Asia Pacific Preclinical Imaging Market
Tamanho do mercado em biliões de dólares
CAGR :
% 
 USD
294.06 Million
USD
451.29 Million
2024
2032
USD
294.06 Million
USD
451.29 Million
2024
2032
| 2025 –2032 | |
| USD 294.06 Million | |
| USD 451.29 Million | |
|
|
|
|
Segmentação do mercado de imagens pré-clínicas da Ásia-Pacífico, por produto (sistemas e serviços), reagentes (reagentes de imagem óptica pré-clínica, reagentes de imagem nuclear pré-clínica, agentes de contraste para ressonância magnética pré-clínica, agentes de contraste para ultrassom pré-clínico e agentes de contraste para tomografia computadorizada pré-clínica), aplicação (pesquisa e desenvolvimento, descoberta de medicamentos, biodistribuição, detecção de células cancerígenas, biomarcadores e outros), usuário final (organização de pesquisa contratada, empresas farmacêuticas e de biotecnologia, institutos de pesquisa acadêmicos e governamentais, centros de diagnóstico e outros) - Tendências e previsões do setor até 2032
Tamanho do mercado de imagens pré-clínicas da Ásia-Pacífico
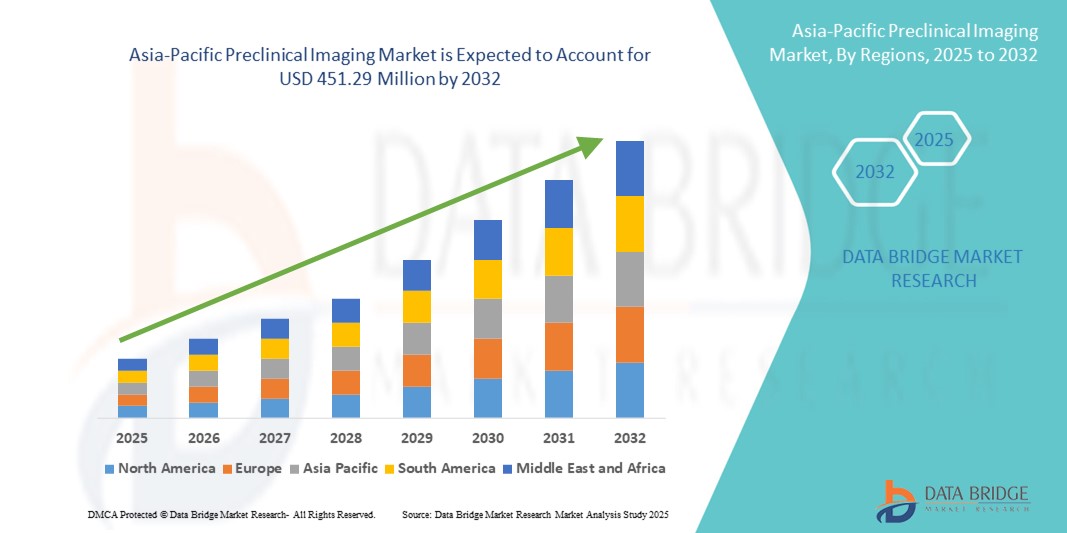
- O tamanho do mercado de imagens pré-clínicas da Ásia-Pacífico foi avaliado em US$ 294,06 milhões em 2024 e deve atingir US$ 451,29 milhões até 2032 , com um CAGR de 5,50% durante o período previsto.
- O crescimento do mercado é impulsionado em grande parte pela crescente adoção de modalidades avançadas de imagem e pelo crescente investimento em pesquisa pré-clínica e descoberta de medicamentos em toda a região, especialmente em países como China, Japão e Índia.
- Além disso, o foco crescente em pesquisa translacional, diagnóstico precoce de doenças e técnicas de imagem não invasivas está incentivando o uso de sistemas de imagem pré-clínica em pesquisas farmacêuticas e biotecnológicas . Esses fatores convergentes estão acelerando a adoção de soluções inovadoras de imagem, impulsionando significativamente o crescimento do setor.
Análise do Mercado de Imagem Pré-clínica da Ásia-Pacífico
- A imagem pré-clínica, abrangendo modalidades avançadas como ressonância magnética, tomografia computadorizada, PET , SPECT e imagem óptica, está se tornando um componente essencial da descoberta de medicamentos, pesquisa translacional e diagnóstico precoce de doenças em ambientes de pesquisa acadêmica, farmacêutica e biotecnológica devido às suas capacidades não invasivas, imagem de alta resolução e capacidade de acelerar estudos pré-clínicos.
- A crescente demanda por imagens pré-clínicas é alimentada principalmente pelo aumento dos investimentos em P&D farmacêutico, pelo foco crescente na medicina de precisão e pela crescente adoção de tecnologias avançadas de imagem para reduzir os prazos de desenvolvimento de medicamentos e aprimorar os resultados da pesquisa translacional.
- A China dominou o mercado de imagens pré-clínicas da Ásia-Pacífico com a maior participação na receita de 39% em 2024, caracterizada por financiamento governamental substancial para pesquisa biomédica, rápida expansão da fabricação farmacêutica e adoção de sistemas avançados de imagens em oncologia, neurologia e estudos de biomarcadores .
- Espera-se que a Índia seja o país com crescimento mais rápido no mercado de imagens pré-clínicas da Ásia-Pacífico durante o período previsto, impulsionado pelo aumento dos volumes de ensaios clínicos, pelo aumento das atividades de pesquisa farmacêutica e biotecnológica e pela crescente adoção de soluções de imagens com boa relação custo-benefício.
- O segmento de reagentes de imagem óptica pré-clínica dominou o mercado de imagem pré-clínica da Ásia-Pacífico com uma participação de mercado de 38,7% em 2024, devido à sua ampla aplicação em imagem molecular, pesquisa de câncer e modelagem de doenças in vivo
Escopo do relatório e segmentação do mercado de imagens pré-clínicas da Ásia-Pacífico
|
Atributos |
Principais insights do mercado de imagens pré-clínicas da Ásia-Pacífico |
|
Segmentos abrangidos |
|
|
Países abrangidos |
Ásia-Pacífico
|
|
Principais participantes do mercado |
|
|
Oportunidades de mercado |
|
|
Conjuntos de informações de dados de valor agregado |
Além dos insights sobre cenários de mercado, como valor de mercado, taxa de crescimento, segmentação, cobertura geográfica e principais participantes, os relatórios de mercado selecionados pela Data Bridge Market Research também incluem análises aprofundadas de especialistas, análises de preços, análises de participação de marca, pesquisas com consumidores, análises demográficas, análises da cadeia de suprimentos, análises da cadeia de valor, visão geral de matérias-primas/consumíveis, critérios de seleção de fornecedores, análise PESTLE, análise de Porter e estrutura regulatória. |
Tendências do mercado de imagens pré-clínicas da Ásia-Pacífico
Avanços em Imagem Multimodal e Integração de IA
- Uma tendência significativa e crescente no mercado de imagens pré-clínicas da Ásia-Pacífico é a integração de sistemas de imagens multimodais com inteligência artificial (IA) e plataformas avançadas de análise de dados. Essa combinação está aprimorando a resolução das imagens, acelerando a análise e permitindo estudos pré-clínicos mais precisos e preditivos.
- Por exemplo, a combinação de sistemas PET/MRI com reconstrução de imagens orientada por IA na China permite que pesquisadores conduzam estudos longitudinais com maior precisão e tempos de varredura reduzidos. Da mesma forma, o Japão testemunhou a adoção de plataformas de imagem óptica alimentadas por IA para pesquisas de alto rendimento sobre câncer, aprimorando a detecção precoce e a avaliação terapêutica.
- A integração com IA permite segmentação automatizada de imagens, reconhecimento de padrões e modelagem preditiva, o que auxilia na melhor compreensão da progressão da doença e da eficácia dos medicamentos. Por exemplo, a análise de ressonância magnética assistida por IA na Índia melhora a identificação de biomarcadores e reduz erros de interpretação manual, enquanto a imagem multimodal fornece insights abrangentes ao combinar dados anatômicos e funcionais.
- A integração perfeita de sistemas de imagem com plataformas centralizadas de gerenciamento de informações de laboratório permite que os pesquisadores gerenciem dados de vários estudos pré-clínicos, facilitando fluxos de trabalho de alto rendimento e resultados reproduzíveis.
- Essa tendência em direção a soluções de imagem mais inteligentes, precisas e interconectadas está remodelando fundamentalmente os padrões da pesquisa pré-clínica. Consequentemente, empresas como a MILabs e a Bruker estão desenvolvendo sistemas de imagem pré-clínica habilitados por IA com funcionalidade multimodal e recursos aprimorados de análise de imagem.
- A demanda por sistemas de imagem pré-clínica com IA e integração multimodal está crescendo rapidamente nos setores farmacêutico, biotecnológico e de pesquisa acadêmica, à medida que essas tecnologias aceleram a descoberta de medicamentos e melhoram os resultados da pesquisa translacional.
Dinâmica do mercado de imagens pré-clínicas da Ásia-Pacífico
Motorista
Aumento do investimento em P&D farmacêutico e pesquisa translacional
- O crescente investimento em P&D farmacêutico e biotecnológico na região da Ásia-Pacífico, particularmente na China, Japão e Índia, é um importante impulsionador da adoção de imagens pré-clínicas. Sistemas avançados de imagem apoiam o desenvolvimento de medicamentos em estágio inicial, a validação de biomarcadores e a modelagem de doenças.
- Por exemplo, em março de 2024, um importante consórcio de pesquisa chinês expandiu sua infraestrutura de imagem pré-clínica para dar suporte a ensaios de medicamentos oncológicos, integrando sistemas de PET/CT e MRI com ferramentas de análise baseadas em IA.
- À medida que as empresas farmacêuticas buscam reduzir os prazos e os custos de desenvolvimento de medicamentos, a imagem pré-clínica oferece insights não invasivos e de alta resolução sobre a progressão da doença e a eficácia terapêutica
- O foco crescente na medicina de precisão, na detecção precoce de doenças e na pesquisa translacional está aumentando a demanda por sistemas de imagem integrados e de alto desempenho em ambientes de pesquisa acadêmica e comercial.
- Iniciativas e subsídios governamentais em países como Japão e Coreia do Sul, destinados a apoiar a pesquisa biomédica, estão fornecendo financiamento adicional para infraestrutura de imagem, acelerando ainda mais o crescimento do mercado
- As colaborações entre fabricantes de equipamentos de imagem e institutos de pesquisa para o desenvolvimento conjunto de soluções de imagem especializadas estão abrindo novas oportunidades de mercado
Restrição/Desafio
Alto custo de equipamento e necessidade de mão de obra qualificada
- O alto custo de sistemas avançados de imagem pré-clínica e reagentes continua sendo uma barreira significativa à sua ampla adoção, especialmente para institutos de pesquisa menores e empresas de biotecnologia emergentes. Sistemas como PET/RM ou imagens ópticas multimodais exigem investimentos de capital substanciais.
- Além disso, a operação de sistemas complexos de imagem exige pessoal altamente treinado para aquisição, análise e manutenção de imagens. A escassez de especialistas qualificados em imagem em mercados emergentes como a Índia e o Sudeste Asiático pode retardar a penetração no mercado.
- Por exemplo, um relatório de 2024 destacou que vários laboratórios de biotecnologia de médio porte na Índia atrasaram a adoção do PET/CT devido a restrições orçamentárias e à falta de operadores treinados.
- Embora algumas empresas ofereçam soluções de imagem menores e econômicas, os sistemas multimodais premium com integração de IA continuam a ter preços elevados, limitando a acessibilidade
- A conformidade regulatória e as aprovações para equipamentos de imagem pré-clínica, especialmente na China e no Japão, podem atrasar a implantação e aumentar os custos operacionais
- Os desafios de gerenciamento e integração de dados, incluindo o manuseio de grandes volumes de dados de imagem de forma segura e eficiente, continuam sendo uma barreira para instituições menores que não possuem infraestrutura de TI
- Superar esses desafios por meio de programas de treinamento, iniciativas de pesquisa colaborativa e desenvolvimento de plataformas de imagem acessíveis será crucial para o crescimento sustentado do mercado no setor de imagem pré-clínica da Ásia-Pacífico.
Escopo do mercado de imagens pré-clínicas da Ásia-Pacífico
O mercado é segmentado com base no produto, reagentes, aplicação e usuário final.
- Por produto
Com base no produto, o mercado de imagens pré-clínicas da Ásia-Pacífico é segmentado em sistemas e serviços. O segmento de sistemas dominou o mercado, com a maior participação na receita, de 65,4% em 2024, impulsionado pela crescente adoção de modalidades avançadas de imagem, como ressonância magnética, tomografia computadorizada, PET, SPECT e imagens ópticas em pesquisas pré-clínicas. Sistemas de imagem de alta resolução são cada vez mais preferidos por empresas farmacêuticas e instituições acadêmicas para estudos anatômicos e funcionais detalhados. A confiabilidade, a reprodutibilidade e a utilidade a longo prazo dos sistemas os tornam indispensáveis para a descoberta de medicamentos e a pesquisa translacional. Os institutos de pesquisa priorizam os sistemas por sua capacidade de apoiar estudos de imagem multimodal e experimentos longitudinais. Além disso, o foco crescente em medicina de precisão e identificação de biomarcadores está acelerando ainda mais a adoção de sistemas de imagem na região.
Espera-se que o segmento de serviços testemunhe o crescimento mais rápido entre 2025 e 2032, impulsionado pela crescente terceirização de estudos de imagem para prestadores de serviços pré-clínicos especializados. Os serviços permitem que laboratórios menores e CROs acessem tecnologias avançadas de imagem sem o ônus de altos investimentos de capital. Os prestadores de serviços de imagem pré-clínica oferecem soluções abrangentes, incluindo geração de imagens, análise de dados e geração de relatórios, que auxiliam na rápida tomada de decisões no desenvolvimento de medicamentos. O segmento se beneficia da crescente demanda por estudos pré-clínicos baseados em contrato em oncologia, neurologia e pesquisa cardiovascular. Além disso, modelos de serviço flexíveis e soluções de imagem personalizadas estão incentivando a adoção em mercados emergentes na Ásia-Pacífico. A relação custo-benefício e a conveniência desses serviços os tornam atraentes para uma base mais ampla de usuários finais.
- Por reagentes
Com base nos reagentes, o mercado de imagens pré-clínicas da Ásia-Pacífico é segmentado em reagentes de imagem óptica pré-clínica, reagentes de imagem nuclear pré-clínica, agentes de contraste pré-clínicos para ressonância magnética, agentes de contraste pré-clínicos para ultrassom e agentes de contraste pré-clínicos para tomografia computadorizada. Os reagentes de imagem óptica pré-clínica dominaram o mercado com uma participação de 38,7% em 2024, devido à sua ampla aplicação em imagens moleculares, pesquisa do câncer e modelagem de doenças in vivo. Esses reagentes são amplamente utilizados em estudos longitudinais, permitindo imagens repetidas do mesmo indivíduo para monitorar a progressão da doença ou a resposta terapêutica. Sua compatibilidade com sistemas de imagem multimodal aumenta sua utilidade na pesquisa translacional. Os reagentes de imagem óptica também permitem a detecção de alta sensibilidade de biomoléculas específicas e eventos celulares em modelos pré-clínicos. A capacidade de visualizar interações moleculares em animais vivos apoia o rápido desenvolvimento de medicamentos e a validação de biomarcadores.
Espera-se que os agentes de contraste para ressonância magnética pré-clínica apresentem o crescimento mais rápido durante o período previsto, impulsionado pela crescente adoção da ressonância magnética para imagens de tecidos moles e avaliação de biomarcadores. Inovações em agentes de contraste que melhoram a resolução, reduzem a toxicidade e fornecem imagens específicas do tecido estão impulsionando a expansão do mercado. O uso crescente de agentes de contraste para ressonância magnética em estudos longitudinais permite que os pesquisadores acompanhem as mudanças na estrutura e função dos tecidos ao longo do tempo. As empresas farmacêuticas estão cada vez mais utilizando agentes avançados de ressonância magnética para estudar a progressão da doença e a eficácia terapêutica. Colaborações em pesquisa com fabricantes de equipamentos de imagem estão acelerando o desenvolvimento de reagentes de contraste para ressonância magnética de última geração. Além disso, o apoio governamental à pesquisa biomédica na Ásia-Pacífico está contribuindo para a adoção desses agentes em estudos pré-clínicos.
- Por aplicação
Com base na aplicação, o mercado de imagens pré-clínicas da Ásia-Pacífico é segmentado em pesquisa e desenvolvimento, descoberta de fármacos, biodistribuição, detecção de células cancerígenas, biomarcadores e outros. O segmento de pesquisa e desenvolvimento dominou o mercado com uma participação de 44,5% em 2024, apoiado pelo aumento do investimento em descoberta de fármacos em estágio inicial e pesquisa translacional. A alta adoção de sistemas de imagem em P&D acadêmico e farmacêutico permite que pesquisadores estudem modelos de doenças in vivo e avaliem a eficácia dos fármacos em tempo real. A imagem pré-clínica facilita estudos mecanísticos detalhados, ajudando a otimizar candidatos a fármacos antes dos ensaios clínicos. A integração de IA e software avançado de análise de imagens está melhorando a eficiência e a precisão dos fluxos de trabalho de P&D. Pesquisadores dependem cada vez mais de imagens para validar alvos terapêuticos, analisar farmacocinética e avaliar toxicidade. Isso posicionou a imagem pré-clínica como um pilar da pesquisa biomédica moderna na Ásia-Pacífico.
Espera-se que o segmento de detecção de células cancerígenas testemunhe o CAGR mais rápido entre 2025 e 2032, devido ao aumento da prevalência do câncer e ao papel crítico da imagem pré-clínica na avaliação de terapias anticâncer. As modalidades de imagem permitem o rastreamento do crescimento tumoral, da metástase e da resposta ao tratamento em modelos animais. O uso de agentes de imagem molecular e óptica permite a visualização precisa de células cancerígenas em estágios iniciais. As empresas farmacêuticas dependem da imagem pré-clínica para testar novos compostos terapêuticos e imunoterapias. O foco crescente na medicina personalizada e na terapia direcionada está impulsionando ainda mais a demanda. Além disso, parcerias entre desenvolvedores de reagentes de imagem e institutos de pesquisa estão aprimorando as capacidades de detecção de células cancerígenas de alta sensibilidade.
- Por usuário final
Com base no usuário final, o mercado de imagens pré-clínicas da Ásia-Pacífico é segmentado em organizações de pesquisa contratadas (CROs), empresas farmacêuticas e de biotecnologia, institutos de pesquisa acadêmicos e governamentais, centros de diagnóstico e outros. As empresas farmacêuticas e de biotecnologia dominaram o mercado com uma participação de 47,1% em 2024, devido aos seus significativos gastos em P&D e à crescente demanda por avaliação de medicamentos em estágio inicial. Essas empresas utilizam imagens pré-clínicas para triagem de candidatos a medicamentos, avaliação de toxicidade e validação de biomarcadores. Os sistemas de imagem permitem decisões mais rápidas de aprovação/reprovação, reduzindo os custos e os prazos de desenvolvimento. A integração com plataformas automatizadas e de alto rendimento fortalece ainda mais sua adoção. A tendência crescente de medicina de precisão e terapias direcionadas aumentou a dependência de tecnologias de imagem para estudos pré-clínicos. Colaborações com fabricantes de equipamentos de imagem e reagentes também apoiam a expansão neste segmento.
Espera-se que os institutos de pesquisa acadêmicos e governamentais testemunhem o crescimento mais rápido durante o período previsto, impulsionados pelo aumento do financiamento governamental para pesquisa biomédica e pela expansão da infraestrutura de pesquisa. Os institutos estão adotando imagens pré-clínicas para avançar na pesquisa fundamental, estudar mecanismos de doenças e desenvolver novas estratégias terapêuticas. Parcerias com fornecedores globais de equipamentos de imagem permitem acesso a sistemas de alta resolução e reagentes de ponta. Programas de treinamento e projetos de pesquisa colaborativa estão promovendo o uso mais amplo de imagens em laboratórios acadêmicos. A adoção de imagens multimodais e ferramentas de análise baseadas em IA está aprimorando ainda mais as capacidades de pesquisa.
Análise regional do mercado de imagens pré-clínicas da Ásia-Pacífico
- A China dominou o mercado de imagens pré-clínicas da Ásia-Pacífico com a maior participação na receita de 39% em 2024, caracterizada por financiamento governamental substancial para pesquisa biomédica, rápida expansão da fabricação farmacêutica e adoção de sistemas avançados de imagens em oncologia, neurologia e estudos de biomarcadores.
- Pesquisadores e instituições da região valorizam muito os recursos de imagem não invasivos e de alta resolução fornecidos pelos sistemas pré-clínicos, que aceleram a descoberta de medicamentos, a pesquisa translacional e a detecção precoce de doenças.
- Essa ampla adoção é ainda apoiada pelo aumento do investimento em P&D farmacêutico e biotecnológico, pelo aumento dos volumes de ensaios clínicos e pelo foco crescente na medicina de precisão, estabelecendo a imagem pré-clínica como uma ferramenta crítica para organizações de pesquisa acadêmica e comercial na região da Ásia-Pacífico.
Visão geral do mercado de imagens pré-clínicas da China
O mercado chinês de imagens pré-clínicas conquistou a maior fatia da receita na região Ásia-Pacífico em 2024, impulsionado por amplo financiamento governamental para pesquisa biomédica, rápida expansão da indústria farmacêutica e crescente adoção de sistemas de imagem multimodais em oncologia, neurologia e estudos de biomarcadores. A ênfase do país em pesquisa de alta tecnologia e desenvolvimento translacional de medicamentos está promovendo a implantação de soluções avançadas de imagem em laboratórios acadêmicos e comerciais.
Visão do mercado de imagens pré-clínicas do Japão
O mercado japonês de imagens pré-clínicas está ganhando impulso devido ao seu forte ecossistema de P&D, alta adoção de tecnologias e foco em medicina de precisão. A adoção é ainda mais impulsionada pela integração de IA e imagens multimodais em estudos pré-clínicos, permitindo modelagem de doenças e avaliação terapêutica mais precisas. A infraestrutura de pesquisa bem estabelecida do país e o apoio governamental à inovação biomédica continuam a impulsionar o crescimento do mercado.
Visão do mercado de imagens pré-clínicas da Índia
Espera-se que o mercado indiano de imagens pré-clínicas testemunhe o crescimento mais rápido na região da Ásia-Pacífico durante o período previsto, devido à rápida expansão da infraestrutura de pesquisa, ao aumento da atividade de ensaios clínicos e à crescente adoção de sistemas de imagem com boa relação custo-benefício. Iniciativas governamentais que promovem a pesquisa biomédica, o surgimento de CROs e colaborações com fornecedores globais de tecnologia de imagem estão impulsionando a demanda nos setores acadêmico e farmacêutico.
Visão do mercado de imagens pré-clínicas da Coreia do Sul
O mercado de imagem pré-clínica da Coreia do Sul está em constante expansão, impulsionado pelo crescente investimento em P&D em biotecnologia, pela adoção de imagens avançadas em pesquisas sobre câncer e neurologia e pelo aumento da colaboração entre institutos de pesquisa locais e fabricantes globais de sistemas de imagem. O foco do país em saúde digital e soluções inovadoras de imagem está incentivando uma maior penetração no mercado.
Participação no mercado de imagens pré-clínicas da Ásia-Pacífico
O setor de imagens pré-clínicas da Ásia-Pacífico é liderado principalmente por empresas bem estabelecidas, incluindo:
- Bruker (EUA)
- PerkinElmer (EUA)
- FUJIFILM VisualSonics, Inc. (Canadá)
- Aspect Imaging Ltda. (Israel)
- TriFoil Imaging (EUA)
- LI-COR Biosciences (EUA)
- Mediso Ltd. (Hungria)
- MILabs BV (Holanda)
- MR Solutions Ltd. (Reino Unido)
- GE Healthcare (EUA)
- Siemens Healthineers AG (Alemanha)
- Canon Medical Systems Corporation (Japão)
- Shanghai United Imaging Healthcare Co., Ltd. (China)
- Mindray Medical International Limited (China)
- Neusoft Medical Systems Co., Ltd. (China)
- Hitachi Medical Corporation (Japão)
- Koninklijke Philips NV (Holanda)
- Olympus Corporation (Japão)
- Hamamatsu Photonics KK (Japão)
- 3DHISTECH Ltd. (Hungria)
Quais são os desenvolvimentos recentes no mercado de imagens pré-clínicas da Ásia-Pacífico?
- Em dezembro de 2024, a Intas Pharmaceuticals anunciou um acordo para adquirir o negócio UDENYCA (pegfilgrastim-cbqv) da Coherus BioSciences, Inc. por até US$ 558 milhões. A aquisição, concluída no primeiro trimestre de 2025, inclui o medicamento biossimilar e todos os ativos relacionados. O UDENYCA é um biossimilar do Neulasta, usado para tratar neutropenia induzida por quimioterapia. Este movimento estratégico fortalece o portfólio de biossimilares da Intas e consolida sua posição como um importante fornecedor global de pegfilgrastim.
- Em outubro de 2024, a Organização Mundial da Saúde (OMS) sediou uma Reunião de Parceiros em Farmacovigilância em Nova Déli, Índia. A reunião, realizada durante a 19ª Conferência Internacional de Autoridades Reguladoras de Medicamentos (ICDRA), reuniu reguladores de 68 países para revisar um rascunho da Estratégia Global de Farmacovigilância Inteligente da OMS. O objetivo desta iniciativa é promover uma evolução convergente das atividades de farmacovigilância entre os países-membros, resultando em requisitos regulatórios harmonizados e pragmáticos.
- Em abril de 2024, a WuXi STA, subsidiária da WuXi AppTec, detalhou um plano de expansão global com múltiplas unidades, incluindo uma nova unidade de fabricação de ingredientes farmacêuticos ativos (IFA) de 169 acres em Taixing, China. Essa expansão, juntamente com outras instalações, visa aprimorar a capacidade e a capacidade de fabricação para atender à crescente demanda por serviços de desenvolvimento de medicamentos, incluindo estudos pré-clínicos, na região da Ásia-Pacífico e globalmente.
- Em novembro de 2022, a Bruker Corporation anunciou a aquisição da Inscopix, Inc., uma empresa de neurociência especializada em microscópios miniaturizados, ou "miniscópios", para imagens cerebrais de animais em movimento livre. Esta aquisição aprimora o portfólio de neurociência da Bruker, adicionando produtos e serviços que permitem uma exploração mais aprofundada da função da rede neural em animais, crucial para a compreensão de distúrbios neurológicos.
- Em maio de 2022, a FUJIFILM VisualSonics Inc. lançou o Vevo F2, o primeiro sistema de imagem ultrassônica e fotoacústica de ultra-alta a baixa frequência (71 MHz-1 MHz) do mundo para uso pré-clínico. Este sistema conta com tecnologia de processamento de imagem em HD e um caminho de sinal completamente novo do transdutor ao monitor, permitindo maior clareza de imagem e taxas de quadros mais altas. Este avanço é particularmente adequado para pesquisas biológicas e fisiológicas multifuncionais, incluindo oncologia, biologia do desenvolvimento, neurobiologia e cardiologia.
SKU-
Obtenha acesso online ao relatório sobre a primeira nuvem de inteligência de mercado do mundo
- Painel interativo de análise de dados
- Painel de análise da empresa para oportunidades de elevado potencial de crescimento
- Acesso de analista de pesquisa para personalização e customização. consultas
- Análise da concorrência com painel interativo
- Últimas notícias, atualizações e atualizações Análise de tendências
- Aproveite o poder da análise de benchmark para um rastreio abrangente da concorrência
Metodologia de Investigação
A recolha de dados e a análise do ano base são feitas através de módulos de recolha de dados com amostras grandes. A etapa inclui a obtenção de informações de mercado ou dados relacionados através de diversas fontes e estratégias. Inclui examinar e planear antecipadamente todos os dados adquiridos no passado. Da mesma forma, envolve o exame de inconsistências de informação observadas em diferentes fontes de informação. Os dados de mercado são analisados e estimados utilizando modelos estatísticos e coerentes de mercado. Além disso, a análise da quota de mercado e a análise das principais tendências são os principais fatores de sucesso no relatório de mercado. Para saber mais, solicite uma chamada de analista ou abra a sua consulta.
A principal metodologia de investigação utilizada pela equipa de investigação do DBMR é a triangulação de dados que envolve a mineração de dados, a análise do impacto das variáveis de dados no mercado e a validação primária (especialista do setor). Os modelos de dados incluem grelha de posicionamento de fornecedores, análise da linha de tempo do mercado, visão geral e guia de mercado, grelha de posicionamento da empresa, análise de patentes, análise de preços, análise da quota de mercado da empresa, normas de medição, análise global versus regional e de participação dos fornecedores. Para saber mais sobre a metodologia de investigação, faça uma consulta para falar com os nossos especialistas do setor.
Personalização disponível
A Data Bridge Market Research é líder em investigação formativa avançada. Orgulhamo-nos de servir os nossos clientes novos e existentes com dados e análises que correspondem e atendem aos seus objetivos. O relatório pode ser personalizado para incluir análise de tendências de preços de marcas-alvo, compreensão do mercado para países adicionais (solicite a lista de países), dados de resultados de ensaios clínicos, revisão de literatura, mercado remodelado e análise de base de produtos . A análise de mercado dos concorrentes-alvo pode ser analisada desde análises baseadas em tecnologia até estratégias de carteira de mercado. Podemos adicionar quantos concorrentes necessitar de dados no formato e estilo de dados que procura. A nossa equipa de analistas também pode fornecer dados em tabelas dinâmicas de ficheiros Excel em bruto (livro de factos) ou pode ajudá-lo a criar apresentações a partir dos conjuntos de dados disponíveis no relatório.