Asia Pacific Vaccines Market
Tamanho do mercado em biliões de dólares
CAGR :
% 
 USD
9,048.51 Million
USD
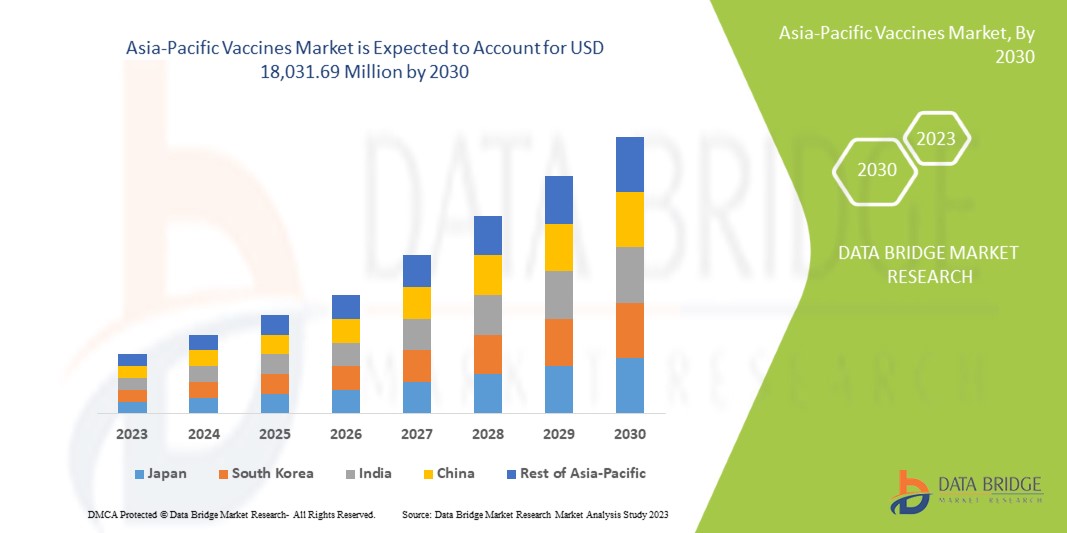
18,031.69 Million
2022
2030
USD
9,048.51 Million
USD
18,031.69 Million
2022
2030
| 2023 –2030 | |
| USD 9,048.51 Million | |
| USD 18,031.69 Million | |
|
|
|
Mercado de vacinas Ásia-Pacífico, por composição (vacinas combinadas e monovacinas), tipo (vacinas subunidade, recombinante, polissacárido e conjugada, vacinas vivas atenuadas, vacinas inativadas e vacinas toxóides), tipo (vacina de rotina, vacina recomendada e Vacina necessária ), Idade de administração (vacina pediátrica e vacina para adultos), doenças (doença pneumocócica, sarampo, papeira e varicela, DPT, hepatite, gripe, febre tifóide, meningocócica, raiva, encefalite japonesa, febre amarela e outras), via de Administração (Injetável, Oral e Nasal), Utilizador Final (Hospitais Comunitários, Hospitais, Centros de Especialidades, Clínicas e Outros), Canal de Distribuição (Farmácia Hospitalar, Farmácia de Retalho e Farmácia Online) - Tendências do Setor e Previsão para 2030.
Análise e insights do mercado de vacinas da Ásia-Pacífico
A crescente prevalência de doenças infeciosas, incluindo doenças bacterianas e virais, proporciona ao mercado um crescimento rentável. Paralelamente, o aumento do apoio governamental e o lançamento de vacinas mais recentes estão também a impulsionar o mercado das vacinas. Outro factor que impulsiona o crescimento do mercado das vacinas é o aumento da sensibilização para a vacinação e a procura de vacinas eficazes contra a COVID-19.
O mercado de vacinas da Ásia-Pacífico deverá crescer no período de previsão de 2023 a 2030. A Data Bridge Market Research analisa que o mercado está a crescer com um CAGR de 9,4% no período de previsão de 2023 a 2030 e deverá atingir os US$ 18.031,69.
|
Métrica de reporte |
Detalhes |
|
Período de previsão |
2023 a 2030 |
|
Ano base |
2022 |
|
Anos históricos |
2021 (personalizável para 2015-2020) |
|
Unidades Quantitativas |
Receita em milhões de dólares |
|
Segmentos cobertos |
Por composição (vacinas combinadas e monovacinas), tipo (vacinas de subunidades, recombinantes, polissacáridos e conjugadas, vacinas vivas atenuadas, vacinas inativadas e vacinas toxóides), tipo (vacina de rotina, vacina recomendada e vacina necessária), idade de Administração (vacina pediátrica e vacina para adultos), doenças (doença pneumocócica, sarampo, papeira e varicela, DPT, hepatite, gripe, febre tifóide, meningocócica, raiva, encefalite japonesa, febre amarela e outras), via de administração (injetável, oral, e Nasal ), Utilizador Final (Hospitais Comunitários, Hospitais, Centros de Especialidades, Clínicas e Outros), Canal de Distribuição (Farmácia Hospitalar, Farmácia de Retalho e Farmácia Online) |
|
Países abrangidos |
Japão, China, Austrália, Índia, Coreia do Sul, Singapura, Indonésia, Tailândia, Malásia, Filipinas, Vietname e Resto da Ásia-Pacífico |
|
Participantes do mercado abrangidos |
Bharat Biotech, Biological E Limited, Bio Farma, Serum Institute of India Unip. Ltd., Takeda Pharmaceutical Company Limited, Merck Sharp & Dohme Corp. (uma subsidiária da Merck & Co., Inc.), Abbott, AstraZeneca, Sanofi, Pfizer Inc., Janssen Global Services, LLC (uma subsidiária da Johnson & Johnson Services , Inc.), F. Hoffmann-La Roche Ltd, Panacea Biotec Ltd e BAXTER VACCINES (uma subsidiária da Baxter), entre outros |
Definição do mercado do mercado de vacinas da Ásia-Pacífico
As vacinas são produtos que estimulam o sistema imunitário individual a induzir imunidade contra uma determinada doença. As vacinas funcionam com base no princípio da memória e do reconhecimento. Quando micróbios enfraquecidos ou mortos são injetados no organismo, estes micróbios fazem com que as células B, células de memória do sistema imunitário, reconheçam o agente patogénico. No futuro, se o mesmo agente patogénico atacar o corpo, atuará contra eles. Foram descobertas vacinas para doenças infeciosas, incluindo doença pneumocócica, sarampo, papeira, rubéola, hepatite, gripe, febre tifóide, varicela e raiva.
As vacinas são de dois tipos: vacinas combinadas (contendo diferentes estirpes do agente patogénico) e monovacinas (contendo uma única estirpe do agente patogénico). Foram desenvolvidos diferentes tipos de vacinas com base no material extraído do patogéneo, que pode ser uma capa polissacárida, ADN, ARN e todo o organismo, inativado ou vivo.
Estas são as vacinas que resultaram na erradicação de doenças como a poliomielite. De acordo com a preferência e eficiência das vacinas, estas podem ser injetadas por diversas vias de administração que podem ser injetáveis, orais ou nasais. Contudo, a via injectável de administração da vacina é altamente preferida, uma vez que induz uma resposta sistémica. A vacinação pode ser realizada em hospitais, clínicas comunitárias e clínicas especializadas, entre outros, por pessoal com formação e conhecimentos adequados sobre os dispositivos de administração de vacinas.
A crescente prevalência de doenças infeciosas, incluindo doenças bacterianas e virais, proporciona ao mercado um crescimento rentável. Juntamente com isto, o aumento do apoio governamental e o lançamento de vacinas mais recentes também impulsionam o mercado das vacinas. Outro factor que impulsiona o crescimento do mercado das vacinas é o aumento da sensibilização para a vacinação e a procura de vacinas eficazes contra a COVID-19.
Dinâmica do mercado de vacinas da Ásia-Pacífico
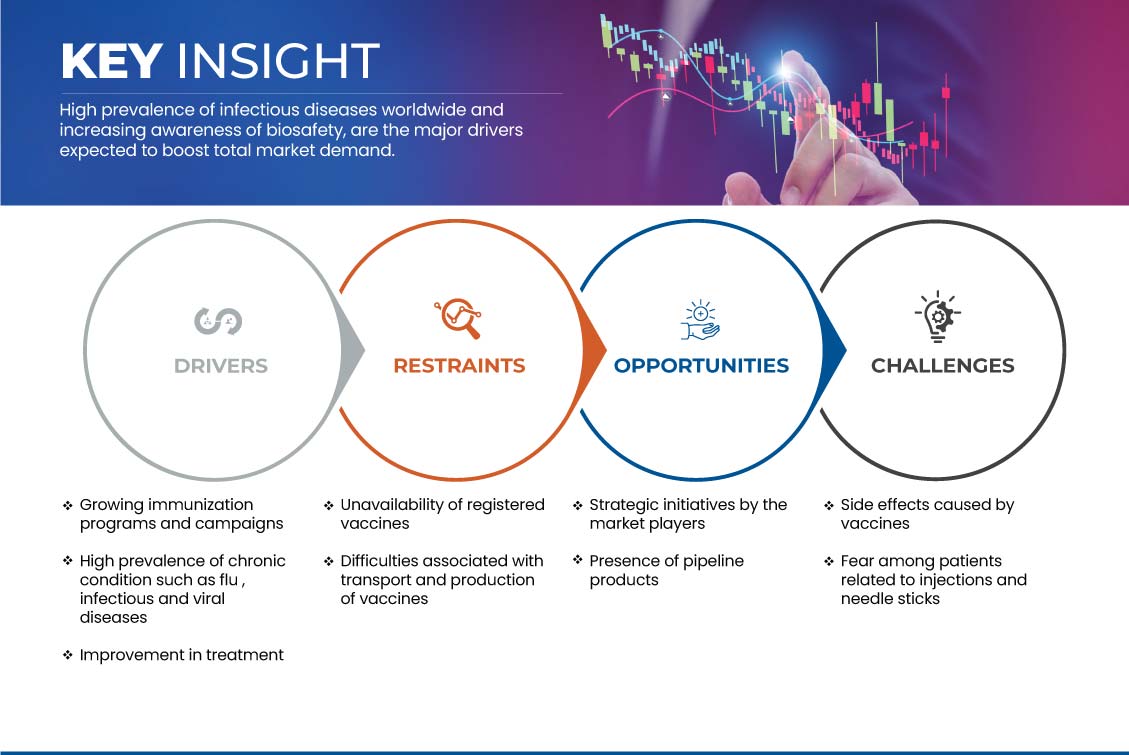
Esta secção trata da compreensão dos impulsionadores do mercado, vantagens, oportunidades, restrições e desafios. Tudo isto é discutido em detalhe abaixo:
MOTORISTAS
CRESCIMENTO NOS PROGRAMAS E CAMPANHAS DE IMUNIZAÇÃO
Os programas e campanhas de imunização estão a aumentar em todo o mundo devido a um número crescente de doenças crónicas. Dado que a hepatite, a difteria, a tosse convulsa e a poliomielite, entre outras doenças infecciosas, prevalecem no ambiente, existe uma necessidade urgente de aumentar a sensibilização relativamente à vacinação, o que pode ser conseguido através do lançamento de diversas campanhas e programas. Foi relatado que o número de programas de imunização está a aumentar com o aumento das doenças infeciosas. A cobertura vacinal está também a aumentar em todo o mundo, com o objectivo de combater doenças debilitantes.
Ainda assim, cerca de 20 milhões de pessoas continuam não vacinadas e subvacinadas, aumentando uma grande procura de vacinação. A prevalência das doenças crónicas está a aumentar em todo o mundo. Daí que a necessidade de se vacinar seja grande. Isto significa que se espera que um programa de imunização crescente e a campanha façam crescer o mercado das vacinas.
ELEVADA PREVALÊNCIA DE CONDIÇÕES CRÓNICAS, COMO GRIPE, DOENÇAS INFECCIOSAS E VIRAIS
A prevalência de doenças infecciosas está a aumentar em todo o mundo, e verifica-se que a gripe e as doenças infecciosas bacterianas estão a aumentar rapidamente. Esta taxa crescente de doenças infecciosas criou a necessidade de prevenção de doenças que podem ser evitadas por vacinação ou imunização. À medida que as doenças aumentam significativamente, há uma necessidade urgente de vacinação em massa. As vacinações em massa requerem muitas doenças, pelo que se espera que proporcionem um crescimento rentável ao mercado das vacinas.
À medida que os casos de doença aumentam, aumenta também o foco na vacinação em massa para prevenir o grande tamanho da população. Muitas pessoas que não foram vacinadas em idades mais jovens são imunizadas em todo o mundo. Assim, para satisfazer estas necessidades, a procura de novas vacinas está a aumentar e, por isso, espera-se que funcione como um motor do mercado de vacinas da Ásia-Pacífico.
RESTRIÇÃO
INDISPONIBILIDADE DE VACINAS REGISTADAS
As aprovações regulamentares rigorosas e os procedimentos demorados de desenvolvimento das vacinas são alguns dos factores responsáveis pela indisponibilidade das vacinas registadas. As autoridades reguladoras encontram dificuldades na avaliação, licenciamento, controlo e vigilância das vacinas. O fornecimento mundial de vacinas está atrasado devido a estas regulamentações.
Assim sendo, os processos de regulação e formação de documentos das empresas fabricantes em diferentes países podem atuar como uma restrição e dificultar o crescimento do mercado das vacinas.
OPORTUNIDADE
INICIATIVAS ESTRATÉGICAS DOS JOGADORES DO MERCADO
Os players do mercado adotam diversas iniciativas estratégicas no mercado das vacinas que envolvem a expansão, a colaboração e a aquisição. Estas iniciativas permitem-lhes aumentar o portefólio de produtos da empresa, levando à expansão do mercado e aumentando a procura de produtos entre os clientes, o que, em última análise, permite aos intervenientes no mercado obter o máximo de receitas.
À medida que a procura por vacinas novas e eficazes aumenta em todo o mundo, estas iniciativas estratégicas tomadas pelos principais intervenientes no mercado visam melhorar as operações comerciais e obter mais rentabilidade no mercado.
Diversas iniciativas estratégicas adoptadas pelos intervenientes no mercado permitiram-lhes expandir a sua raiz nas vacinas e obter mais crescimento do mercado. Assim, os players do mercado que operam nas vacinas estão a adotar diversas iniciativas estratégicas que deverão funcionar como uma oportunidade para o crescimento do mercado das vacinas.
DESAFIO
EFEITOS COLATERAIS CAUSADOS PELAS VACINAS
A vacina é um produto médico que ajuda na prevenção de diversas doenças. Mas, por vezes, surgem efeitos secundários devido ao uso das vacinas. Alguns deles são efeitos secundários ligeiros que são vermelhidão, dor ou inchaço no local da injeção. Mas os efeitos secundários adversos das vacinas são raros, mas colocam a vida em risco.
Os efeitos secundários potencialmente fatais podem gerar medo na população. Além disso, tem impacto na credibilidade dos fabricantes de vacinas, afetando as vendas dos produtos. Isto sugere, portanto, que os efeitos secundários causados pelas vacinas podem dificultar o crescimento do mercado das vacinas.
Desenvolvimentos recentes
- Em outubro de 2022, a Indonésia lançou a sua primeira vacina contra a COVID-19 produzida localmente. A vacina IndoVac foi desenvolvida em conjunto pela empresa farmacêutica estatal indonésia Bio Farma e pelo Baylor College of Medicine, um centro independente de ciências da saúde em Houston, Texas.
- Em novembro de 2020, a Merck Sharp & Dohme Corp., uma subsidiária da Merck & Co., Inc., assinou um acordo para adquirir a OncoImmune, uma empresa biofarmacêutica em fase clínica. A OncoImmune Company está altamente focada no desenvolvimento de opções de tratamento para a COVID-19. Através deste acordo, a empresa espera desenvolver novas vacinas candidatas
- Em Setembro de 2020, a Sanofi e a GSK assinaram um acordo com o governo do Canadá para fornecer 72 milhões de doses de vacinas contra a COVID-19. Existe uma grande procura de vacinas contra a COVID-19, e a procura está a aumentar com o aumento da pandemia. Este acordo permitiu à empresa garantir o potencial futuro
Âmbito do mercado de vacinas da Ásia-Pacífico
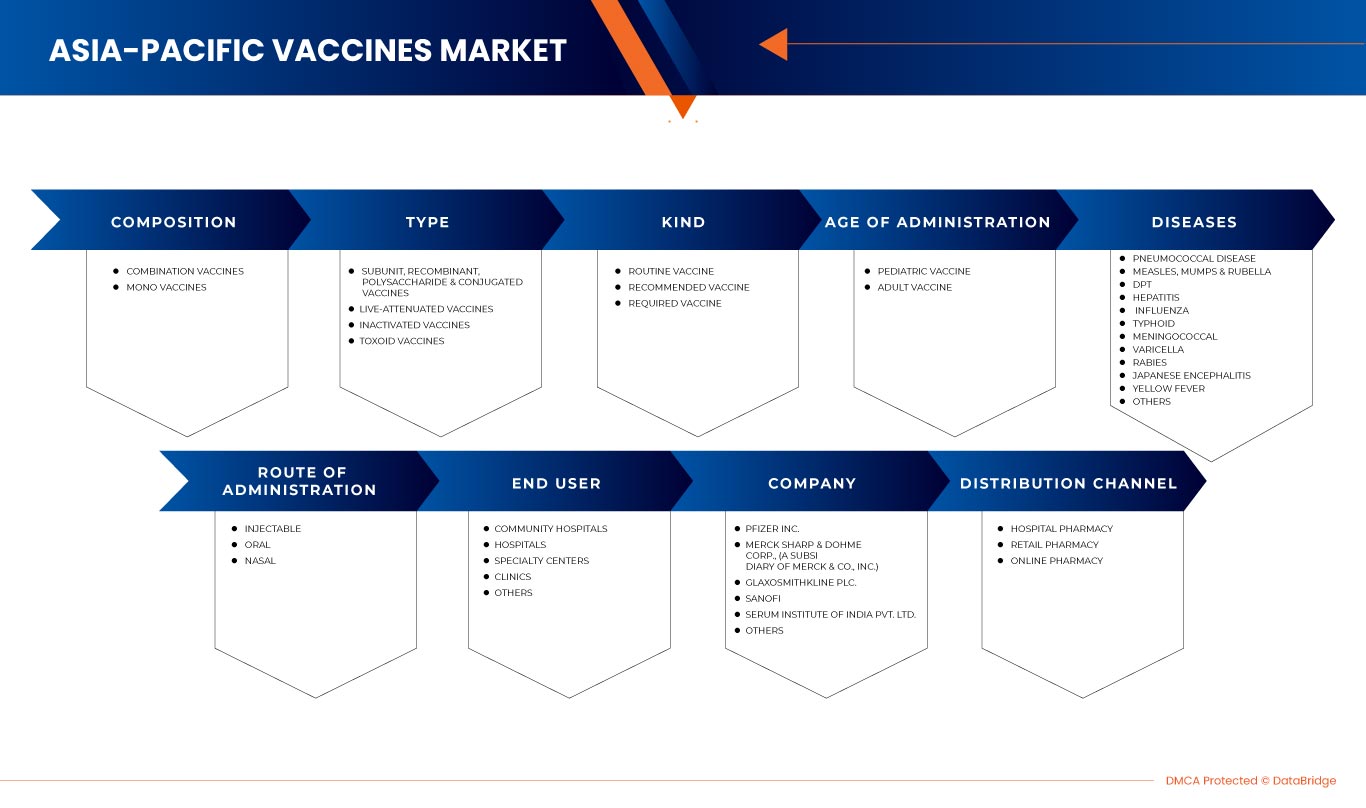
O mercado de vacinas da Ásia-Pacífico está segmentado em composição, tipo, tipo, idade de administração, doenças, via de administração, utilizador final e canal de distribuição. O crescimento entre estes segmentos irá ajudá-lo a analisar os escassos segmentos de crescimento nas indústrias e a fornecer aos utilizadores uma valiosa visão geral do mercado e insights de mercado para tomar decisões estratégicas para identificar as principais aplicações do mercado.
COMPOSIÇÃO
- Vacinas combinadas
- Monovacinas
Com base na composição, o mercado de vacinas da Ásia-Pacífico está segmentado em vacinas combinadas e monovacinas.
TIPO
- Vacinas de subunidades, recombinantes, polissacáridas e conjugadas
- Vacinas vivas atenuadas
- Vacinas inativadas
- Vacinas toxóides
Com base no tipo, o mercado de vacinas Ásia-Pacífico está segmentado em vacinas de subunidades, recombinantes, polissacarídeos e conjugadas, vacinas vivas atenuadas, vacinas inativadas e vacinas toxóides.
AMABILIDADE
- Vacina de rotina
- Vacina recomendada
- Vacina necessária
Com base no tipo, o mercado de vacinas da Ásia-Pacífico está segmentado em vacina de rotina, vacina recomendada e vacina necessária.
IDADE DE ADMINISTRAÇÃO
- Vacina pediátrica
- Vacina para adultos
Com base na idade de administração, o mercado de vacinas da Ásia-Pacífico está segmentado em vacinas pediátricas e vacinas para adultos
DOENÇAS
- Doença pneumocócica
- Sarampo, papeira e varicela
- DPT
- Hepatite
- Gripe
- Tifóide
- Meningocócica
- Varicela
- Raiva
- Encefalite japonesa
- Febre amarela
- Outros
Com base nas doenças, o mercado de vacinas da Ásia-Pacífico está segmentado em doenças pneumocócicas, sarampo, papeira e varicela, DPT, hepatite, gripe, febre tifóide, meningocócica, raiva, encefalite japonesa, febre amarela, entre outras.
VIA DE ADMINISTRAÇÃO
- Injetável
- Nasal
- Oral
Com base na via de administração, o mercado de vacinas da Ásia-Pacífico está segmentado em injetáveis, orais e nasais.
UTILIZADOR FINAL
- Hospitais comunitários
- Hospitais
- Centros especializados
- Clínicas
- Outros
Com base no utilizador final, o mercado de vacinas da Ásia-Pacífico está segmentado em hospitais comunitários, hospitais, centros especializados, clínicas, entre outros.
CANAL DE DISTRIBUIÇÃO
- Farmácia hospitalar
- Farmácia de retalho
- Farmácia on-line
Com base no canal de distribuição, o mercado de vacinas da Ásia-Pacífico está segmentado em farmácia hospitalar, farmácia de retalho e farmácia online.
Análise/perspetivas regionais do mercado de vacinas da Ásia-Pacífico
O mercado de vacinas da Ásia-Pacífico é analisado, e os insights e tendências do tamanho do mercado são fornecidos por país, composição, tipo, tipo, idade de administração, doenças, via de administração, utilizador final e canal de distribuição, conforme mencionado acima.
Os países abrangidos neste relatório de mercado são o Japão, a China, a Coreia do Sul, a Índia, a Austrália, Singapura, a Tailândia, a Malásia, a Indonésia, as Filipinas, o Vietname e o resto da Ásia-Pacífico.
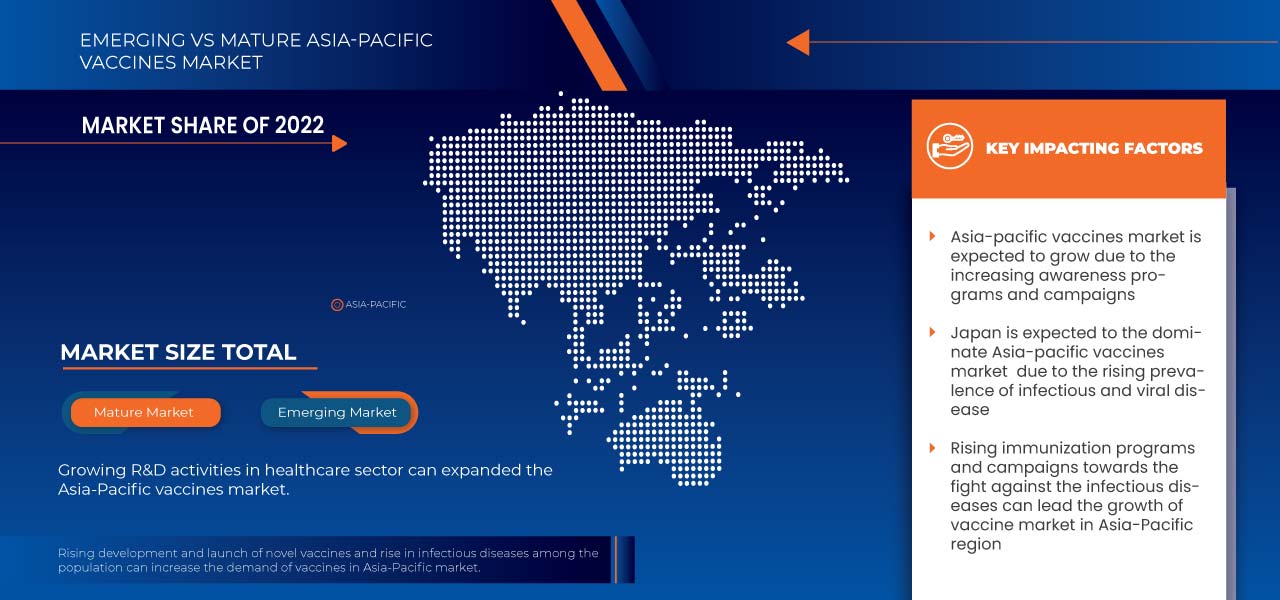
Espera-se que o Japão domine o mercado de vacinas da Ásia-Pacífico em termos de quota de mercado e receitas e continuará a florescer o seu domínio durante o período de previsão. Isto deve-se a uma preferência crescente por exames de saúde preventivos.
A secção do país do relatório também fornece fatores individuais de impacto no mercado e alterações nas regulamentações do mercado que impactam as tendências atuais e futuras do mercado. Pontos de dados, como vendas de novos e de reposição, demografia do país, epidemiologia de doenças e tarifas de importação e exportação, são alguns dos principais indicadores utilizados para prever o cenário de mercado para países individuais. Além disso, são considerados a presença e disponibilidade de marcas globais e os desafios enfrentados devido à concorrência de marcas locais e nacionais e o impacto dos canais de vendas, ao mesmo tempo que se fornece uma análise de previsão dos dados do país.
Análise do panorama competitivo e da quota de mercado de vacinas da Ásia-Pacífico
O panorama competitivo do mercado das vacinas fornece detalhes de um concorrente. Os detalhes incluem a visão geral da empresa, finanças da empresa, receitas geradas, potencial de mercado, investimento em investigação e desenvolvimento, novas iniciativas de mercado, presença global, localizações e instalações de produção, capacidades de produção, pontos fortes e fracos da empresa, lançamento do produto, largura e amplitude do produto e aplicação domínio. Os dados acima estão apenas relacionados com o foco das empresas no mercado das vacinas.
Alguns dos principais players que operam no mercado de vacinas da Ásia-Pacífico são a Bharat Biotech, Biological E Limited, Bio Farma, Serum Institute of India Pvt. Ltd., Takeda Pharmaceutical Company Limited, Merck Sharp & Dohme Corp. (uma subsidiária da Merck & Co., Inc.), Abbott, AstraZeneca, Sanofi, Pfizer Inc., Janssen Global Services, LLC (uma subsidiária da Johnson & Johnson Services , Inc.), F. Hoffmann-La Roche Ltd, Panacea Biotec Ltd e BAXTER VACCINES (uma subsidiária da Baxter), entre outros.
SKU-
Obtenha acesso online ao relatório sobre a primeira nuvem de inteligência de mercado do mundo
- Painel interativo de análise de dados
- Painel de análise da empresa para oportunidades de elevado potencial de crescimento
- Acesso de analista de pesquisa para personalização e customização. consultas
- Análise da concorrência com painel interativo
- Últimas notícias, atualizações e atualizações Análise de tendências
- Aproveite o poder da análise de benchmark para um rastreio abrangente da concorrência
Índice
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF THE ASIA-PACIFIC VACCINES MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 COMPOSITION LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 SECONDARY SOURCES
2.11 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHT
4.1 PESTEL MODEL
4.2 PORTER'S FIVE FORCES
4.3 EPIDEMIOLOGY
4.4 INDUSTRIAL INSIGHTS:
4.5 PIPELINE ANALYSIS
4.6 ASIA-PACIFIC VACCINES MARKET: SUPPLY CHAIN MANAGEMENT OF VACCINES
4.6.1 COLD CHAIN STORAGE:
4.6.2 PROCESS OF LOGISTICS
5 REGULATORY FRAMEWORK
5.1 JAPAN
5.2 CHINA
5.3 SOUTH KOREA
5.4 INDIA
5.5 AUSTRALIA
5.6 SINGAPORE
5.7 THAILAND
5.8 MALAYSIA
5.9 INDONESIA
5.1 VIETNAM
5.11 PHILIPPINES
5.12 REST OF ASIA-PACIFIC
5.12.1 TAIWAN
5.12.2 CAMBODIA
6 MARKET OVERVIEW
6.1 DRIVERS
6.1.1 GROWING IMMUNIZATION PROGRAMS AND CAMPAIGNS
6.1.2 HIGH PREVALENCE OF CHRONIC CONDITIONS SUCH AS FLU, INFECTIOUS AND VIRAL DISEASES
6.1.3 IMPROVEMENT IN TREATMENT
6.1.4 LAUNCH OF NEWER VACCINES
6.1.5 INCREASING GOVERNMENT SUPPORT
6.2 RESTRAINTS
6.2.1 UNAVAILABILITY OF REGISTERED VACCINES
6.2.2 DIFFICULTIES ASSOCIATED WITH THE TRANSPORT AND PRODUCTION OF VACCINES
6.3 OPPORTUNITIES
6.3.1 STRATEGIC INITIATIVES BY THE MARKET PLAYERS
6.3.2 PRESENCE OF PIPELINE PRODUCTS
6.3.3 RISE IN EXPENDITURE IN THE HEALTHCARE SECTOR
6.3.4 INCREASING AWARENESS FOR VACCINATION
6.4 CHALLENGES
6.4.1 SIDE EFFECTS CAUSED BY VACCINES
6.4.2 FEAR AMONG PATIENTS RELATED TO INJECTIONS AND NEEDLE STICKS
6.4.3 PRODUCT RECALL
7 ASIA-PACIFIC VACCINES MARKET, BY COMPOSITION
7.1 OVERVIEW
7.2 COMBINATION VACCINES
7.3 MONO VACCINES
8 ASIA-PACIFIC VACCINES MARKET, BY TYPE
8.1 OVERVIEW
8.2 SUBUNIT, RECOMBINANT, POLYSACCHARIDE, AND CONJUGATE VACCINES
8.2.1 PNEUMOCOCCAL DISEASE
8.2.2 HIB (HAEMOPHILUS INFLUENZA TYPE B) DISEASE
8.2.3 HPV (HUMAN PAPILLOMA VIRUS)
8.2.4 HEPATITIS B
8.2.5 MENINGOCOCCAL
8.2.6 SHINGLES
8.2.7 WHOOPING COUGH
8.2.8 OTHERS
8.3 LIVE-ATTENUTAED VACCINES
8.3.1 ROTAVIRUS
8.3.2 MEASLES
8.3.3 MUMPS
8.3.4 RUBELLA
8.3.5 SMALLPOX
8.3.6 YELLOW FEVER
8.3.7 OTHERS
8.4 INACTIVATED VACCINES
8.4.1 FLU (SHOT ONLY)
8.4.2 POLIO (SHOT ONLY)
8.4.3 HEPATITIS A
8.4.4 RABIES
8.4.5 OTHERS
8.5 TOXOID VACCINES
8.5.1 DIPHTHERIA, TETANUS & PERTUSSIS (DTP)
8.5.2 OTHERS
9 ASIA-PACIFIC VACCINES MARKET, BY KIND
9.1 OVERVIEW
9.2 ROUTINE VACCINES
9.2.1 PNEUMOCOCCAL DISEASES
9.3 DIPTHERIA, TETANUS & PERTUSIS(DPT)
9.3.1 HIB (HAEMOPHILUS INFLUENZA TYPE B) DISEASE
9.3.2 MEASLES
9.3.3 MUMPS
9.3.4 HEPATITIS B
9.3.5 RUBELLA
9.3.6 POLIO
9.3.7 OTHERS
9.4 RECOMMENDED VACCINE
9.4.1 TYPHOID FEVER VACCINE
9.5 HEPATITIS A
9.5.1 RABIES
9.5.2 JAPANESE ENCEPHALITIS
9.5.3 TICK-BORNE ENCEPHALITIS
9.5.4 CHOLERA
9.5.5 OTHERS
9.6 REQUIRED VACCINE
9.6.1 MENINGOCOCCAL
9.7 YELLOW FEVER
9.7.1 OTHERS
10 ASIA-PACIFIC VACCINES MARKET, BY AGE OF ADMINISTRATION
10.1 OVERVIEW
10.2 PEDIATRIC VACCINE
10.2.1 PNEUMOCOCCAL DISEASES
10.3 MEASLES, MUMPS & RUBELLA
10.3.1 DIPTHERIA, TETANUS & PERTUSIS (DPT)
10.3.2 ROTAVIRUS
10.3.3 MENINGOCOCCAL
10.3.4 VARICELLA
10.3.5 POLIO
10.3.6 TUBERCULOSIS
10.3.7 MALARIA
10.3.8 OTHERS
10.4 ADULT VACCINE
10.4.1 INFLUENZA
10.5 HPV (HUMAN PAPILLOMA VIRUS)
10.5.1 TYPHOID
10.5.2 HEPATITIS B
10.5.3 JAPANESE ENCEPHALITIS
10.5.4 YELLOW FEVER
10.5.5 CANCER
10.5.6 OTHERS
11 ASIA-PACIFIC VACCINES MARKET, BY DISEASES
11.1 OVERVIEW
11.2 PNEUMOCCOCAL DISEASE
11.3 MEASLES, MUMPS & RUBELLA
11.4 DPT
11.5 HEPATITIS
11.6 INFLUENZA
11.7 TYPHOID
11.8 MENINGOCOCCAL
11.9 VARICELLA
11.1 RABIES
11.11 JAPANESE ENCEPHALITIS
11.12 YELLOW FEVER
11.13 OTHERS
12 ASIA-PACIFIC VACCINES MARKET, BY ROUTE OF ADMINISTRATION
12.1 OVERVIEW
12.2 INJECTABLE
12.2.1 INTRAMUSCULAR
12.2.2 SUBCUTANEOUS
12.2.3 INTRADERMAL
12.3 ORAL
12.4 NASAL
13 ASIA-PACIFIC VACCINES MARKET, BY END USER
13.1 OVERVIEW
13.2 COMMUNITY HOSPITALS
13.3 HOSPITALS
13.4 SPECIALTY CENTERS
13.5 CLINICS
13.6 OTHERS
14 ASIA-PACIFIC VACCINES MARKET, BY DISTRIBUTION CHANNEL
14.1 OVERVIEW
14.2 HOSPITAL PHARMACY
14.3 RETAIL PHARMACY
14.4 ONLINE PHARMACY
15 ASIA-PACIFIC VACCINE MARKET
15.1 ASIA-PACIFIC
15.1.1 JAPAN
15.1.2 CHINA
15.1.3 AUSTRALIA
15.1.4 INDIA
15.1.5 SOUTH KOREA
15.1.6 SINGAPORE
15.1.7 MALAYSIA
15.1.8 THAILAND
15.1.9 INDONESIA
15.1.10 PHILIPPINES
15.1.11 VIETNAM
15.1.12 REST OF ASIA PACIFIC
16 ASIA-PACIFIC VACCINES MARKET: COMPANY LANDSCAPE
16.1 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
16.2 DISEASE SHARE ANALYSIS: PFIZER, INC.
16.3 COUNTRY SHARE ANALYSIS: PFIZER, INC.
16.4 DISEASE SHARE ANALYSIS: MERCK SHARP & DOHME CORP. (A SUBSIDIARY OF MERCK & CO., INC.)
16.5 COUNTRY SHARE ANALYSIS: MERCK SHARP & DOHME CORP. (A SUBSIDIARY OF MERCK & CO., INC.)
16.6 DISEASE SHARE ANALYSIS: GLAXOSMITHKLINE PLC
16.7 COUNTRY SHARE ANALYSIS: GLAXOSMITHKLINE PLC.
16.8 DISEASE SHARE ANALYSIS: SANOFI
16.9 COUNTRY SHARE ANALYSIS: SANOFI
16.1 DISEASE SHARE ANALYSIS: SERUM INSTITUTE OF INDIA PVT. LTD.
16.11 COUNTRY SHARE ANALYSIS: SERUM INSTITUTE OF INDIA PVT. LTD.
17 SWOT ANALYSIS
18 COMPANY PROFILE
18.1 PFIZER INC.
18.1.1 COMPANY SNAPSHOT
18.1.2 REVENUE ANALYSIS
18.1.3 PRODUCT PORTFOLIO
18.1.4 RECENT DEVELOPMENTS
18.2 MERCK SHARP & DOHME CORP. (A SUBSIDIARY OF MERCK & CO., INC.)
18.2.1 COMPANY SNAPSHOT
18.2.2 REVENUE ANALYSIS
18.2.3 COMPANY WEBSITE AND PRESS RELEASES
18.2.4 PRODUCT PORTFOLIO
18.2.5 RECENT DEVELOPMENTS
18.3 GLAXOSMITHKLINE PLC.
18.3.1 COMPANY SNAPSHOT
18.3.2 REVENUE ANALYSIS
18.3.3 PRODUCT PORTFOLIO
18.3.4 RECENT DEVELOPMENTS
18.4 SANOFI
18.4.1 COMPANY SNAPSHOT
18.4.2 REVENUE ANALYSIS
18.4.3 PRODUCT PORTFOLIO
18.4.4 RECENT DEVELOPMENTS
18.5 SERUM INSTITUTE OF INDIA PVT. LTD.
18.5.1 COMPANY SNAPSHOT
18.5.2 PRODUCT PORTFOLIO
18.5.3 RECENT DEVELOPMENTS
18.6 ABBOTT
18.6.1 COMPANY SNAPSHOT
18.6.2 REVENUE ANALYSIS
18.6.3 PRODUCT PORTFOLIO
18.6.4 RECENT DEVELOPMENTS
18.7 ASTRAZENECA (2022)
18.7.1 COMPANY SNAPSHOT
18.7.2 REVENUE ANALYSIS
18.7.3 PRODUCT PORTFOLIO
18.7.4 RECENT DEVELOPMENTS
18.8 ALK
18.8.1 COMPANY SNAPSHOT
18.8.2 REVENUE ANALYSIS
18.8.3 PRODUCT PORTFOLIO
18.8.4 RECENT DEVELOPMENTS
18.9 BAXTER VACCINES (A SUBSIDIARY OF BAXTER)
18.9.1 COMPANY SNAPSHOT
18.9.2 REVENUE ANALYSIS
18.9.3 PRODUCT PORTFOLIO
18.9.4 RECENT DEVELOPMENT
18.1 BHARAT BIOTECH
18.10.1 COMPANY SNAPSHOT
18.10.2 PRODUCT PORTFOLIO
18.10.3 RECENT DEVELOPMENTS
18.11 BIO FARMA
18.11.1 COMPANY SNAPSHOT
18.11.2 PRODUCT PORTFOLIO
18.11.3 RECENT DEVELOPMENTS
18.12 BIOLOGICAL E LIMITED
18.12.1 COMPANY SNAPSHOT
18.12.2 PRODUCT PORTFOLIO
18.12.3 RECENT DEVELOPMENTS
18.13 DAIICHI SANKYO COMPANY, LIMITED
18.13.1 COMPANY SNAPSHOT
18.13.2 REVENUE ANALYSIS
18.13.3 PRODUCT PORTFOLIO
18.13.4 RECENT DEVELOPMENTS
18.14 F. HOFFMANN-LA ROCHE LTD
18.14.1 COMPANY SNAPSHOT
18.14.2 REVENUE ANALYSIS
18.14.3 PRODUCT PORTFOLIO
18.14.4 RECENT DEVELOPMENT
18.15 JANSSEN GLOBAL SERVICES, LLC (A SUBSIDIARY OF JOHNSON & JOHNSON SERVICES, INC.)
18.15.1 COMPANY SNAPSHOT
18.15.2 REVENUE ANALYSIS
18.15.3 PRODUCT PORTFOLIO
18.15.4 RECENT DEVELOPMENTS
18.16 LANZHOU BIOLOGICAL PRODUCTS RESEARCH INSTITUTE CO., LTD.,
18.16.1 COMPANY SNAPSHOT
18.16.2 PRODUCT PORTFOLIO
18.16.3 RECENT DEVELOPMENTS
18.17 PANACEA BIOTEC LTD
18.17.1 COMPANY SNAPSHOT
18.17.2 REVENUE ANALYSIS
18.17.3 PRODUCT PORTFOLIO
18.17.4 RECENT DEVELOPMENTS
18.18 SEQIRUS (A SUBSIDIARY OF CSL LIMITED)
18.18.1 COMPANY SNAPSHOT
18.18.2 REVENUE ANALYSIS
18.18.3 PRODUCT PORTFOLIO
18.18.4 RECENT DEVELOPMENTS
18.19 TAKEDA PHARMACEUTICAL COMPANY LIMITED
18.19.1 COMPANY SNAPSHOT
18.19.2 REVENUE ANALYSIS
18.19.3 COMPANY WEBSITE AND PRESS RELEASES
18.19.4 PRODUCT PORTFOLIO
18.19.5 RECENT DEVELOPMENTS
19 QUESTIONNAIRE
20 RELATED REPORTS
Lista de Tabela
TABLE 1 ASIA-PACIFIC VACCINES MARKET, PIPELINE ANALYSIS
TABLE 2 RECOMMENDED TEMPERATURE AND STORAGE LENGTH AT VARIOUS LEVELS OF THE COLD CHAIN.
TABLE 3 LOGISTICS PROCESS ACROSS DIFFERENT REGIONS.
TABLE 4 LAWS AND REGULATIONS IN TAIWAN
TABLE 5 VACCINES UNDER CLINICAL TRIAL
TABLE 6 THE SIDE EFFECTS RELATED TO THE VACCINES
TABLE 7 ASIA-PACIFIC VACCINES MARKET, BY COMPOSITION, 2021-2030 (USD MILLION)
TABLE 8 ASIA-PACIFIC VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 9 ASIA-PACIFIC SUBUNIT, RECOMBINANT, POLYSACCHARIDE & CONJUGATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 10 ASIA-PACIFIC LIVE-ATTENUATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 11 ASIA-PACIFIC INACTIVATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 12 ASIA-PACIFIC TOXOID VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 13 ASIA-PACIFIC VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 14 ASIA-PACIFIC ROUTINE VACCINES IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 15 ASIA-PACIFIC RECOMMENDED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 16 ASIA-PACIFIC REQUIRED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 17 ASIA-PACIFIC VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 18 ASIA-PACIFIC PEDIATRIC VACCINE IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 19 ASIA-PACIFIC ADULT VACCINE IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 20 ASIA-PACIFIC VACCINES MARKET, BY DISEASE, 2021-2030 (USD MILLION)
TABLE 21 ASIA-PACIFIC VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2022-2030 (USD MILLION)
TABLE 22 ASIA-PACIFIC INJECTABLE IN VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2022-2030 (USD MILLION)
TABLE 23 ASIA-PACIFIC VACCINES MARKET, BY END USER, 2022-2030 (USD MILLION)
TABLE 24 ASIA-PACIFIC VACCINES MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 25 ASIA-PACIFIC KNEE CARTILAGE REPAIR MARKET, BY COUNTRY, 2021-2030 (USD MILLION)
TABLE 26 JAPAN VACCINES MARKET, BY COMPOSITION, 2021-2030 (USD MILLION)
TABLE 27 JAPAN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 28 JAPAN SUBUNIT, RECOMBINANT, POLYSACCHARIDE & CONJUGATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 29 JAPAN LIVE-ATTENUATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 30 JAPAN INACTIVATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 31 JAPAN TOXOID VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 32 JAPAN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 33 JAPAN ROUTINE VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 34 JAPAN RECOMMENDED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 35 JAPAN REQUIRED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 36 JAPAN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 37 JAPAN PEDIATRIC VACCINE IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 38 JAPAN ADULT IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 39 JAPAN VACCINES MARKET, BY DISEASE, 2021-2030 (USD MILLION)
TABLE 40 JAPAN VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 41 JAPAN INJECTABLE IN VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 42 JAPAN VACCINES MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 43 JAPAN VACCINES MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 44 CHINA VACCINES MARKET, BY COMPOSITION, 2021-2030 (USD MILLION)
TABLE 45 CHINA VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 46 CHINA SUBUNIT, RECOMBINANT, POLYSACCHARIDE & CONJUGATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 47 CHINA LIVE-ATTENUATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 48 CHINA INACTIVATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 49 CHINA TOXOID VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 50 CHINA VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 51 CHINA ROUTINE VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 52 CHINA RECOMMENDED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 53 CHINA REQUIRED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 54 CHINA VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 55 CHINA PEDIATRIC VACCINE IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 56 CHINA ADULT IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 57 CHINA VACCINES MARKET, BY DISEASE, 2021-2030 (USD MILLION)
TABLE 58 CHINA VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 59 CHINA INJECTABLE IN VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 60 CHINA VACCINES MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 61 CHINA VACCINES MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 62 AUSTRALIA VACCINES MARKET, BY COMPOSITION, 2021-2030 (USD MILLION)
TABLE 63 AUSTRALIA VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 64 AUSTRALIA SUBUNIT, RECOMBINANT, POLYSACCHARIDE & CONJUGATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 65 AUSTRALIA LIVE-ATTENUATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 66 AUSTRALIA INACTIVATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 67 AUSTRALIA TOXOID VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 68 AUSTRALIA VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 69 AUSTRALIA ROUTINE VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 70 AUSTRALIA RECOMMENDED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 71 AUSTRALIA REQUIRED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 72 AUSTRALIA VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 73 AUSTRALIA PEDIATRIC VACCINE IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 74 AUSTRALIA ADULT IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 75 AUSTRALIA VACCINES MARKET, BY DISEASE, 2021-2030 (USD MILLION)
TABLE 76 AUSTRALIA VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 77 AUSTRALIA INJECTABLE IN VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 78 AUSTRALIA VACCINES MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 79 INDIA VACCINES MARKET, BY COMPOSITION, 2021-2030 (USD MILLION)
TABLE 80 INDIA VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 81 INDIA SUBUNIT, RECOMBINANT, POLYSACCHARIDE & CONJUGATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 82 INDIA LIVE-ATTENUATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 83 INDIA INACTIVATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 84 INDIA TOXOID VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 85 INDIA VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 86 INDIA ROUTINE VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 87 INDIA RECOMMENDED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 88 INDIA REQUIRED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 89 INDIA VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 90 INDIA PEDIATRIC VACCINE IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 91 INDIA ADULT IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 92 INDIA VACCINES MARKET, BY DISEASE, 2021-2030 (USD MILLION)
TABLE 93 INDIA VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 94 INDIA INJECTABLE IN VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 95 INDIA VACCINES MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 96 INDIA VACCINES MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 97 SOUTH KOREA VACCINES MARKET, BY COMPOSITION, 2021-2030 (USD MILLION)
TABLE 98 SOUTH KOREA VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 99 SOUTH KOREA SUBUNIT, RECOMBINANT, POLYSACCHARIDE & CONJUGATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 100 SOUTH KOREA LIVE-ATTENUATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 101 SOUTH KOREA INACTIVATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 102 SOUTH KOREA VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 103 SOUTH KOREA ROUTINE VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 104 SOUTH KOREA RECOMMENDED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 105 SOUTH KOREA REQUIRED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 106 SOUTH KOREA VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 107 SOUTH KOREA PEDIATRIC VACCINE IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 108 SOUTH KOREA ADULT IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 109 SOUTH KOREA VACCINES MARKET, BY DISEASE, 2021-2030 (USD MILLION)
TABLE 110 SOUTH KOREA VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 111 SOUTH KOREA INJECTABLE IN VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 112 SOUTH KOREA VACCINES MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 113 SOUTH KOREA VACCINES MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 114 SINGAPORE VACCINES MARKET, BY COMPOSITION, 2021-2030 (USD MILLION)
TABLE 115 SINGAPORE SUBUNIT, RECOMBINANT, POLYSACCHARIDE & CONJUGATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 116 SINGAPORE LIVE-ATTENUATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 117 SINGAPORE INACTIVATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 118 SINGAPORE TOXOID VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 119 SINGAPORE VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 120 SINGAPORE ROUTINE VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 121 SINGAPORE RECOMMENDED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 122 SINGAPORE REQUIRED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 123 SINGAPORE VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 124 SINGAPORE PEDIATRIC VACCINE IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 125 SINGAPORE ADULT IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 126 SINGAPORE VACCINES MARKET, BY DISEASE, 2021-2030 (USD MILLION)
TABLE 127 SINGAPORE VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 128 SINGAPORE INJECTABLE IN VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 129 SINGAPORE VACCINES MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 130 SINGAPORE VACCINES MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 131 MALAYSIA VACCINES MARKET, BY COMPOSITION, 2021-2030 (USD MILLION)
TABLE 132 MALAYSIA VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 133 MALAYSIA SUBUNIT, RECOMBINANT, POLYSACCHARIDE & CONJUGATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 134 MALAYSIA LIVE-ATTENUATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 135 MALAYSIA INACTIVATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 136 MALAYSIA TOXOID VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 137 MALAYSIA VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 138 MALAYSIA ROUTINE VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 139 MALAYSIA RECOMMENDED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 140 MALAYSIA REQUIRED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 141 MALAYSIA VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 142 MALAYSIA PEDIATRIC VACCINE IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 143 MALAYSIA ADULT IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 144 MALAYSIA VACCINES MARKET, BY DISEASE, 2021-2030 (USD MILLION)
TABLE 145 MALAYSIA VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 146 MALAYSIA INJECTABLE IN VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 147 MALAYSIA VACCINES MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 148 MALAYSIA VACCINES MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 149 THAILAND VACCINES MARKET, BY COMPOSITION, 2021-2030 (USD MILLION)
TABLE 150 THAILAND VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 151 THAILAND SUBUNIT, RECOMBINANT, POLYSACCHARIDE & CONJUGATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 152 THAILAND LIVE-ATTENUATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 153 THAILAND INACTIVATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 154 THAILAND TOXOID VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 155 THAILAND VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 156 THAILAND RECOMMENDED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 157 THAILAND REQUIRED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 158 THAILAND VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 159 THAILAND PEDIATRIC VACCINE IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 160 THAILAND ADULT IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 161 THAILAND VACCINES MARKET, BY DISEASE, 2021-2030 (USD MILLION)
TABLE 162 THAILAND VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 163 THAILAND INJECTABLE IN VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 164 THAILAND VACCINES MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 165 THAILAND VACCINES MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 166 INDONESIA VACCINES MARKET, BY COMPOSITION, 2021-2030 (USD MILLION)
TABLE 167 INDONESIA VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 168 INDONESIA SUBUNIT, RECOMBINANT, POLYSACCHARIDE & CONJUGATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 169 INDONESIA LIVE-ATTENUATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 170 INDONESIA INACTIVATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 171 INDONESIA TOXOID VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 172 INDONESIA VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 173 INDONESIA ROUTINE VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 174 INDONESIA RECOMMENDED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 175 INDONESIA REQUIRED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 176 INDONESIA VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 177 INDONESIA PEDIATRIC VACCINE IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 178 INDONESIA ADULT IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 179 INDONESIA VACCINES MARKET, BY DISEASE, 2021-2030 (USD MILLION)
TABLE 180 INDONESIA VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 181 INDONESIA INJECTABLE IN VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 182 INDONESIA VACCINES MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 183 INDONESIA VACCINES MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 184 PHILIPPINES VACCINES MARKET, BY COMPOSITION, 2021-2030 (USD MILLION)
TABLE 185 PHILIPPINES VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 186 PHILIPPINES SUBUNIT, RECOMBINANT, POLYSACCHARIDE & CONJUGATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 187 PHILIPPINES LIVE-ATTENUATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 188 PHILIPPINES INACTIVATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 189 PHILIPPINES TOXOID VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 190 PHILIPPINES VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 191 PHILIPPINES ROUTINE VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 192 PHILIPPINES RECOMMENDED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 193 PHILIPPINES REQUIRED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 194 PHILIPPINES VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 195 PHILIPPINES PEDIATRIC VACCINE IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 196 PHILIPPINES ADULT IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 197 PHILIPPINES VACCINES MARKET, BY DISEASE, 2021-2030 (USD MILLION)
TABLE 198 PHILIPPINES VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 199 PHILIPPINES INJECTABLE IN VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 200 PHILIPPINES VACCINES MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 201 PHILIPPINES VACCINES MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 202 VIETNAM VACCINES MARKET, BY COMPOSITION, 2021-2030 (USD MILLION)
TABLE 203 VIETNAM VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 204 VIETNAM SUBUNIT, RECOMBINANT, POLYSACCHARIDE & CONJUGATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 205 VIETNAM LIVE-ATTENUATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 206 VIETNAM INACTIVATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 207 VIETNAM TOXOID VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 208 VIETNAM VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 209 VIETNAM ROUTINE VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 210 VIETNAM RECOMMENDED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 211 VIETNAM REQUIRED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 212 VIETNAM VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 213 VIETNAM PEDIATRIC VACCINE IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 214 VIETNAM ADULT IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 215 VIETNAM VACCINES MARKET, BY DISEASE, 2021-2030 (USD MILLION)
TABLE 216 VIETNAM VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 217 VIETNAM INJECTABLE IN VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 218 VIETNAM VACCINES MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 219 VIETNAM VACCINES MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 220 REST OF ASIA-PACIFIC VACCINES MARKET, BY COMPOSITION, 2021-2030 (USD MILLION)
Lista de Figura
FIGURE 1 ASIA-PACIFIC VACCINES MARKET: SEGMENTATION
FIGURE 2 ASIA-PACIFIC VACCINES MARKET: DATA TRIANGULATION
FIGURE 3 ASIA-PACIFIC VACCINES MARKET: DROC ANALYSIS
FIGURE 4 ASIA-PACIFIC VACCINES MARKET: ASIA-PACIFIC VS COUNTRY MARKET ANALYSIS
FIGURE 5 ASIA-PACIFIC VACCINES MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 ASIA-PACIFIC VACCINES MARKET: MULTIVARIATE MODELLING
FIGURE 7 ASIA-PACIFIC VACCINES MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 8 ASIA-PACIFIC VACCINES MARKET: DBMR MARKET POSITION GRID
FIGURE 9 ASIA-PACIFIC VACCINES MARKET: SEGMENTATION
FIGURE 10 GROWING IMMUNIZATION PROGRAMS AND CAMPAIGNS AND THE HIGH PREVALENCE OF CHRONIC CONDITIONS SUCH AS FLU AND BACTERIAL INFECTIOUS DISEASES ARE DRIVING THE ASIA-PACIFIC VACCINES MARKET IN THE FORECAST PERIOD OF 2023 TO 2030
FIGURE 11 THE COMBINATION VACCINES SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE ASIA-PACIFIC VACCINES MARKET IN 2023 & 2030
FIGURE 12 FDA REGULATORY REVIEW PROCESS OF VACCINES
FIGURE 13 PROCESS OF SPECIAL APPROVAL ON VACCINES DURING THE 2019 H1N1PDM PANDEMIC
FIGURE 14 REGULATION OVERVIEW FOR THERAPEUTICS IN SINGAPORE
FIGURE 15 DRIVERS, RESTRAINTS, OPPORTUNITIES AND CHALLENGES OF THE ASIA-PACIFIC VACCINES MARKET
FIGURE 16 ASIA-PACIFIC VACCINES MARKET: BY COMPOSITION, 2022
FIGURE 17 ASIA-PACIFIC VACCINES MARKET: BY COMPOSITION, 2023-2030 (USD MILLION)
FIGURE 18 ASIA-PACIFIC VACCINES MARKET: BY COMPOSITION, CAGR (2023-2030)
FIGURE 19 ASIA-PACIFIC VACCINES MARKET: BY COMPOSITION, LIFELINE CURVE
FIGURE 20 ASIA-PACIFIC VACCINES MARKET: BY TYPE, 2022
FIGURE 21 ASIA-PACIFIC VACCINES MARKET: BY TYPE, 2023-2030 (USD MILLION)
FIGURE 22 ASIA-PACIFIC VACCINES MARKET: BY TYPE, CAGR (2023-2030)
FIGURE 23 ASIA-PACIFIC VACCINES MARKET: BY TYPE, LIFELINE CURVE
FIGURE 24 ASIA-PACIFIC VACCINES MARKET: BY KIND, 2022
FIGURE 25 ASIA-PACIFIC VACCINES MARKET: BY KIND, 2023-2030 (USD MILLION)
FIGURE 26 ASIA-PACIFIC VACCINES MARKET: BY KIND, CAGR (2023-2030)
FIGURE 27 ASIA-PACIFIC VACCINES MARKET: BY KIND, LIFELINE CURVE
FIGURE 28 ASIA-PACIFIC VACCINES MARKET: BY AGE OF ADMINISTRATION, 2022
FIGURE 29 ASIA-PACIFIC VACCINES MARKET: BY AGE OF ADMINISTRATION, 2023-2030 (USD MILLION)
FIGURE 30 ASIA-PACIFIC VACCINES MARKET: BY AGE OF ADMINISTRATION, CAGR (2023-2030)
FIGURE 31 ASIA-PACIFIC VACCINES MARKET: BY AGE OF ADMINISTRATION, LIFELINE CURVE
FIGURE 32 ASIA-PACIFIC VACCINES MARKET: BY DISEASES, 2022
FIGURE 33 ASIA-PACIFIC VACCINES MARKET: BY DISEASES, 2023-2030 (USD MILLION)
FIGURE 34 ASIA-PACIFIC VACCINES MARKET: BY DISEASES, CAGR (2023-2030)
FIGURE 35 ASIA-PACIFIC VACCINES MARKET: BY DISEASES, LIFELINE CURVE
FIGURE 36 ASIA-PACIFIC VACCINES MARKET: BY ROUTE OF ADMINISTRATION, 2022
FIGURE 37 ASIA-PACIFIC VACCINES MARKET: BY ROUTE OF ADMINISTRATION, 2023-2030 (USD MILLION)
FIGURE 38 ASIA-PACIFIC VACCINES MARKET: BY ROUTE OF ADMINISTRATION, CAGR (2023-2030)
FIGURE 39 ASIA-PACIFIC VACCINES MARKET: BY ROUTE OF ADMINISTRATION, LIFELINE CURVE
FIGURE 40 ASIA-PACIFIC VACCINES MARKET: BY END USER, 2022
FIGURE 41 ASIA-PACIFIC VACCINES MARKET: BY END USER, 2023-2030 (USD MILLION)
FIGURE 42 ASIA-PACIFIC VACCINES MARKET: BY END USER, CAGR (2023-2030)
FIGURE 43 ASIA-PACIFIC VACCINES MARKET: BY END USER, LIFELINE CURVE
FIGURE 44 ASIA-PACIFIC VACCINES MARKET: BY DISTRIBUTION CHANNEL, 2022
FIGURE 45 ASIA-PACIFIC VACCINES MARKET: BY DISTRIBUTION CHANNEL, 2023-2030 (USD MILLION)
FIGURE 46 ASIA-PACIFIC VACCINES MARKET: BY DISTRIBUTION CHANNEL, CAGR (2023-2030)
FIGURE 47 ASIA-PACIFIC VACCINES MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 48 ASIA-PACIFIC VACCINE MARKET: SNAPSHOT (2022)
FIGURE 49 ASIA-PACIFIC VACCINE MARKET: BY COUNTRY (2022)
FIGURE 50 ASIA-PACIFIC VACCINE MARKET: BY COUNTRY (2023 & 2030)
FIGURE 51 ASIA-PACIFIC VACCINE MARKET: BY COUNTRY (2022 & 2030)
FIGURE 52 ASIA-PACIFIC VACCINE MARKET: BY COMPOSITION (2023-2030)
FIGURE 53 ASIA-PACIFIC VACCINES MARKET: COMPANY SHARE 2022 (%)
FIGURE 54 PFIZER, INC., ASIA-PACIFIC VACCINES MARKET: DISEASE SHARE 2022 (%)
FIGURE 55 PFIZER, INC., ASIA-PACIFIC VACCINES MARKET: COUNTRY SHARE 2022 (%)
FIGURE 56 MERCK SHARP & DOHME CORP. (A SUBSIDIARY OF MERCK & CO., INC.) ASIA-PACIFIC VACCINES MARKET: DISEASE SHARE 2022 (%)
FIGURE 57 MERCK SHARP & DOHME CORP. (A SUBSIDIARY OF MERCK & CO., INC.) ASIA-PACIFIC VACCINES MARKET: COUNTRY SHARE 2022 (%)
FIGURE 58 GLAXOSMITHKLINE PLC, ASIA-PACIFIC VACCINES MARKET: DISEASE SHARE 2022 (%)
FIGURE 59 GLAXOSMITHKLINE PLC, ASIA-PACIFIC VACCINES MARKET: COMPANY SHARE 2022 (%)
FIGURE 60 SANOFI, ASIA-PACIFIC VACCINES MARKET: DISEASE SHARE 2022 (%)
FIGURE 61 SANOFI ASIA-PACIFIC VACCINES MARKET: COUNTRY SHARE 2022 (%)
FIGURE 62 SERUM INSTITUTE OF INDIA PVT. LTD., ASIA-PACIFIC VACCINES MARKET: DISEASE SHARE 2022 (%)
FIGURE 63 SERUM INSTITUTE OF INDIA PVT. LTD. ASIA-PACIFIC VACCINES MARKET: COUNTRY SHARE 2022 (%)

Metodologia de Investigação
A recolha de dados e a análise do ano base são feitas através de módulos de recolha de dados com amostras grandes. A etapa inclui a obtenção de informações de mercado ou dados relacionados através de diversas fontes e estratégias. Inclui examinar e planear antecipadamente todos os dados adquiridos no passado. Da mesma forma, envolve o exame de inconsistências de informação observadas em diferentes fontes de informação. Os dados de mercado são analisados e estimados utilizando modelos estatísticos e coerentes de mercado. Além disso, a análise da quota de mercado e a análise das principais tendências são os principais fatores de sucesso no relatório de mercado. Para saber mais, solicite uma chamada de analista ou abra a sua consulta.
A principal metodologia de investigação utilizada pela equipa de investigação do DBMR é a triangulação de dados que envolve a mineração de dados, a análise do impacto das variáveis de dados no mercado e a validação primária (especialista do setor). Os modelos de dados incluem grelha de posicionamento de fornecedores, análise da linha de tempo do mercado, visão geral e guia de mercado, grelha de posicionamento da empresa, análise de patentes, análise de preços, análise da quota de mercado da empresa, normas de medição, análise global versus regional e de participação dos fornecedores. Para saber mais sobre a metodologia de investigação, faça uma consulta para falar com os nossos especialistas do setor.
Personalização disponível
A Data Bridge Market Research é líder em investigação formativa avançada. Orgulhamo-nos de servir os nossos clientes novos e existentes com dados e análises que correspondem e atendem aos seus objetivos. O relatório pode ser personalizado para incluir análise de tendências de preços de marcas-alvo, compreensão do mercado para países adicionais (solicite a lista de países), dados de resultados de ensaios clínicos, revisão de literatura, mercado remodelado e análise de base de produtos . A análise de mercado dos concorrentes-alvo pode ser analisada desde análises baseadas em tecnologia até estratégias de carteira de mercado. Podemos adicionar quantos concorrentes necessitar de dados no formato e estilo de dados que procura. A nossa equipa de analistas também pode fornecer dados em tabelas dinâmicas de ficheiros Excel em bruto (livro de factos) ou pode ajudá-lo a criar apresentações a partir dos conjuntos de dados disponíveis no relatório.