concorrência
Índice
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF EUROPE ADALIMUMAB MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 INDICATION LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 VENDOR SHARE ANALYSIS
2.11 SECONDARY SOURCES
2.12 ASSUMPTIONS
3 EXECUTIVE SUMMARY
3.1 PIPELINE ANALYSIS
4 REGULATORY FRAMEWORK OF EUROPE ADALIMUMAB MARKET
5 EPIDEMIOLOGY
6 ADALIMUMAB PRESCRIPTION
7 EUROPE ADALIMUMAB MARKET: REIMBURSEMENT SCENARIO
7.1 REIMBURSEMENT SCENARIO IN THE U.S.
7.2 REIMBURSEMENT SCENARIO IN CHINA
7.3 REIMBURSEMENT SCENARIO IN JAPAN
7.4 REIMBURSEMENT IN CENTRAL AND EASTERN EUROPE
7.5 REIMBURSEMENT SCENARIO IN DENMARK
7.6 REIMBURSEMENT SCENARIO IN IRELAND
8 IMPACT OF BIOSIMILAR
9 MARKET OVERVIEW
9.1 DRIVERS
9.1.1 RISE IN THE PREVALENCE OF RHEUMATOID ARHTRITIS
9.1.2 INCREASING GERIATRIC POPULATION
9.1.3 INCREASING NUMBER OF CONTRACT RESEARCH ORGANIZATIONS
9.1.4 INTRODUCTION TO BIOSIMILARS
9.1.5 EXPLORATION OF EMERGING MARKETS
9.2 RESTRAINTS
9.2.1 HIGH COSTS OF DRUGS
9.2.2 SIDE EFFECTS OF DRUGS
9.2.3 CANCER CAUSING DRUGS
9.3 OPPORTUNITIES
9.3.1 PRESENCE OF PRODUCT PIPELINE
9.3.2 STRATEGIC INITIATIVES BY MARKET PLAYERS
9.3.3 INCREASING HEALTHCARE EXPENDITURE
9.3.4 PRESENCE OF REIMBURSEMENT POLICIES
9.4 CHALLENGES
9.4.1 LOSS OF PATENTS
9.4.2 AVAILABILITY OF ALTERNATIVES
9.4.3 LONG APPROVAL PROCEDURE
10 COVID-19 IMPACT ON ADALIMUMAB IN HEALTHCARE INDUSTRY
10.1 OVERVIEW
10.2 ADALIMUMAB AND COVID-19
10.3 PRICE IMPACT OF COVID-19
10.4 IMPACT ON DEMAND
10.5 IMPACT ON SUPPLY CHAIN
10.6 STRATEGIC DECISIONS FOR MANUFACTURERS
10.7 CONCLUSION
11 EUROPE ADALIMUMAB MARKET, BY INDICATION
11.1 OVERVIEW
11.2 RHEUMATOID ARTHRITIS
11.3 ANKYLOSING SPONDYLITIS
11.4 CHRONIC PLAQUE PSORIASIS
11.5 CROHN’S DISEASE
11.6 ULCERATIVE COLITIS
11.7 PSORIATIC ARTHRITIS
11.8 JUVENILE IDIOPATHIC ARTHRITIS
11.9 HIDRADENITIS SUPPURATIVA
11.1 NON-INFECTIOUS INTERMEDIATE
11.11 OTHERS
12 EUROPE ADALIMUMAB MARKET, BY TYPE
12.1 OVERVIEW
12.2 BIOLOGICS
12.3 BIOSIMILARS
12.3.1 ADALIMUMAB-ATTO
12.3.2 ADALIMUMAB-BWWD
12.3.3 ADALIMUMAB-ADBM
12.3.4 ADALIMUMAB-ADAZ
12.3.5 ADALIMUMAB-FKJP
12.3.6 ADALIMUMAB-AFZB
12.3.7 OTHERS
13 EUROPE ADALIMUMAB MARKET, BY DOSAGE STRENGTH
13.1 OVERVIEW
13.2MG/0.4ML
13.3MG/0.8ML
13.4MG/0.4ML
13.5MG/0.1ML
13.6 OTHERS
14 EUROPE ADALIMUMAB MARKET, BY DRUG TYPE
14.1 OVERVIEW
14.2 BRANDED
14.3 GENERICS
14.3.1 AMJEVITA
14.3.2 HYRIMOZ
14.3.3 HULIO
14.3.4 OTHERS
15 EUROPE ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION
15.1 OVERVIEW
15.2 PARENTERAL
15.3 ORAL
16 EUROPE ADALIMUMAB MARKET, BY POPULATION TYPE
16.1 OVERVIEW
16.2 ADULTS
16.3 CHILDREN
17 EUROPE ADALIMUMAB MARKET, BY END USER
17.1 OVERVIEW
17.2 HOSPITALS
17.3 SPECIALTY CLINICS
17.4 HOME HEALTHCARE
17.5 OTHERS
18 EUROPE ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL
18.1 OVERVIEW
18.2 HOSPITAL PHARMACIES
18.3 RETAIL PHARMACIES
18.4 ONLINE PHARMACIES
18.5 DIRECT TENDER
18.6 OTHERS
19 EUROPE ADALIMUMAB MARKET, BY GEOGRAPHY
19.1 EUROPE
19.1.1 GERMANY
19.1.2 U.K
19.1.3 ITALY
19.1.4 FRANCE
19.1.5 SPAIN
19.1.6 NETHERLANDS
19.1.7 RUSSIA
19.1.8 SWITZERLAND
19.1.9 BELGIUM
19.1.10 TURKEY
19.1.11 AUSTRIA
19.1.12 NORWAY
19.1.13 HUNGARY
19.1.14 LITHUANIA
19.1.15 IRELAND
19.1.16 POLAND
19.1.17 REST OF EUROPE
20 EUROPE ADALIMUMAB MARKET: COMPANY LANDSCAPE
20.1 COMPANY SHARE ANALYSIS: EUROPE
21 SWOT
22 COMPANY PROFILES
22.1 ABBVIE INC.
22.1.1 COMPANY SNAPSHOT
22.1.2 REVENUE ANALYSIS
22.1.3 COMPANY SHARE ANALYSIS
22.1.4 PRODUCT PORTFOLIO
22.1.5 RECENT DEVELOPMENTS
22.2 AMGEN (EUROPE) GMBH (A SUBSIDIARY OF AMGEN INC.)
22.2.1 COMPANY SNAPSHOT
22.2.2 REVENUE ANALYSIS
22.2.3 COMPANY SHARE ANALYSIS
22.2.4 PRODUCT PORTFOLIO
22.2.5 RECENT DEVELOPMENTS
22.3 BIOGEN
22.3.1 COMPANY SNAPSHOT
22.3.2 REVENUE ANALYSIS
22.3.3 PRODUCT PORTFOLIO
22.3.4 RECENT DEVELOPMENTS
22.4 SANDOZ INTERNATIONAL GMBH {A SUBSIDIARY OF SANDOZ (A DIVISION OF NOVARTIS AG)}
22.4.1 COMPANY SNAPSHOT
22.4.2 REVENUE ANALYSIS
22.4.3 PRODUCT PORTFOLIO
22.4.4 RECENT DEVELOPMENTS
22.5 MYLAN N.V.
22.5.1 COMPANY SNAPSHOT
22.5.2 REVENUE ANALYSIS
22.5.3 PRODUCT PORTFOLIO
22.5.4 RECENT DEVELOPMENTS
22.6 BOEHRINGER INGELHEIM INTERNATIONAL GMBH
22.6.1 COMPANY SNAPSHOT
22.6.2 REVENUE ANALYSIS
22.6.3 PRODUCT PORTFOLIO
22.6.4 RECENT DEVELOPMENTS
22.7 CELLTRION INC.
22.7.1 COMPANY SNAPSHOT
22.7.2 REVENUE ANALYSIS
22.7.3 PRODUCT PORTFOLIO
22.7.4 RECENT DEVELOPMENTS
22.8 COHERUS BIOSCIENCES
22.8.1 COMPANY SNAPSHOT
22.8.2 PRODUCT PORTFOLIO
22.8.3 RECENT DEVELOPMENTS
22.9 FRESENIUS KABI DEUTSCHLAND GMBH (A SUBSIDIARY OF FRESENIUS KABI AG)
22.9.1 COMPANY SNAPSHOT
22.9.2 REVENUE ANALYSIS
22.9.3 PRODUCT PORTFOLIO
22.9.4 RECENT DEVELOPMENTS
22.1 HETERO BIOPHARMA LTD.
22.10.1 COMPANY SNAPSHOT
22.10.2 PRODUCT PORTFOLIO
22.10.3 RECENT DEVELOPMENTS
22.11 INNOVENT BIOLOGICS, INC.
22.11.1 COMPANY SNAPSHOT
22.11.2 REVENUE ANALYSIS
22.11.3 PRODUCT PORTFOLIO
22.11.4 RECENT DEVELOPMENTS
22.12 PFIZER INC.
22.12.1 COMPANY SNAPSHOT
22.12.2 REVENUE ANALYSIS
22.12.3 PRODUCT PORTFOLIO
22.12.4 RECENT DEVELOPMENTS
22.13 RELIANCE LIFE SCIENCES (A SUBSIDIARY OF RELIANCE INDUSTRIES LIMITED)
22.13.1 COMPANY SNAPSHOT
22.13.2 REVENUE ANALYSIS
22.13.3 PRODUCT PORTFOLIO
22.13.4 RECENT DEVELOPMENTS
22.14 SAMSUNG BIOEPIS (A SUBSIDIARY OF SAMSUNG BIOLOGICS)
22.14.1 COMPANY SNAPSHOT
22.14.2 REVENUE ANALYSIS
22.14.3 PRODUCT PORTFOLIO
22.14.4 RECENT DEVELOPMENTS
22.15 ZYDUS CADILA
22.15.1 COMPANY SNAPSHOT
22.15.2 REVENUE ANALYSIS
22.15.3 PRODUCT PORTFOLIO
22.15.4 RECENT DEVELOPMENT
23 QUESTIONNAIRE
24 RELATED REPORTS
Lista de Tabela
LIST OF TABLES
TABLE 1 EUROPE ADALIMUMAB MARKET, PIPELINE ANALYSIS
TABLE 2 BIOSIMILAR OF ADALIMUMAB LAUNCHED IN THE U.S.
TABLE 3 PREVALENCE AND INCIDENCE RATES OF RA WORLDWIDE (CASE PER 100 INHABITANTS)
TABLE 4 BIOLOGIC DRUGS SUBJECTED TO PATENT LOSS
TABLE 5 ALTERNATIVE DRUGS FOR INFLAMMATORY DISEASES TREATMENT
TABLE 6 EUROPE ADALIMUMAB MARKET, BY INDICATION 2019-2027 (USD MILLION)
TABLE 7 EUROPE RHEUMATOID ARTHRITIS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 8 EUROPE ANKYLOSING SPONDYLITIS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 9 EUROPE CHRONIC PLAQUE PSORIASIS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 10 EUROPE CROHN’S DISEASE IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 11 EUROPE ULCERATIVE COLITIS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 12 EUROPE PSORIATIC ARTHRITIS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 13 EUROPE JUVENILE IDIOPATHIC ARTHRITIS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 14 EUROPE HIDRADENITIS SUPPURATIVA IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 15 EUROPE NONINFECTIOUS INTERMEDIATE IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 16 EUROPE OTHERS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 17 EUROPE ADALIMUMAB MARKET, BY TYPE 2019-2027 (USD MILLION)
TABLE 18 EUROPE BIOLOGICS IN ADALIMUMAB MARKET, BY TYPE 2019-2027 (USD MILLION)
TABLE 19 EUROPE BIOSIMILARS IN ADALIMUMAB MARKET, BY TYPE 2019-2027 (USD MILLION)
TABLE 20 EUROPE BIOSIMILARS IN ADALIMUMAB MARKET, BY TYPE 2019-2027 (USD MILLION)
TABLE 21 EUROPE ADALIMUMAB MARKET, BY DOSAGE STRENGHT, 2019-2027 (USD MILLION)
TABLE 22 EUROPE 40MG/0.4ML IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 23 EUROPE 80MG/0.8ML IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 24 EUROPE 20MG/0.4ML IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 25 EUROPE 10MG/0.1ML IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 26 EUROPE OTHERS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 27 EUROPE ADALIMUMAB MARKET, BY DRUG TYPE, 2019-2027 (USD MILLION)
TABLE 28 EUROPE BRANDED IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 29 EUROPE GENERICS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 30 EUROPE GENERICS ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 31 EUROPE ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION 2019-2027 (USD MILLION)
TABLE 32 EUROPE PARENTERAL IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 33 EUROPE ADALIMUMAB MARKET, BY POPULATION TYPE, 2019-2027 (USD MILLION)
TABLE 34 EUROPE ADULTS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 35 EUROPE CHILDREN IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 36 EUROPE ADALIMUMAB MARKET, BY END USER, 2019-2027 (USD MILLION)
TABLE 37 EUROPE HOSPITALS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 38 EUROPE SPECIALTY CLINICS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 39 EUROPE HOME HEALTHCARE IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 40 EUROPE OTHERS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 41 EUROPE ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2019-2027 (USD MILLION)
TABLE 42 EUROPE HOSPITAL PHARMACIES IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 43 EUROPE RETAIL PHARMACIES IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 44 EUROPE ONLINE PHARMACIES IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 45 EUROPE DIRECT TENDER IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 46 EUROPE OTHERS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 47 EUROPE ADALIMUMAB MARKET, BY COUNTRY, 2018-2027 (USD MILLION)
TABLE 48 EUROPE ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
TABLE 49 EUROPE ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 50 EUROPE BIOSIMILARS OF ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 51 EUROPE ADALIMUMAB MARKET, BY DOSAGE STRENGTH, 2018-2027 (USD MILLION)
TABLE 52 EUROPE ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 53 EUROPE GENERICS IN ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 54 EUROPE ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION, 2018-2027 (USD MILLION)
TABLE 55 EUROPE ADALIMUMAB MARKET, BY POPULATION TYPE, 2018-2027 (USD MILLION)
TABLE 56 EUROPE ADALIMUMAB MARKET, BY END USER, 2018-2027 (USD MILLION)
TABLE 57 EUROPE ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2018-2027 (USD MILLION)
TABLE 58 GERMANYADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
TABLE 59 GERMANYADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 60 GERMANYBIOSIMILARS OF ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 61 GERMANYADALIMUMAB MARKET, BY DOSAGE STRENGTH, 2018-2027 (USD MILLION)
TABLE 62 GERMANYADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 63 GERMANY GENERICS IN ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 64 GERMANYADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION, 2018-2027 (USD MILLION)
TABLE 65 GERMANYADALIMUMAB MARKET, BY POPULATION TYPE, 2018-2027 (USD MILLION)
TABLE 66 GERMANYADALIMUMAB MARKET, BY END USER, 2018-2027 (USD MILLION)
TABLE 67 GERMANYADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2018-2027 (USD MILLION)
TABLE 68 U.K ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
TABLE 69 U.K ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 70 U.K BIOSIMILARS OF ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 71 U.K ADALIMUMAB MARKET, BY DOSAGE STRENGTH, 2018-2027 (USD MILLION)
TABLE 72 U.K ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 73 U.K GENERICS IN ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 74 U.K ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION, 2018-2027 (USD MILLION)
TABLE 75 U.K ADALIMUMAB MARKET, BY POPULATION TYPE, 2018-2027 (USD MILLION)
TABLE 76 U.K ADALIMUMAB MARKET, BY END USER, 2018-2027 (USD MILLION)
TABLE 77 U.K ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2018-2027 (USD MILLION)
TABLE 78 ITALY ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
TABLE 79 ITALY ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 80 ITALY BIOSIMILARS OF ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 81 ITALY ADALIMUMAB MARKET, BY DOSAGE STRENGTH, 2018-2027 (USD MILLION)
TABLE 82 ITALY ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 83 ITALY GENERICS IN ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 84 ITALY ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION, 2018-2027 (USD MILLION)
TABLE 85 ITALY ADALIMUMAB MARKET, BY POPULATION TYPE, 2018-2027 (USD MILLION)
TABLE 86 ITALY ADALIMUMAB MARKET, BY END USER, 2018-2027 (USD MILLION)
TABLE 87 ITALY ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2018-2027 (USD MILLION)
TABLE 88 FRANCE ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
TABLE 89 FRANCE ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 90 FRANCE BIOSIMILARS OF ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 91 FRANCE ADALIMUMAB MARKET, BY DOSAGE STRENGTH, 2018-2027 (USD MILLION)
TABLE 92 FRANCE ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 93 FRANCE GENERICS IN ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 94 FRANCE ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION, 2018-2027 (USD MILLION)
TABLE 95 FRANCE ADALIMUMAB MARKET, BY POPULATION TYPE, 2018-2027 (USD MILLION)
TABLE 96 FRANCE ADALIMUMAB MARKET, BY END USER, 2018-2027 (USD MILLION)
TABLE 97 FRANCE ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2018-2027 (USD MILLION)
TABLE 98 SPAIN ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
TABLE 99 SPAIN ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 100 SPAIN BIOSIMILARS OF ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 101 SPAIN ADALIMUMAB MARKET, BY DOSAGE STRENGTH, 2018-2027 (USD MILLION)
TABLE 102 SPAIN ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 103 SPAIN GENERICS IN ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 104 SPAIN ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION, 2018-2027 (USD MILLION)
TABLE 105 SPAIN ADALIMUMAB MARKET, BY POPULATION TYPE, 2018-2027 (USD MILLION)
TABLE 106 SPAIN ADALIMUMAB MARKET, BY END USER, 2018-2027 (USD MILLION)
TABLE 107 SPAIN ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2018-2027 (USD MILLION)
TABLE 108 NETHERLANDS ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
TABLE 109 NETHERLANDS ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 110 NETHERLANDS BIOSIMILARS OF ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 111 NETHERLANDS ADALIMUMAB MARKET, BY DOSAGE STRENGTH, 2018-2027 (USD MILLION)
TABLE 112 NETHERLANDS ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 113 NETHERLANDS GENERICS IN ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 114 NETHERLANDS ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION, 2018-2027 (USD MILLION)
TABLE 115 NETHERLANDS ADALIMUMAB MARKET, BY POPULATION TYPE, 2018-2027 (USD MILLION)
TABLE 116 NETHERLANDS ADALIMUMAB MARKET, BY END USER, 2018-2027 (USD MILLION)
TABLE 117 NETHERLANDS ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2018-2027 (USD MILLION)
TABLE 118 RUSSIA ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
TABLE 119 RUSSIA ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 120 RUSSIA BIOSIMILARS OF ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 121 RUSSIA ADALIMUMAB MARKET, BY DOSAGE STRENGTH, 2018-2027 (USD MILLION)
TABLE 122 RUSSIA ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 123 RUSSIA GENERICS IN ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 124 RUSSIA ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION, 2018-2027 (USD MILLION)
TABLE 125 RUSSIA ADALIMUMAB MARKET, BY POPULATION TYPE, 2018-2027 (USD MILLION)
TABLE 126 RUSSIA ADALIMUMAB MARKET, BY END USER, 2018-2027 (USD MILLION)
TABLE 127 RUSSIA ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2018-2027 (USD MILLION)
TABLE 128 SWITZERLAND ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
TABLE 129 SWITZERLAND ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 130 SWITZERLAND BIOSIMILARS OF ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 131 SWITZERLAND ADALIMUMAB MARKET, BY DOSAGE STRENGTH, 2018-2027 (USD MILLION)
TABLE 132 SWITZERLAND ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 133 SWITZERLAND GENERICS IN ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 134 SWITZERLAND ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION, 2018-2027 (USD MILLION)
TABLE 135 SWITZERLAND ADALIMUMAB MARKET, BY POPULATION TYPE, 2018-2027 (USD MILLION)
TABLE 136 SWITZERLAND ADALIMUMAB MARKET, BY END USER, 2018-2027 (USD MILLION)
TABLE 137 SWITZERLAND ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2018-2027 (USD MILLION)
TABLE 138 BELGIUM ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
TABLE 139 BELGIUM ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 140 BELGIUM BIOSIMILARS OF ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 141 BELGIUM ADALIMUMAB MARKET, BY DOSAGE STRENGTH, 2018-2027 (USD MILLION)
TABLE 142 BELGIUM ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 143 BELGIUM GENERICS IN ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 144 BELGIUM ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION, 2018-2027 (USD MILLION)
TABLE 145 BELGIUM ADALIMUMAB MARKET, BY POPULATION TYPE, 2018-2027 (USD MILLION)
TABLE 146 BELGIUM ADALIMUMAB MARKET, BY END USER, 2018-2027 (USD MILLION)
TABLE 147 BELGIUM ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2018-2027 (USD MILLION)
TABLE 148 TURKEY ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
TABLE 149 TURKEY ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 150 TURKEY BIOSIMILARS OF ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 151 TURKEY ADALIMUMAB MARKET, BY DOSAGE STRENGTH, 2018-2027 (USD MILLION)
TABLE 152 TURKEY ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 153 TURKEY GENERICS IN ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 154 TURKEY ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION, 2018-2027 (USD MILLION)
TABLE 155 TURKEY ADALIMUMAB MARKET, BY POPULATION TYPE, 2018-2027 (USD MILLION)
TABLE 156 TURKEY ADALIMUMAB MARKET, BY END USER, 2018-2027 (USD MILLION)
TABLE 157 TURKEY ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2018-2027 (USD MILLION)
TABLE 158 AUSTRIA ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
TABLE 159 AUSTRIA ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 160 AUSTRIA BIOSIMILARS OF ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 161 AUSTRIA ADALIMUMAB MARKET, BY DOSAGE STRENGTH, 2018-2027 (USD MILLION)
TABLE 162 AUSTRIA ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 163 AUSTRIA GENERICS IN ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 164 AUSTRIA ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION, 2018-2027 (USD MILLION)
TABLE 165 AUSTRIA ADALIMUMAB MARKET, BY POPULATION TYPE, 2018-2027 (USD MILLION)
TABLE 166 AUSTRIA ADALIMUMAB MARKET, BY END USER, 2018-2027 (USD MILLION)
TABLE 167 AUSTRIA ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2018-2027 (USD MILLION)
TABLE 168 NORWAY ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
TABLE 169 NORWAY ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 170 NORWAY BIOSIMILARS OF ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 171 NORWAY ADALIMUMAB MARKET, BY DOSAGE STRENGTH, 2018-2027 (USD MILLION)
TABLE 172 NORWAY ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 173 NORWAY GENERICS IN ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 174 NORWAY ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION, 2018-2027 (USD MILLION)
TABLE 175 NORWAY ADALIMUMAB MARKET, BY POPULATION TYPE, 2018-2027 (USD MILLION)
TABLE 176 NORWAY ADALIMUMAB MARKET, BY END USER, 2018-2027 (USD MILLION)
TABLE 177 NORWAY ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2018-2027 (USD MILLION)
TABLE 178 HUNGARY ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
TABLE 179 HUNGARY ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 180 HUNGARY BIOSIMILARS OF ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 181 HUNGARY ADALIMUMAB MARKET, BY DOSAGE STRENGTH, 2018-2027 (USD MILLION)
TABLE 182 HUNGARY ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 183 HUNGARY GENERICS IN ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 184 HUNGARY ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION, 2018-2027 (USD MILLION)
TABLE 185 HUNGARY ADALIMUMAB MARKET, BY POPULATION TYPE, 2018-2027 (USD MILLION)
TABLE 186 HUNGARY ADALIMUMAB MARKET, BY END USER, 2018-2027 (USD MILLION)
TABLE 187 HUNGARY ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2018-2027 (USD MILLION)
TABLE 188 LITHUANIA ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
TABLE 189 LITHUANIA ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 190 LITHUANIA BIOSIMILARS OF ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 191 LITHUANIA ADALIMUMAB MARKET, BY DOSAGE STRENGTH, 2018-2027 (USD MILLION)
TABLE 192 LITHUANIA ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 193 LITHUANIA GENERICS IN ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 194 LITHUANIA ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION, 2018-2027 (USD MILLION)
TABLE 195 LITHUANIA ADALIMUMAB MARKET, BY POPULATION TYPE, 2018-2027 (USD MILLION)
TABLE 196 LITHUANIA ADALIMUMAB MARKET, BY END USER, 2018-2027 (USD MILLION)
TABLE 197 LITHUANIA ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2018-2027 (USD MILLION)
TABLE 198 IRELAND ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
TABLE 199 IRELAND ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 200 IRELAND BIOSIMILARS OF ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 201 IRELAND ADALIMUMAB MARKET, BY DOSAGE STRENGTH, 2018-2027 (USD MILLION)
TABLE 202 IRELAND ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 203 IRELAND GENERICS IN ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 204 IRELAND ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION, 2018-2027 (USD MILLION)
TABLE 205 IRELAND ADALIMUMAB MARKET, BY POPULATION TYPE, 2018-2027 (USD MILLION)
TABLE 206 IRELAND ADALIMUMAB MARKET, BY END USER, 2018-2027 (USD MILLION)
TABLE 207 IRELAND ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2018-2027 (USD MILLION)
TABLE 208 POLAND ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
TABLE 209 POLAND ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 210 POLAND BIOSIMILARS OF ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 211 POLAND ADALIMUMAB MARKET, BY DOSAGE STRENGTH, 2018-2027 (USD MILLION)
TABLE 212 POLAND ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 213 POLAND GENERICS IN ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 214 POLAND ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION, 2018-2027 (USD MILLION)
TABLE 215 POLAND ADALIMUMAB MARKET, BY POPULATION TYPE, 2018-2027 (USD MILLION)
TABLE 216 POLAND ADALIMUMAB MARKET, BY END USER, 2018-2027 (USD MILLION)
TABLE 217 POLAND ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2018-2027 (USD MILLION)
TABLE 218 REST OF EUROPE ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
Lista de Figura
LIST OF FIGURES
FIGURE 1 EUROPE ADALIMUMAB MARKET: SEGMENTATION
FIGURE 2 EUROPE ADALIMUMAB MARKET: DATA TRIANGULATION
FIGURE 3 EUROPE ADALIMUMAB MARKET: DROC ANALYSIS
FIGURE 4 EUROPE ADALIMUMAB MARKET: EUROPE VS REGIONAL MARKET ANALYSIS
FIGURE 5 EUROPE ADALIMUMAB MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 EUROPE ADALIMUMAB MARKET: MULTIVARIATE MODELLING
FIGURE 7 EUROPE ADALIMUMAB MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 8 EUROPE ADALIMUMAB MARKET: DBMR MARKET POSITION GRID
FIGURE 9 EUROPE ADALIMUMAB MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 EUROPE ADALIMUMAB MARKET: SEGMENTATION
FIGURE 11 RISE IN THE PREVALENCE OF RHEUMATOID ARTHRITIS AND INCREASING GERIATRIC POPULATION IS DRIVING THE EUROPE ADALIMUMAB MARKET IN THE FORECAST PERIOD OF 2020 TO 2027
FIGURE 12 RHEUMATOID ARTHRITIS IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE EUROPE ADALIMUMAB MARKET IN 2020 & 2027
FIGURE 13 DRIVERS, RESTRAINTS, OPPORTUNITIES AND CHALLENGES OF EUROPE ADALIMUMAB MARKET
FIGURE 14 MARKET GROWTH IN CLINICAL CRO (IN USD MILLIONS)
FIGURE 15 FUNCTION OF CRO
FIGURE 16 HEALTHCARE EXPENDITURE IN 2016 AND 2019
FIGURE 17 EUROPE ADALIMUMAB MARKET: BY INDICATION, 2019
FIGURE 18 EUROPE ADALIMUMAB MARKET: BY INDICATION, 2019-2027 (USD MILLION)
FIGURE 19 EUROPE ADALIMUMAB MARKET: BY INDICATION, CAGR (2020-2027)
FIGURE 20 EUROPE ADALIMUMAB MARKET: BY INDICATION, LIFELINE CURVE
FIGURE 21 EUROPE ADALIMUMAB MARKET: BY TYPE, 2019
FIGURE 22 EUROPE ADALIMUMAB MARKET: BY TYPE 2019-2027 (USD MILLION)
FIGURE 23 EUROPE ADALIMUMAB MARKET: BY TYPE, CAGR (2020-2027)
FIGURE 24 EUROPE ADALIMUMAB MARKET: BY TYPE, LIFELINE CURVE
FIGURE 25 EUROPE ADALIMUMAB MARKET: BY DOSAGE STRENGTH, 2019
FIGURE 26 EUROPE ADALIMUMAB MARKET: BY DOSAGE STRENGTH 2019-2027 (USD MILLION)
FIGURE 27 EUROPE ADALIMUMAB MARKET: BY DOSAGE STRENGTH, CAGR (2020-2027)
FIGURE 28 EUROPE ADALIMUMAB MARKET: BY DOSAGE STRENGTH, LIFELINE CURVE
FIGURE 29 EUROPE ADALIMUMAB MARKET: BY DRUG TYPE, 2019
FIGURE 30 EUROPE ADALIMUMAB MARKET: BY DRUG TYPE , 2019-2027 (USD MILLION)
FIGURE 31 EUROPE ADALIMUMAB MARKET: BY DRUG TYPE, CAGR (2020-2027)
FIGURE 32 EUROPE ADALIMUMAB MARKET: BY DRUG TYPE, LIFELINE CURVE
FIGURE 33 EUROPE ADALIMUMAB MARKET: BY ROUTE OF ADMINISTRATION, 2019
FIGURE 34 EUROPE ADALIMUMAB MARKET: BY ROUTE OF ADMINISTRATION, 2019-2027 (USD MILLION)
FIGURE 35 EUROPE ADALIMUMAB MARKET: BY ROUTE OF ADMINISTRATION, CAGR (2020-2027)
FIGURE 36 EUROPE ADALIMUMAB MARKET: BY ROUTE OF ADMINISTRATION, LIFELINE CURVE
FIGURE 37 EUROPE ADALIMUMAB MARKET: BY POPULATION TYPE, 2019
FIGURE 38 EUROPE ADALIMUMAB MARKET: BY POPULATION TYPE, 2019-2027 (USD MILLION)
FIGURE 39 EUROPE ADALIMUMAB MARKET: BY POPULATION TYPE, CAGR (2020-2027)
FIGURE 40 EUROPE ADALIMUMAB MARKET: BY POPULATION TYPE, LIFELINE CURVE
FIGURE 41 EUROPE ADALIMUMAB MARKET: BY END USER, 2019
FIGURE 42 EUROPE ADALIMUMAB MARKET: BY END USER, 2019-2027 (USD MILLION)
FIGURE 43 EUROPE ADALIMUMAB MARKET: BY END USER, CAGR (2020-2027)
FIGURE 44 EUROPE ADALIMUMAB MARKET: BY END USER, LIFELINE CURVE
FIGURE 45 EUROPE ADALIMUMAB MARKET: BY DISTRIBUTION CHANNEL, 2019
FIGURE 46 EUROPE ADALIMUMAB MARKET: BY DISTRIBUTION CHANNEL, 2019-2027 (USD MILLION)
FIGURE 47 EUROPE ADALIMUMAB MARKET: BY DISTRIBUTION CHANNEL, CAGR (2020-2027)
FIGURE 48 EUROPE ADALIMUMAB MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 49 EUROPE ADALIMUMAB MARKET: SNAPSHOT (2019)
FIGURE 50 EUROPE ADALIMUMAB MARKET: BY COUNTRY (2019)
FIGURE 51 EUROPE ADALIMUMAB MARKET: BY COUNTRY (2020 & 2027)
FIGURE 52 EUROPE ADALIMUMAB MARKET: BY COUNTRY (2019 & 2027)
FIGURE 53 EUROPE ADALIMUMAB MARKET: BY POPULATION TYPE (2020-2027)
FIGURE 54 EUROPE ADALIMUMAB MARKET: COMPANY SHARE 2019 (%)

Metodologia de Investigação
A recolha de dados e a análise do ano base são feitas através de módulos de recolha de dados com amostras grandes. A etapa inclui a obtenção de informações de mercado ou dados relacionados através de diversas fontes e estratégias. Inclui examinar e planear antecipadamente todos os dados adquiridos no passado. Da mesma forma, envolve o exame de inconsistências de informação observadas em diferentes fontes de informação. Os dados de mercado são analisados e estimados utilizando modelos estatísticos e coerentes de mercado. Além disso, a análise da quota de mercado e a análise das principais tendências são os principais fatores de sucesso no relatório de mercado. Para saber mais, solicite uma chamada de analista ou abra a sua consulta.
A principal metodologia de investigação utilizada pela equipa de investigação do DBMR é a triangulação de dados que envolve a mineração de dados, a análise do impacto das variáveis de dados no mercado e a validação primária (especialista do setor). Os modelos de dados incluem grelha de posicionamento de fornecedores, análise da linha de tempo do mercado, visão geral e guia de mercado, grelha de posicionamento da empresa, análise de patentes, análise de preços, análise da quota de mercado da empresa, normas de medição, análise global versus regional e de participação dos fornecedores. Para saber mais sobre a metodologia de investigação, faça uma consulta para falar com os nossos especialistas do setor.
Personalização disponível
A Data Bridge Market Research é líder em investigação formativa avançada. Orgulhamo-nos de servir os nossos clientes novos e existentes com dados e análises que correspondem e atendem aos seus objetivos. O relatório pode ser personalizado para incluir análise de tendências de preços de marcas-alvo, compreensão do mercado para países adicionais (solicite a lista de países), dados de resultados de ensaios clínicos, revisão de literatura, mercado remodelado e análise de base de produtos . A análise de mercado dos concorrentes-alvo pode ser analisada desde análises baseadas em tecnologia até estratégias de carteira de mercado. Podemos adicionar quantos concorrentes necessitar de dados no formato e estilo de dados que procura. A nossa equipa de analistas também pode fornecer dados em tabelas dinâmicas de ficheiros Excel em bruto (livro de factos) ou pode ajudá-lo a criar apresentações a partir dos conjuntos de dados disponíveis no relatório.