Asia Pacific Molecular Diagnostics Services Market
Tamanho do mercado em biliões de dólares
CAGR :
% 
 USD
25.84 Million
USD
49.99 Million
2024
2032
USD
25.84 Million
USD
49.99 Million
2024
2032
| 2025 –2032 | |
| USD 25.84 Million | |
| USD 49.99 Million | |
|
|
|
|
Segmentação do mercado de serviços de diagnóstico molecular da Ásia-Pacífico, por tipo de serviço (serviços de reparo de instrumentos, serviços de treinamento, serviços de conformidade , serviços de calibração , serviços de manutenção, serviços de automação escalável, serviços turnkey, serviços de realocação de instrumentos, personalização de hardware, serviços de garantia de desempenho, serviços de design e desenvolvimento, soluções de cadeia de suprimentos, serviços de introdução de novos produtos, serviços de fabricação, serviços ambientais e regulatórios, certificação e auditoria de sistemas de gestão médica , serviços de pesquisa clínica, serviços de consultoria e outros serviços), tecnologia (PCR, PCR em tempo real, sequenciamento de última geração e outras tecnologias), usuário final ( hospitais , centros de diagnóstico, instituições acadêmicas e de pesquisa e outros), tendências e previsões do setor até 2030.
Tamanho do mercado de serviços de diagnóstico molecular da Ásia-Pacífico
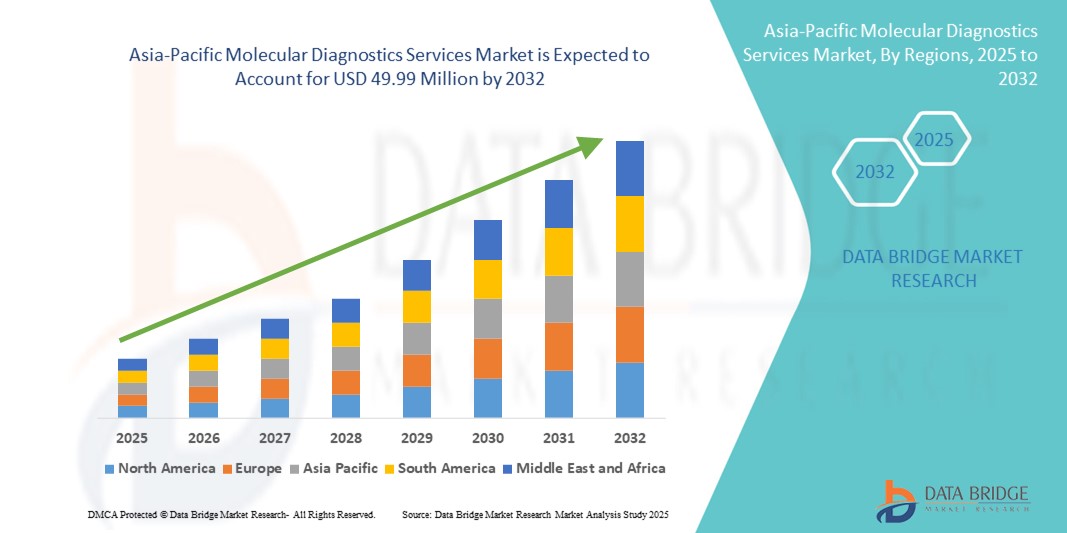
- O tamanho do mercado de serviços de diagnóstico molecular da Ásia-Pacífico foi avaliado em US$ 25,84 milhões em 2024 e deve atingir US$ 49,99 milhões até 2032 , com um CAGR de 8,60% durante o período previsto.
- O crescimento do mercado é amplamente impulsionado pela crescente conscientização, pelo aumento do acesso à saúde e pelos avanços em tecnologias de diagnóstico em toda a região da Ásia-Pacífico, permitindo a detecção e o tratamento oportunos de uma ampla gama de doenças genéticas, infecciosas e crônicas. Países como Índia, China e Indonésia estão testemunhando um aumento no desenvolvimento da infraestrutura de saúde, contribuindo para a crescente adoção de serviços de diagnóstico molecular.
- Além disso, o aumento dos investimentos em instalações laboratoriais, a expansão dos serviços de diagnóstico em áreas rurais e semiurbanas e o aumento das parcerias público-privadas estão impulsionando a inovação e a disponibilidade de tecnologias avançadas de testes moleculares. Iniciativas governamentais de saúde, aliadas à crescente presença de empresas internacionais de diagnóstico e à capacidade de fabricação local, estão impulsionando significativamente o crescimento do mercado de Serviços de Diagnóstico Molecular da Ásia-Pacífico.
Análise de Mercado de Serviços de Diagnóstico Molecular da Ásia-Pacífico
- O mercado de serviços de diagnóstico molecular da Ásia-Pacífico está testemunhando um crescimento significativo, impulsionado pelo aumento dos investimentos em plataformas avançadas de diagnóstico, pela crescente prevalência de doenças infecciosas e genéticas e pela crescente adoção de iniciativas de medicina de precisão. Países como China, Índia, Japão e Coreia do Sul estão expandindo sua infraestrutura laboratorial e capacidades de pesquisa, contribuindo para o aumento da demanda por serviços de diagnóstico molecular de alta qualidade.
- O foco crescente em saúde personalizada e pesquisa clínica na região é apoiado por financiamento governamental reforçado, crescentes investimentos privados em saúde e expansão de centros de diagnóstico especializados. A crescente conscientização sobre a detecção precoce de doenças, aliada aos avanços em tecnologias de testes moleculares, como PCR, PCR em Tempo Real e Sequenciamento de Nova Geração (NGS), está impulsionando uma adoção mais ampla em hospitais e institutos de pesquisa.
- A China dominou o mercado de serviços de diagnóstico molecular da Ásia-Pacífico, respondendo pela maior fatia de receita de 35,1% em 2024, impulsionada por sua rede hospitalar bem estabelecida, grande população de pacientes e rápida integração de tecnologias avançadas de diagnóstico molecular em fluxos de trabalho clínicos e de pesquisa.
- A Índia deverá registrar o CAGR mais rápido, de 13,6%, no mercado de serviços de diagnóstico molecular da Ásia-Pacífico durante o período previsto, impulsionado pela expansão do acesso à saúde, pela crescente demanda por testes moleculares acessíveis e pelo crescente investimento em infraestrutura laboratorial. Iniciativas como programas de diagnóstico apoiados pelo governo e o aumento das colaborações com o setor privado estão acelerando a adoção do diagnóstico molecular em regiões urbanas e semiurbanas.
- Os serviços baseados em PCR dominaram o mercado de serviços de diagnóstico molecular da Ásia-Pacífico, com 36,5% de participação em 2024, devido à sua ampla adoção em testes moleculares para doenças infecciosas, exames genéticos e aplicações de diagnóstico de rotina. A PCR continua sendo uma tecnologia fundamental devido à sua confiabilidade, acessibilidade e adaptabilidade a múltiplos fluxos de trabalho clínicos.
Escopo do Relatório e Segmentação do Mercado de Serviços de Diagnóstico Molecular da Ásia-Pacífico
|
Atributos |
Principais insights de mercado dos serviços de diagnóstico molecular da Ásia-Pacífico |
|
Segmentos abrangidos |
|
|
Países abrangidos |
Ásia-Pacífico
|
|
Principais participantes do mercado |
|
|
Oportunidades de mercado |
|
|
Conjuntos de informações de dados de valor agregado |
Além dos insights sobre cenários de mercado, como valor de mercado, taxa de crescimento, segmentação, cobertura geográfica e principais participantes, os relatórios de mercado selecionados pela Data Bridge Market Research também incluem análises aprofundadas de especialistas, análises de preços, análises de participação de marca, pesquisas com consumidores, análises demográficas, análises da cadeia de suprimentos, análises da cadeia de valor, visão geral de matérias-primas/consumíveis, critérios de seleção de fornecedores, análise PESTLE, análise de Porter e estrutura regulatória. |
Tendências do mercado de serviços de diagnóstico molecular na Ásia-Pacífico
Avanço da terapêutica e expansão da pesquisa clínica na Ásia-Pacífico
- Uma tendência significativa e crescente no mercado de serviços de diagnóstico molecular da Ásia-Pacífico é o foco crescente em inovações terapêuticas e pesquisa clínica, particularmente em áreas como oncologia, doenças infecciosas e distúrbios genéticos. Testes moleculares avançados estão permitindo diagnósticos mais precoces e abordagens de tratamento mais direcionadas.
- Por exemplo, diversas empresas de diagnóstico e institutos de pesquisa na região Ásia-Pacífico estão investindo em sequenciamento de última geração (NGS), ensaios baseados em PCR e painéis multiplex de biomarcadores. Esses desenvolvimentos visam fornecer resultados diagnósticos mais rápidos, precisos e econômicos, essenciais para a medicina personalizada e o gerenciamento de doenças.
- A crescente adoção de modelos de medicina de precisão em hospitais e clínicas especializadas está permitindo intervenções terapêuticas mais eficazes. Esses modelos utilizam perfis moleculares avançados e bioinformática para orientar a seleção do tratamento, monitorar a progressão da doença e prever a resposta do paciente.
- As parcerias entre empresas de tecnologia de diagnóstico, centros de pesquisa acadêmica e programas apoiados pelo governo também estão ajudando a expandir o acesso aos testes moleculares, melhorando as estruturas de reembolso, padronizando as práticas laboratoriais e aprimorando o treinamento clínico.
- À medida que a região Ásia-Pacífico continua a priorizar cuidados de saúde de precisão e resultados baseados em valor, o mercado de serviços de diagnóstico molecular está preparado para um crescimento sustentado, impulsionado pela inovação, pela melhoria da precisão diagnóstica e pela crescente procura por deteção precoce e estratégias terapêuticas personalizadas.
Dinâmica do Mercado de Serviços de Diagnóstico Molecular da Ásia-Pacífico
Motorista
Necessidade crescente devido ao aumento das taxas de diagnóstico e aos avanços na pesquisa genética
- A crescente prevalência de doenças complexas e crônicas na região da Ásia-Pacífico, apoiada pela crescente conscientização e pela melhoria das capacidades de diagnóstico, está impulsionando significativamente o crescimento do mercado. Países como China, Índia, Japão e Coreia do Sul estão aprimorando sua infraestrutura de saúde e programas de diagnóstico, permitindo a detecção precoce e a intervenção oportuna em condições como câncer, doenças infecciosas e distúrbios genéticos.
- Por exemplo, em abril de 2024, a Anavex Life Sciences relatou progresso positivo em seu ensaio clínico de Fase III para o Anavex 2-73 (blarcamesina), visando doenças neurodegenerativas por meio de diagnósticos de precisão e biomarcadores moleculares. Espera-se que tais inovações catalisem a adoção de diagnósticos moleculares avançados, acelerando assim o mercado de Serviços de Diagnóstico Molecular da Ásia-Pacífico durante o período previsto.
- O crescente interesse na medicina personalizada e a disponibilidade de tecnologias moleculares de última geração — incluindo PCR, PCR em tempo real e sequenciamento de última geração — estão provocando uma mudança de mercado de métodos de diagnóstico convencionais para soluções de teste mais precisas e específicas para cada paciente.
- Órgãos reguladores na Ásia-Pacífico, como a Agência de Produtos Farmacêuticos e Dispositivos Médicos (PMDA) no Japão e a Administração Nacional de Produtos Médicos (NMPA) na China, estão cada vez mais apoiando a inovação em diagnóstico por meio de aprovações rápidas, suporte a ensaios clínicos e diretrizes de conformidade simplificadas, promovendo acesso rápido ao mercado para serviços avançados de diagnóstico molecular.
- Colaborações entre empresas regionais de biotecnologia, centros de pesquisa acadêmica e associações de saúde estão fortalecendo o ecossistema de inovação na Ásia-Pacífico. Essas parcerias são fundamentais para expandir o acesso dos pacientes a diagnósticos moleculares avançados, ampliar iniciativas de pesquisa clínica e aumentar a conscientização sobre a detecção precoce de doenças e testes genéticos em diversas populações.
Restrição/Desafio
Infraestrutura limitada e variabilidade na adoção clínica
- O alto custo associado aos serviços avançados de diagnóstico molecular — incluindo instrumentação sofisticada, reagentes e sequenciamento de alto rendimento — representa uma barreira substancial à adoção generalizada, especialmente em áreas rurais ou subfinanciadas.
- Mesmo quando apoiados por incentivos governamentais, os testes de diagnóstico molecular geralmente envolvem fluxos de trabalho complexos, pessoal especializado e controle de qualidade rigoroso, tornando-os menos acessíveis para sistemas de saúde com orçamentos limitados.
- Além disso, as instalações laboratoriais especializadas e o pessoal treinado concentram-se frequentemente nos centros urbanos, obrigando os pacientes em áreas remotas a percorrer longas distâncias ou a sofrer atrasos nos testes e nos relatórios.
- Outro desafio é a falta de protocolos padronizados para determinados ensaios moleculares e testes genéticos. A experiência clínica limitada e as capacidades laboratoriais variáveis contribuem para a adoção inconsistente entre os prestadores de cuidados de saúde.
- Para superar esses desafios, as reformas políticas, o aumento do financiamento governamental, a colaboração transfronteiriça em pesquisas e o estabelecimento de centros dedicados de diagnóstico molecular em toda a Ásia-Pacífico serão essenciais para expandir o acesso e alcançar o crescimento sustentável no mercado de serviços de diagnóstico molecular da Ásia-Pacífico.
Escopo de mercado de serviços de diagnóstico molecular na Ásia-Pacífico
O mercado é segmentado com base no tipo de serviço, tecnologia e usuário final.
- Por tipo de serviço
Com base no tipo de serviço, o mercado de serviços de diagnóstico molecular da Ásia-Pacífico é segmentado em serviços de reparo de instrumentos, serviços de treinamento, serviços de conformidade, serviços de calibração, serviços de manutenção, serviços de automação escalável, serviços turnkey, serviços de realocação de instrumentos, personalização de hardware, serviços de garantia de desempenho, serviços de design e desenvolvimento, soluções para a cadeia de suprimentos, serviços de introdução de novos produtos, serviços de fabricação, serviços ambientais e regulatórios, certificação e auditoria de sistemas de gestão médica, serviços de pesquisa clínica, serviços de consultoria e outros serviços. Os Serviços de Manutenção dominaram o mercado com a maior participação na receita, de 23,7% em 2024, devido ao seu papel essencial em garantir o funcionamento contínuo e preciso dos instrumentos de diagnóstico molecular. Esses serviços são essenciais para minimizar o tempo de inatividade, estender a vida útil dos equipamentos e manter a precisão diagnóstica consistente em todas as redes hospitalares e laboratoriais.
A projeção é que os serviços de pesquisa clínica apresentem o maior CAGR, de 9,1%, entre 2025 e 2032, impulsionado pelo foco crescente em pesquisa translacional e em medicina de precisão. Esse crescimento é sustentado por maiores investimentos em descoberta de biomarcadores, genômica e iniciativas de saúde personalizada, bem como pela expansão das colaborações entre instituições de pesquisa e provedores de serviços de diagnóstico.
- Por Tecnologia
Com base na tecnologia, o mercado de serviços de diagnóstico molecular da Ásia-Pacífico é segmentado em PCR, PCR em Tempo Real, Sequenciamento de Nova Geração (NGS) e Outras Tecnologias. Os serviços baseados em PCR lideraram o mercado com uma participação de 36,5% em 2024, devido à sua ampla adoção em testes moleculares para doenças infecciosas, triagens genéticas e aplicações de diagnóstico de rotina. A PCR continua sendo uma tecnologia fundamental devido à sua confiabilidade, acessibilidade e adaptabilidade a múltiplos fluxos de trabalho clínicos.
A projeção é de que o Sequenciamento de Nova Geração (NGS) cresça a uma taxa composta de crescimento anual (CAGR) de 14,2% entre 2025 e 2032, impulsionado pela crescente demanda por análises genômicas de alto rendimento, oncologia de precisão e perfilamento abrangente de doenças. O NGS permite que pesquisadores e médicos realizem estudos genômicos em larga escala com maior precisão, auxiliando no diagnóstico precoce, na estratificação do tratamento e em abordagens terapêuticas personalizadas.
- Por usuário final
Com base no usuário final, o mercado de serviços de diagnóstico molecular da Ásia-Pacífico é segmentado em hospitais, centros de diagnóstico, instituições acadêmicas e de pesquisa, entre outros. Os hospitais detinham a maior participação, com 41,8% em 2024, apoiados por seu grande volume de pacientes, infraestrutura laboratorial integrada e adoção consistente de plataformas avançadas de diagnóstico molecular. Os hospitais utilizam esses serviços não apenas para diagnósticos de rotina, mas também para dar suporte a departamentos especializados, como oncologia, doenças infecciosas e aconselhamento genético.
Espera-se que as instituições acadêmicas e de pesquisa cresçam a uma taxa composta de crescimento anual (CAGR) de 10,3% durante o período previsto, impulsionadas pelo aumento de iniciativas de pesquisa molecular, financiamento governamental e privado para estudos genômicos e clínicos, e pela adoção de tecnologias de diagnóstico de ponta para pesquisa translacional. Essas instituições desempenham um papel crucial no fomento da inovação e na validação de novas soluções de diagnóstico molecular antes da comercialização.
Análise regional do mercado de serviços de diagnóstico molecular da Ásia-Pacífico
- A região da Ásia-Pacífico dominou o mercado global de serviços de diagnóstico molecular, com a maior participação na receita, de 32,5% em 2024, impulsionada pela expansão da infraestrutura de saúde da região, pelo aumento da prevalência de doenças crônicas e infecciosas e pela rápida adoção de tecnologias avançadas de diagnóstico molecular. Investimentos em instalações médicas, o número crescente de centros de diagnóstico e iniciativas governamentais que promovem a detecção precoce de doenças impulsionam ainda mais o crescimento do mercado.
- Estruturas regulatórias robustas, ampla cobertura de seguros e alta conscientização dos pacientes estão fomentando o crescimento nos setores de saúde pública e privada. O aumento do financiamento governamental para programas de diagnóstico, aliado a parcerias público-privadas e iniciativas de saúde pós-pandemia, está acelerando a adoção de serviços avançados de diagnóstico molecular.
- Além disso, a Ásia-Pacífico abriga vários provedores de serviços de diagnóstico, instituições acadêmicas e centros de P&D líderes, facilitando a inovação contínua no desenvolvimento de ensaios, avaliação clínica e integração de tecnologias de última geração.
Visão do mercado de serviços de diagnóstico molecular na China Ásia-Pacífico
O mercado chinês de serviços de diagnóstico molecular detinha a maior participação de mercado na região Ásia-Pacífico, com 35,1% em 2024, impulsionado por uma grande base populacional, aumento da prevalência de doenças infecciosas e genéticas e expansão do acesso a centros de diagnóstico especializados. Reformas governamentais no sistema de saúde, aumento da cobertura de seguros e políticas de reembolso favoráveis estão incentivando prestadores de serviços nacionais e internacionais a expandir suas ofertas de diagnóstico molecular. Empresas locais também estão investindo fortemente em P&D para atender à crescente demanda por soluções avançadas de diagnóstico.
Visão do mercado de serviços de diagnóstico molecular na Ásia-Pacífico e no Japão
O mercado japonês de serviços de diagnóstico molecular representou 21,5% da participação de mercado da Ásia-Pacífico em 2024, apoiado por sua infraestrutura de saúde altamente desenvolvida, forte cobertura de seguros e ambiente laboratorial tecnologicamente avançado. A crescente adoção de sequenciamento de última geração (NGS), testes baseados em PCR e outras tecnologias de diagnóstico molecular, especialmente em hospitais e centros de pesquisa, está impulsionando a demanda do mercado. A ênfase do Japão na detecção precoce de doenças e na medicina de precisão está reforçando sua posição de liderança na região.
Visão do mercado de serviços de diagnóstico molecular na Índia e Ásia-Pacífico
O mercado indiano de serviços de diagnóstico molecular deverá ser o de crescimento mais rápido na região Ásia-Pacífico, com um CAGR de 13,6% entre 2025 e 2032, impulsionado pela crescente conscientização sobre saúde, pela crescente acessibilidade aos serviços de diagnóstico e pelo aumento da renda disponível. Programas nacionais de detecção de doenças, expansão da infraestrutura de diagnóstico em cidades de segundo e terceiro níveis e a crescente participação do setor privado estão acelerando a adoção. A Índia também está emergindo como um polo de diagnóstico molecular com boa relação custo-benefício, aumentando sua competitividade regional.
Participação no mercado de serviços de diagnóstico molecular na Ásia-Pacífico
O setor de serviços de diagnóstico molecular da Ásia-Pacífico é liderado principalmente por empresas bem estabelecidas, incluindo:
- F. Hoffmann-La Roche Ltd (Suíça)
- Danaher Corporation (EUA)
- BIOMÉRIEUX (França)
- QIAGEN NV (Holanda)
- Thermo Fisher Scientific Inc. (EUA)
- Bio-Rad Laboratories, Inc. (EUA)
- Abbott (EUA)
- DiaSorin SpA (Itália)
- Hologic, Inc. (EUA)
Últimos desenvolvimentos no mercado de serviços de diagnóstico molecular da Ásia-Pacífico
- Em agosto de 2025 , as empresas farmacêuticas chinesas de P&D passaram a adquirir cada vez mais reagentes de laboratório de fornecedores nacionais, como a Shanghai Titan Scientific e a Nanjing Vazyme Biotech, para reduzir custos e encurtar os prazos de entrega. Essa mudança ocorreu em resposta ao aumento das tarifas de importação e às preocupações com a confiabilidade da cadeia de suprimentos em meio às tensões comerciais com os EUA. Anteriormente dominado por empresas ocidentais como Thermo Fisher e Merck, o mercado chinês de reagentes, avaliado em US$ 5,76 bilhões, viu uma movimentação significativa em direção a empresas locais.
- Em março de 2025 , a Qiagen anunciou que descontinuaria seus Sistemas Integrados de Teste de PCR NeuMoDx 96 e 288 devido aos desenvolvimentos do mercado e às mudanças nas necessidades dos clientes após a pandemia de COVID-19. A empresa iniciou discussões com os clientes para entender o impacto nas vendas de 2024 e apoiaria os clientes existentes até 2025.
- Em fevereiro de 2024 , a PlexBio apresentou sua tecnologia avançada de detecção de câncer de pulmão no Medlab Dubai, destacando seu compromisso em expandir as capacidades de diagnóstico molecular em oncologia
- Em janeiro de 2024 , a Revvity, anteriormente parte da PerkinElmer, aumentou significativamente seus investimentos em pesquisa, software e operações internas após sua cisão e reformulação da marca. A empresa se concentrou em aprimorar seus setores de ciências da vida e diagnóstico, visando aumentar os gastos em P&D e investir em eficiência operacional, incluindo uma nova plataforma de e-commerce e otimização da cadeia de suprimentos.
SKU-
Obtenha acesso online ao relatório sobre a primeira nuvem de inteligência de mercado do mundo
- Painel interativo de análise de dados
- Painel de análise da empresa para oportunidades de elevado potencial de crescimento
- Acesso de analista de pesquisa para personalização e customização. consultas
- Análise da concorrência com painel interativo
- Últimas notícias, atualizações e atualizações Análise de tendências
- Aproveite o poder da análise de benchmark para um rastreio abrangente da concorrência
Metodologia de Investigação
A recolha de dados e a análise do ano base são feitas através de módulos de recolha de dados com amostras grandes. A etapa inclui a obtenção de informações de mercado ou dados relacionados através de diversas fontes e estratégias. Inclui examinar e planear antecipadamente todos os dados adquiridos no passado. Da mesma forma, envolve o exame de inconsistências de informação observadas em diferentes fontes de informação. Os dados de mercado são analisados e estimados utilizando modelos estatísticos e coerentes de mercado. Além disso, a análise da quota de mercado e a análise das principais tendências são os principais fatores de sucesso no relatório de mercado. Para saber mais, solicite uma chamada de analista ou abra a sua consulta.
A principal metodologia de investigação utilizada pela equipa de investigação do DBMR é a triangulação de dados que envolve a mineração de dados, a análise do impacto das variáveis de dados no mercado e a validação primária (especialista do setor). Os modelos de dados incluem grelha de posicionamento de fornecedores, análise da linha de tempo do mercado, visão geral e guia de mercado, grelha de posicionamento da empresa, análise de patentes, análise de preços, análise da quota de mercado da empresa, normas de medição, análise global versus regional e de participação dos fornecedores. Para saber mais sobre a metodologia de investigação, faça uma consulta para falar com os nossos especialistas do setor.
Personalização disponível
A Data Bridge Market Research é líder em investigação formativa avançada. Orgulhamo-nos de servir os nossos clientes novos e existentes com dados e análises que correspondem e atendem aos seus objetivos. O relatório pode ser personalizado para incluir análise de tendências de preços de marcas-alvo, compreensão do mercado para países adicionais (solicite a lista de países), dados de resultados de ensaios clínicos, revisão de literatura, mercado remodelado e análise de base de produtos . A análise de mercado dos concorrentes-alvo pode ser analisada desde análises baseadas em tecnologia até estratégias de carteira de mercado. Podemos adicionar quantos concorrentes necessitar de dados no formato e estilo de dados que procura. A nossa equipa de analistas também pode fornecer dados em tabelas dinâmicas de ficheiros Excel em bruto (livro de factos) ou pode ajudá-lo a criar apresentações a partir dos conjuntos de dados disponíveis no relatório.