Mercado de diagnóstico de cancro do rim na Ásia-Pacífico, por tipo de teste (imagiologia, teste de biomarcador, teste sanguíneo, biópsia, teste genético e outros), estádio do cancro (estádio I, estádio II, estádio III e estádio IV) , tipo de tumor (renal Carcinoma de células, carcinoma de células renais de células claras, carcinoma de células renais de células não claras), produto (produtos baseados em plataforma, produtos baseados em instrumentos, kits e reagentes e outros consumíveis), tecnologia ( hibridização in situ fluorescente , sequenciação de última geração, fluoroimunoensaio, ensaio comparativo Hibridização Genómica, Imunohistoquímica e Outros), Aplicação (Triagem, Diagnóstico e Preditivo, Prognóstico e Investigação), Utilizador Final (Hospitais, Centros de Diagnóstico, Centros de Investigação do Cancro, Institutos Académicos, Centros Cirúrgicos de Ambulatório e Outros), Canal de Distribuição (Direct Licitações, Vendas a Retalho e Outros), Tendências e Previsões do Sector até 2030.
Análise e dimensão do mercado de diagnósticos de cancro renal na Ásia-Pacífico
O cancro do rim começa quando as células saudáveis num ou em ambos os rins mudam e crescem descontroladamente, formando uma massa chamada tumor cortical. O tumor pode ser maligno, indolente ou benigno. A malignidade é cancro, o que significa que pode crescer e espalhar-se para outras partes do corpo. Um tumor indolente é também cancro, mas este tipo de tumor raramente se espalha para outras partes do corpo. Um tumor benigno significa que o tumor pode crescer, mas não se espalhar.
A crescente consciencialização sobre o cancro renal na Ásia-Pacífico aumentou a procura pelo mercado. O aumento dos gastos em saúde para melhores serviços de saúde também contribui para o crescimento do mercado. Os principais participantes do mercado concentram-se em vários lançamentos e aprovações de serviços durante este período crucial. Além disso, o aumento de procedimentos de diagnóstico melhorados para o cancro renal também contribui para a crescente procura de testes de diagnóstico de cancro renal.
Espera-se que o mercado de diagnóstico de cancro renal na Ásia-Pacífico cresça no ano previsto devido ao aumento de participantes no mercado e à disponibilidade de serviços avançados. A par disto, os fabricantes estão envolvidos em atividades de I&D para lançar novos serviços no mercado. Espera-se que o aumento da investigação na área do diagnóstico e desenvolvimento renal impulsione ainda mais o crescimento do mercado. No entanto, prevê-se que os danos nos tecidos devido à elevada exposição à radiação dos exames de imagem prejudiquem o crescimento do mercado de diagnóstico do cancro do rim na Ásia-Pacífico no período previsto.
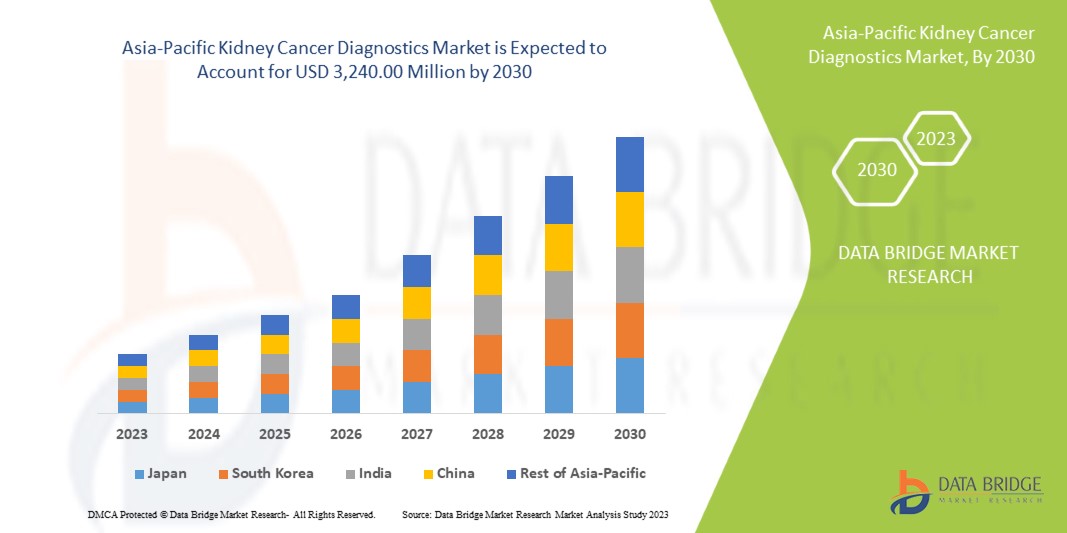
A Data Bridge Market Research analisa que o mercado de diagnóstico de cancro renal deverá atingir um valor de 3.240,00 milhões de dólares até 2030, com um CAGR de 6,9% durante o período previsto. Os exames de imagem representam o maior segmento de tipo de teste no mercado devido à crescente procura de dispositivos inteligentes, e o aumento dos gastos com a saúde acelerou a procura de dispositivos médicos inteligentes.
|
Métrica de Reporte |
Detalhes |
|
Período de previsão |
2023 a 2030 |
|
Ano base |
2022 |
|
Anos históricos |
2021 (Personalizável para 2020-2016) |
|
Unidades quantitativas |
Receita em milhões de dólares americanos, volumes em unidades, preços em dólares americanos |
|
Segmentos abrangidos |
Por tipo de teste (imagiologia, teste de biomarcador, análise ao sangue, biópsia, teste genético e outros), estádio do cancro (estádio I, estádio II, estádio III e estádio IV), tipo de tumor (carcinoma de células renais, carcinoma de células renais de células claras , Carcinoma de células renais de células não claras), Produto (produtos baseados em plataforma, produtos baseados em instrumentos, kits e reagentes e outros consumíveis), Tecnologia (hibridização in situ fluorescente, sequenciação de última geração, fluoroimunoensaio , hibridização genómica comparativa, imunohistoquímica e outros) , Aplicação (Rastreio, Diagnóstico e Preditivo, Prognóstico e Investigação), Utilizador Final (Hospitais, Centros de Diagnóstico, Centros de Investigação do Cancro, Institutos Académicos, Centros Cirúrgicos de Ambulatório e Outros), Canal de Distribuição (Licitação Directa, Vendas a Retalho e Outros) . |
|
Países abrangidos |
China, Japão, Índia, Austrália, Nova Zelândia, Coreia do Sul, Singapura, Malásia, Tailândia, Indonésia, Filipinas, Vietname, Taiwan e restante Ásia-Pacífico. |
|
Atores do mercado abrangidos |
Siemens Healthcare GmbH, Koninklijke Philips NV, FUJIFILM Corporation, Grail, Laboratory Corporation of America Holdings, Thermo Fisher Scientific, Myriad Genetics, Inc., CANON MEDICAL SYSTEMS CORPORATION, QIAGEN, Illumina Inc., Ambry Genetics, Invitae Corporation, General Electric Company, Centogene NV, GenPath, Creative Diagnostics, GeneDx LLC, Blueprint Genetics Oy, BioVendor R&D e CD Genomics, e BD, entre outros |
Definição de Mercado
O cancro do rim, vulgarmente conhecido como cancro do rim, é uma condição na qual as células renais se desenvolvem em tumores malignos (cancerígenos) e se expandem descontroladamente. Um dos 10 tipos de cancro mais prevalentes é o cancro do rim. O cancro do rim é fatal e o processo de diagnóstico também apresenta problemas de segurança; não é rentável. Os doentes oncológicos podem ser hospitalizados e receber uma variedade de terapias, como cirurgia, radioterapia e terapia sistémica. Cerca de 40% dos tumores renais são massas pequenas e localizadas. Localizado refere-se a um tumor que não se espalhou a partir do seu local original. As massas renais não podem ser detectadas com procedimentos laboratoriais regulares. O diagnóstico do cancro do rim inclui procedimentos de biópsia, análises ao sangue e exames de imagem. Terapias avançadas para o cancro renal, como a imunoterapia, radioterapia, etc., são recomendadas. Devido aos métodos de ponta, procedimentos não cirúrgicos como a crioablação (que congela as células cancerígenas) e a ablação por radiofrequência são por vezes utilizados para tratar tumores renais mais pequenos (células cancerígenas aquecidas).
O cancro do rim pode ser difícil de diagnosticar porque, apesar da vasta gama de sinais e sintomas, não são específicos e podem estar relacionados com outras condições médicas mais comuns. Mais de 43.000 homens e 25.000 mulheres são diagnosticados com cancro do rim e da pélvis renal por ano, e 9.000 homens e 5.000 mulheres morrem em consequência desta doença. No entanto, espera-se que as regulamentações e normas rigorosas para a aprovação e comercialização de produtos de diagnóstico do cancro do rim restrinjam o crescimento do mercado.
Dinâmica do mercado de diagnóstico de cancro renal na Ásia-Pacífico
Esta secção trata da compreensão dos impulsionadores, vantagens, oportunidades, restrições e desafios do mercado. Tudo isto é discutido em detalhe abaixo:
Motoristas
- Prevalência crescente de cancro renal
Todas as idades podem ser afetadas por este tipo de cancro. O cancro do rim pode ser difícil de diagnosticar porque, apesar da vasta gama de sinais e sintomas, não são específicos e podem estar relacionados com outras condições médicas mais comuns. O cancro do rim geralmente não apresenta sinais ou sintomas nos seus estágios iniciais. Com o tempo, podem ocorrer sinais e sintomas, incluindo sangue na urina, que pode parecer cor-de-rosa, vermelho ou cor de cola, dor nas costas ou nos lados que não desaparece, perda de apetite, perda de peso inexplicável, fadiga e febre. Nos adultos, o cancro do rim é o tipo mais comum de cancro. As crianças pequenas têm maior probabilidade de desenvolver um tipo de cancro renal chamado tumor de Wilms. O cancro do rim (também conhecido como cancro do rim ou adenocarcinoma de células renais) é o 14º cancro mais comum no mundo. É o 9º entre os homens e o 14º entre as mulheres. Em 2020, foram diagnosticados mais de 30.000 novos casos de cancro renal.
Devido a vários fatores de risco, como o tabagismo, a obesidade, a hipertensão arterial (hipertensão) ou o historial familiar de cancro do rim, o número de doentes com cancro do rim está a aumentar na Ásia-Pacífico e a tornar-se um problema socioeconómico significativo. Assim, o número crescente de doentes com cancro renal aumenta a procura de produtos de diagnóstico de cancro renal, que atuam como um impulsionador no mercado de diagnóstico de cancro renal na Ásia-Pacífico.
- Aumento dos procedimentos diagnósticos para o cancro renal
As técnicas utilizadas para diagnosticar o cancro do rim incluem a ecografia, a tomografia computorizada (TC), a ressonância magnética (RM) e, por vezes, a tomografia por emissão de positrões (PET). O tratamento para o cancro renal com taxa de crescimento lenta pode envolver a monitorização. A quimioterapia para o cancro é ocasionalmente combinada com radioterapia e transplante de células estaminais. O aumento das taxas de cancro tem sido um fator impulsionador para a crescente aprovação de produtos de diagnóstico.
Assim, o aumento das aprovações de produtos de diagnóstico levou a um aumento do número de produtos altamente eficientes no mercado para o tratamento de diagnóstico do cancro do rim. Espera-se que isto atue como um impulsionador do crescimento do mercado de diagnóstico de cancro renal na Ásia-Pacífico.
Oportunidade
-
Preferência crescente por exames de saúde preventivos
O check-up preventivo de saúde é uma ação preventiva realizada para a deteção inicial da doença do cancro do rim. Além disso, a crescente preferência por exames de saúde preventivos oferece uma proteção contra a possível exposição a qualquer doença no futuro.
A sensibilização para promover o rastreio é a componente mais importante da prevenção do cancro do rim. O check-up consiste na identificação do cancro e exames dos fatores de risco para limitar a perda numa fase inicial.
O check-up preventivo do cancro do rim é realizado com a ajuda de vários exames de diagnóstico, que incluem biópsia, imunohistoquímica, rastreio do cancro, ressonância magnética, entre outros.
As pessoas são relativamente mais propensas a doenças de cancro renal. Por conseguinte, precisam de ser submetidos a exames regulares para facilitar que os médicos desenvolvam uma compreensão das doenças e forneçam um melhor tratamento a um paciente que sofre de cancro, pois espera-se que a crescente preferência por exames preventivos de saúde atue como um impulsionador para o crescimento do mercado de diagnóstico de cancro renal na Ásia-Pacífico.
Restrição/Desafio
- Regulamentação e normas rigorosas para aprovação e comercialização de produtos de diagnóstico de cancro renal
As regulamentações rigorosas para a comercialização de qualquer produto no mercado estão a revelar-se um grande desafio para os fabricantes de produtos de diagnóstico de cancro na Ásia-Pacífico, que têm regulamentos e um organismo diferente para os procedimentos regulamentares.
Os fabricantes precisam primeiro de verificar a aprovação da marca CE para a comercialização dos seus produtos no mercado da Ásia-Pacífico. Espera-se que as políticas regulamentares rigorosas dificultem o desenvolvimento do mercado de diagnóstico do cancro. A exigência regulamentar para aprovações de comercialização ou certificação CE e aplicação de leis e regulamentos pode levar a grandes mudanças nos negócios ou ao pagamento de penalizações, incluindo a potencial perda de licenças comerciais. Os recursos e os custos necessários para cumprir estas leis, regras e regulamentos são elevados.
Os requisitos regulamentares para aprovações de comercialização, declaração de conformidade e o tempo necessário para a revisão regulamentar podem variar para diferentes produtos. A empresa que não consegue obter a aprovação regulamentar prejudica o negócio porque, sem obter a aprovação ou sem obter a aprovação da marca CE nos produtos, os fabricantes não conseguem lançar os seus produtos no mercado da Ásia-Pacífico e, por esse motivo, as regulamentações rigorosas e Espera-se que as normas para a aprovação e comercialização de produtos de diagnóstico do cancro do rim atuem como uma restrição para o mercado de diagnóstico do cancro do rim na Ásia-Pacífico.
Desenvolvimentos recentes
- Em novembro de 2022, a Koninklijke Philips NV anunciou o lançamento na Ásia-Pacífico de uma solução de ultrassons portáteis compacta de última geração na reunião anual da Radiological Society of North America (RSNA) para levar a qualidade de diagnóstico associada aos sistemas de ultrassons premium baseados em carrinho a mais doentes . É portátil e versátil, com boa qualidade de imagem e desempenho. É compatível com os sistemas de ultrassons Philips Affiniti e transdutor EPIQ. Isto ajudou a empresa a expandir o seu portfólio de produtos.
- Em outubro de 2022, a General Electric Company colaborou com vários institutos de investigação, como os Hospitais da Universidade de Cambridge, a Sophia Genetics e, anteriormente, com a Optellum, para utilizar dados de imagem em colaboração com a inteligência artificial. Isto ajudará a reduzir o tempo de diagnóstico de vários tipos de cancro e a prestar cuidados personalizados aos doentes. Isto ajudou a empresa a ampliar os seus horizontes em diagnósticos de cancro.
- Em julho de 2022, a Canon Medical Systems USA Inc. anunciou a conclusão da aquisição da NXC Imaging, um distribuidor de equipamentos de imagiologia médica e prestador de serviços localizado em Minnesota, EUA. -Pacífico.
Âmbito do mercado de diagnóstico de cancro renal na Ásia-Pacífico
O mercado de diagnóstico do cancro do rim da Ásia-Pacífico está segmentado em oito segmentos notáveis com base no tipo de teste, estádio do cancro, tipo de tumor, produto, aplicação, tecnologia, utilizador final e canal de distribuição. O crescimento entre segmentos ajuda-o a analisar os nichos de crescimento e as estratégias para abordar o mercado e determinar as suas principais áreas de aplicação e a diferença nos seus mercados-alvo.
Tipo de teste
- TESTE DE IMAGEM
- TESTE DE BIOMARCADOR
- EXAME DE SANGUE
- BIÓPSIA
- TESTE GENÉTICO
- OUTROS
Com base no tipo de teste, o mercado de diagnóstico do cancro do rim na Ásia-Pacífico está segmentado em exames de imagem, testes de biomarcadores, análises ao sangue, biópsias, testes genéticos e outros.
Estágio do cancro
- ESTÁGIO I
- ESTÁGIO II
- ESTÁGIO III
- ESTÁGIO IV
Com base no estádio do cancro, o mercado de diagnóstico do cancro do rim da Ásia-Pacífico está segmentado no estádio I, estádio II, estádio III e estádio IV.
Tipo de tumor
- CARCINOMA DE CÉLULAS RENAIS
- CARCINOMA DE CÉLULAS RENAIS DE CÉLULAS CLARAS
- CARCINOMA DE CÉLULAS RENAIS DE CÉLULAS NÃO CLARAS
Com base no tipo de tumor, o mercado de diagnóstico do cancro do rim na Ásia-Pacífico está segmentado em carcinoma de células renais, carcinoma de células renais de células claras e carcinoma de células renais de células não claras.
Produto
- PRODUTOS BASEADOS EM PLATAFORMA
- PRODUTOS BASEADOS EM INSTRUMENTOS
- KITS E REAGENTES
- OUTROS CONSUMÍVEIS
Com base no produto, o mercado de diagnóstico do cancro do rim da Ásia-Pacífico está segmentado em produtos baseados em instrumentos, produtos baseados em plataformas, kits e reagentes e outros consumíveis.
Tecnologia
- HIBRIDIZAÇÃO IN SITU FLUORESCENTE
- SEQUENCIAÇÃO DE PRÓXIMA GERAÇÃO
- FLUOROIMUNOENSAIO
- HIBRIDIZAÇÃO GENÓMICA COMPARATIVA
- IMUNO-HISTOQUÍMICA
- OUTROS
Com base na tecnologia, o mercado de diagnóstico do cancro do rim na Ásia-Pacífico está segmentado em hibridização in situ fluorescente, sequenciação de última geração, fluoroimunoensaio, hibridização genómica comparativa, imunohistoquímica e outros.
Aplicação
- TRIAGEM
- DIAGNÓSTICO E PREDITIVO
- PROGNÓSTICO
- INVESTIGAÇÃO
Com base na aplicação, o mercado de diagnóstico do cancro do rim na Ásia-Pacífico está segmentado em rastreio, diagnóstico e previsão, prognóstico e investigação.
Utilizador final
- HOSPITAIS
- CENTROS DE INVESTIGAÇÃO SOBRE CÂNCER
- INSTITUTOS ACADÉMICOS
- CENTROS DE DIAGNÓSTICO
- CENTROS CIRÚRGICOS AMBULATORIAIS
- OUTROS
Com base nos utilizadores finais, o mercado de diagnóstico de cancro renal da Ásia-Pacífico está segmentado em hospitais, centros de diagnóstico, centros de investigação de cancro, institutos académicos, centros de cirurgia ambulatória e outros.
Canal de Distribuição
- LICITAÇÕES DIRETAS
- VENDAS NO VAREJO
- OUTROS
Com base no canal de distribuição, o mercado de diagnóstico do cancro do rim na Ásia-Pacífico está segmentado em licitação direta, vendas a retalho e outros.
Análise/Insights Regionais do Mercado de Diagnóstico do Cancro do Rim da Ásia-Pacífico
O mercado de diagnóstico do cancro do rim da Ásia-Pacífico é analisado e são fornecidas informações sobre o tamanho do mercado com base no país, tipo de teste, estádio do cancro, tipo de tumor, produto, aplicação, tecnologia, utilizador final e canal de distribuição.
Os países abrangidos neste relatório de mercado são a China, Japão, Índia, Austrália, Nova Zelândia, Coreia do Sul, Singapura, Malásia, Tailândia, Indonésia, Filipinas, Vietname, Taiwan e o resto da Ásia-Pacífico.
A China domina a região da Ásia-Pacífico devido à crescente consciencialização sobre os exames de saúde regulares.
A secção de países do relatório também fornece fatores individuais que impactam o mercado e alterações na regulamentação do mercado nacional que impactam as tendências atuais e futuras do mercado. Pontos de dados como novas vendas, vendas de reposição, demografia do país, atos regulamentares e tarifas de importação e exportação são alguns dos principais indicadores utilizados para prever o cenário de mercado para países individuais. Além disso, a presença e a disponibilidade de marcas da Ásia-Pacífico e os seus desafios enfrentados devido à grande ou escassa concorrência de marcas locais e nacionais e o impacto dos canais de vendas são considerados ao fornecer uma análise de previsão dos dados do país.
Análise do panorama competitivo e da quota de mercado de diagnósticos de cancro renal na Ásia-Pacífico
O panorama competitivo do mercado de diagnóstico do cancro do rim fornece detalhes por concorrente. Os detalhes incluídos são a visão geral da empresa, finanças da empresa, receitas geradas, potencial de mercado, investimento em I&D, novas iniciativas de mercado, localizações e instalações de produção, pontos fortes e fracos da empresa, lançamento de produtos, aprovações de produtos, amplitude e amplitude do produto, domínio da aplicação e tipo de produto curva da linha de vida. Os pontos de dados fornecidos acima estão apenas relacionados com o foco da empresa no mercado de diagnóstico de cancro renal.
Alguns dos principais participantes que operam no mercado são a Siemens Healthcare GmbH, Koninklijke Philips NV, FUJIFILM Corporation, Grail, Laboratory Corporation of America Holdings, Thermo Fisher Scientific, Myriad Genetics, Inc., CANON MEDICAL SYSTEMS CORPORATION, QIAGEN, Illumina Inc., Ambry Genetics, Invitae Corporation, General Electric Company, Centogene NV, GenPath, Creative Diagnostics, GeneDx LLC, Blueprint Genetics Oy, BioVendor R&D e CD Genomics, BD, entre outros.
SKU-
Obtenha acesso online ao relatório sobre a primeira nuvem de inteligência de mercado do mundo
- Painel interativo de análise de dados
- Painel de análise da empresa para oportunidades de elevado potencial de crescimento
- Acesso de analista de pesquisa para personalização e customização. consultas
- Análise da concorrência com painel interativo
- Últimas notícias, atualizações e atualizações Análise de tendências
- Aproveite o poder da análise de benchmark para um rastreio abrangente da concorrência
Índice
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF THE ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATIONS
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 DBMR TRIPOD DATA VALIDATION MODEL
2.5 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.6 MULTIVARIATE MODELLING
2.7 MARKET APPLICATION COVERAGE GRID
2.8 TEST TYPE LIFELINE CURVE
2.9 DBMR MARKET POSITION GRID
2.1 VENDOR SHARE ANALYSIS
2.11 SECONDARY SOURCES
2.12 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL ANALYSIS
4.2 PORTER'S 5 FORCES
4.3 EPIDEMIOLOGY
4.3.1 KIDNEY CANCER INCIDENCES, 2020, BY BOTH SEXES
4.3.2 KIDNEY CANCER MORTALITY, 2020, BY BOTH SEXES
5 INDUSTRY INSIGHTS
6 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET, REGULATIONS
7 MARKET OVERVIEW
7.1 DRIVERS
7.1.1 GROWING PREVALENCE OF KIDNEY CANCER
7.1.2 INCREASE IN DIAGNOSTIC PROCEDURES FOR KIDNEY CANCER
7.1.3 RISE IN HEALTHCARE EXPENDITURE FOR CANCER DIAGNOSIS AND TREATMENT
7.1.4 RISING AWARENESS TOWARDS KIDNEY CANCER
7.2 RESTRAINTS
7.2.1 STRICT REGULATIONS AND STANDARDS FOR THE APPROVAL AND COMMERCIALIZATION OF KIDNEY CANCER DIAGNOSTIC PRODUCTS
7.2.2 TISSUE DAMAGE DUE TO HIGH RADIATION EXPOSURE FROM IMAGING TESTS
7.3 OPPORTUNITIES
7.3.1 RISING PREFERENCE FOR PREVENTIVE HEALTH CHECK-UPS
7.3.2 GOVERNMENT INITIATIVES TOWARD KIDNEY CANCER DIAGNOSTICS
7.3.3 GROWING DEMAND FOR BETTER QUALITY HEALTHCARE
7.3.4 INCREASED DEMAND FOR NON-INVASIVE TESTING METHODS
7.4 CHALLENGES
7.4.1 LACK OF SKILLED AND CERTIFIED PROFESSIONALS
7.4.2 HIGH COST OF DIAGNOSTICS PROCEDURE FOR KIDNEY CANCERS
8 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE
8.1 OVERVIEW
8.2 IMAGING
8.2.1 COMPUTED TOMOGRAPHY
8.2.2 ULTRASOUND
8.2.3 MAGNETIC RESONANCE IMAGING (MRI)
8.2.4 ANGIOGRAPHY
8.2.5 X-RAY
8.2.6 OTHERS
8.3 BLOOD TEST
8.4 BIOPSY
8.4.1 FINE NEEDLE ASPIRATION
8.4.2 NEEDLE CORE BIOPSY
8.5 BIOMARKER TEST
8.5.1 AQUAPORIN 1 (AQP1)
8.5.2 PERILIPIN (PLIN2)
8.5.3 N-METHYLTRANSFERASE (NMNT)
8.5.4 L-PLASTIN (LCP-1)
8.5.5 NM23A
8.6 GENETIC TEST
8.7 OTHERS
9 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET, BY CANCER STAGE
9.1 OVERVIEW
9.2 STAGE I
9.3 STAGE II
9.4 STAGE III
9.5 STAGE IV
10 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE
10.1 OVERVIEW
10.2 RENAL CELL CARCINOMA
10.2.1 IMAGING
10.2.2 BLOOD TEST
10.2.3 BIOPSY
10.2.4 BIOMARKER TEST
10.2.5 GENETIC TEST
10.2.6 OTHERS
10.3 CLEAR CELL RENAL CELL CARCINOMA
10.3.1 IMAGING
10.3.2 BLOOD TEST
10.3.3 BIOPSY
10.3.4 BIOMARKER TEST
10.3.5 GENETIC TEST
10.3.6 OTHERS
10.4 NON CLEAR CELL RENAL CELL CARCINOMA
10.4.1 PAPILLARY RENAL CELL CARCINOMA
10.4.1.1 IMAGING
10.4.1.2 BLOOD TEST
10.4.1.3 BIOPSY
10.4.1.4 BIOMARKER TEST
10.4.1.5 GENETIC TEST
10.4.1.6 OTHERS
10.4.2 CHROMOPHOBE RENAL CELL CARCINOMA
10.4.2.1 IMAGING
10.4.2.2 BLOOD TEST
10.4.2.3 BIOPSY
10.4.2.4 BIOMARKER TEST
10.4.2.5 GENETIC TEST
10.4.2.6 OTHERS
11 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT
11.1 OVERVIEW
11.2 INSTRUMENT BASED PRODUCTS
11.2.1 IMAGING
11.2.2 BIOPSY
11.3 PLATFORM BASED PRODUCTS
11.3.1 NEXT GENERATION SEQUENCING
11.3.2 MICROARRAYS
11.3.3 PCR
11.3.4 OTHERS
11.4 KITS AND REAGENTS
11.4.1 RENAL CANCER PANELS
11.4.2 RENAL CANCER ANTIBODIES
11.5 OTHER CONSUMABLES
12 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET, BY TECHNOLOGY
12.1 OVERVIEW
12.2 FLUORESCENT IN SITU HYBRIDIZATION
12.3 NEXT GENERATION SEQUENCING
12.4 FLUORIMMUNOASSAY
12.5 COMPARATIVE GENOMIC HYBRIDIZATION
12.6 IMMUNOHISTOCHEMICAL
12.7 OTHERS
13 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET, BY APPLICATION
13.1 OVERVIEW
13.2 SCREENING
13.2.1 INSTRUMENT BASED PRODUCTS
13.2.2 PLATFORM BASED PRODUCTS
13.2.3 KITS AND REAGENTS
13.2.4 OTHER CONSUMABLES
13.3 DIAGNOSTIC AND PREDICTIVE
13.3.1 INSTRUMENT BASED PRODUCTS
13.3.2 PLATFORM BASED PRODUCTS
13.3.3 KITS AND REAGENTS
13.3.4 OTHER CONSUMABLES
13.4 PROGNOSTIC
13.4.1 INSTRUMENT BASED PRODUCTS
13.4.2 PLATFORM BASED PRODUCTS
13.4.3 KITS AND REAGENTS
13.4.4 OTHER CONSUMABLES
13.5 RESEARCH
13.5.1 INSTRUMENT BASED PRODUCTS
13.5.2 PLATFORM BASED PRODUCTS
13.5.3 KITS AND REAGENTS
13.5.4 OTHER CONSUMABLES
14 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET, BY END USER
14.1 OVERVIEW
14.2 HOSPITALS
14.3 DIAGNOSTIC CENTERS
14.4 CANCER RESEARCH CENTERS
14.5 ACADEMIC INSTITUTES
14.6 AMBULATORY SURGICAL CENTERS
14.7 OTHERS
15 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL
15.1 OVERVIEW
15.2 DIRECT TENDER
15.3 RETAIL SALES
15.4 OTHERS
16 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION
16.1 ASIA-PACIFIC
16.1.1 CHINA
16.1.2 JAPAN
16.1.3 INDIA
16.1.4 SOUTH KOREA
16.1.5 AUSTRALIA
16.1.6 TAIWAN
16.1.7 NEW ZEALAND
16.1.8 INDONESIA
16.1.9 THAILAND
16.1.10 PHILIPPINES
16.1.11 MALAYSIA
16.1.12 VIETNAM
16.1.13 SINGAPORE
16.1.14 REST OF ASIA PACIFIC
17 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET: COMPANY LANDSCAPE
17.1 COMPANY SHARE ANALYSIS: ASIA PACIFIC
18 SWOT ANALYSIS
19 COMPANY PROFILE
19.1 CANON MEDICAL SYSTEMS CORPORATION
19.1.1 COMPANY SNAPSHOT
19.1.2 REVENUE ANALYSIS
19.1.3 COMPANY PROFILE
19.1.4 PRODUCT PORTFOLIO
19.1.5 RECENT DEVELOPMENT
19.2 KONINKLIJKE PHILIPS N.V.
19.2.1 COMPANY SNAPSHOT
19.2.2 REVENUE ANALYSIS
19.2.3 COMPANY PROFILE
19.2.4 PRODUCT PORTFOLIO
19.2.5 RECENT DEVELOPMENTS
19.3 GENERAL ELECTRIC COMPANY
19.3.1 COMPANY SNAPSHOT
19.3.2 REVENUE ANALYSIS
19.3.3 COMPANY PROFILE
19.3.4 PRODUCT PORTFOLIO
19.3.5 RECENT DEVELOPMENTS
19.4 SIEMENS HEALTHCARE GMBH
19.4.1 COMPANY SNAPSHOT
19.4.2 REVENUE ANALYSIS
19.4.3 COMPANY PROFILE
19.4.4 PRODUCT PORTFOLIO
19.4.5 RECENT DEVELOPMENT
19.5 GRAIL
19.5.1 COMPANY SNAPSHOT
19.5.2 PRODUCT PORTFOLIO
19.5.3 RECENT DEVELOPMENTS
19.6 AMBRY GENETICS
19.6.1 COMPANY SNAPSHOT
19.6.2 PRODUCT PORTFOLIO
19.6.3 RECENT DEVELOPMENT
19.7 BIOVENDOR R&D
19.7.1 COMPANY SNAPSHOT
19.7.2 PRODUCT PORTFOLIO
19.7.3 RECENT DEVELOPMENT
19.8 BLUEPRINT GENETICS OY.
19.8.1 COMPANY SNAPSHOT
19.8.2 PRODUCT PORTFOLIO
19.8.3 RECENT DEVELOPMENT
19.9 CD GENOMICS
19.9.1 COMPANY SNAPSHOT
19.9.2 PRODUCT PORTFOLIO
19.9.3 RECENT DEVELOPMENT
19.1 CENTOGENE N.V.
19.10.1 COMPANY SNAPSHOT
19.10.2 REVENUE ANALYSIS
19.10.3 PRODUCT PORTFOLIO
19.10.4 RECENT DEVELOPMENT
19.11 CREATIVE DIAGNOSTICS
19.11.1 COMPANY SNAPSHOT
19.11.2 PRODUCT PORTFOLIO
19.11.3 RECENT DEVELOPMENT
19.12 FUJIFILM CORPORATION
19.12.1 COMPANY SNAPSHOT
19.12.2 REVENUE ANALYSIS
19.12.3 PRODUCT PORTFOLIO
19.12.4 RECENT DEVELOPMENTS
19.13 GENEDX, LLC
19.13.1 COMPANY SNAPSHOT
19.13.2 REVENUE ANALYSIS
19.13.3 PRODUCT PORTFOLIO
19.13.4 RECENT DEVELOPMENT
19.14 GENPATH, A DIVISION OF BIOREFERENCE LABORATORIES, AN OPKO HEALTH INC. COMPANY
19.14.1 COMPANY SNAPSHOT
19.14.2 PRODUCT PORTFOLIO
19.14.3 RECENT DEVELOPMENT
19.15 ILLUMINA, INC.
19.15.1 COMPANY SNAPSHOT
19.15.2 REVENUE ANALYSIS
19.15.3 PRODUCT PORTFOLIO
19.15.4 RECENT DEVELOPMENT
19.16 INVITAE CORPORATION
19.16.1 COMPANY SNAPSHOT
19.16.2 REVENUE ANALYSIS
19.16.3 PRODUCT PORTFOLIO
19.16.4 RECENT DEVELOPMENT
19.17 LABORATORY CORPORATION OF AMERICA HOLDINGS
19.17.1 COMPANY SNAPSHOT
19.17.2 REVENUE ANALYSIS
19.17.3 PRODUCT PORTFOLIO
19.17.4 RECENT DEVELOPMENTS
19.18 MYRIAD GENETICS, INC.
19.18.1 COMPANY SNAPSHOT
19.18.2 REVENUE ANALYSIS
19.18.3 PRODUCT PORTFOLIO
19.18.4 RECENT DEVELOPMENT
19.19 THERMO FISHER SCIENTIFIC INC.
19.19.1 COMPANY SNAPSHOT
19.19.2 REVENUE ANALYSIS
19.19.3 PRODUCT PORTFOLIO
19.19.4 RECENT DEVELOPMENT
19.2 QIAGEN
19.20.1 COMPANY SNAPSHOT
19.20.2 REVENUE ANALYSIS
19.20.3 PRODUCT PORTFOLIO
19.20.4 RECENT DEVELOPMENT
20 QUESTIONNAIRE
21 RELATED REPORTS
Lista de Tabela
TABLE 1 ESTIMATED NEW CANCER CASES AND DEATHS
TABLE 2 APPROVED DIAGNOSTICS OF KIDNEY CANCER
TABLE 3 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 4 ASIA PACIFIC IMAGING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 5 ASIA PACIFIC IMAGING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 6 ASIA PACIFIC BLOOD TEST IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 7 ASIA PACIFIC BIOPSY IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 8 ASIA PACIFIC BIOPSY IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 9 ASIA PACIFIC BIOMARKER TEST IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 10 ASIA PACIFIC BIOMARKER TEST IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 11 ASIA PACIFIC GENETIC TEST IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 12 ASIA PACIFIC OTHERS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 13 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 14 ASIA PACIFIC STAGE I IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 15 ASIA PACIFIC STAGE II IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 16 ASIA PACIFIC STAGE III IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 17 ASIA PACIFIC STAGE IV IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 18 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 19 ASIA PACIFIC RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 20 ASIA PACIFIC RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 21 ASIA PACIFIC CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 22 ASIA PACIFIC CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 23 ASIA PACIFIC NON CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 24 ASIA PACIFIC NON CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 25 ASIA PACIFIC PAPILLARY RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 26 ASIA PACIFIC CHROMOPHOBE RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 27 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 28 ASIA PACIFIC INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 29 ASIA PACIFIC INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 30 ASIA PACIFIC INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 31 ASIA PACIFIC INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 32 ASIA PACIFIC PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 33 ASIA PACIFIC PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 34 ASIA PACIFIC PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 35 ASIA PACIFIC PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 36 ASIA PACIFIC KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 37 ASIA PACIFIC KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 38 ASIA PACIFIC KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 39 ASIA PACIFIC KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 40 ASIA PACIFIC OTHER CONSUMABLES IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 41 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 42 ASIA PACIFIC FLUORESCENT IN SITU HYBRIDIZATION IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 43 ASIA PACIFIC NEXT GENERATION SEQUENCING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 44 ASIA PACIFIC FLUORIMMUNOASSAY IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 45 ASIA PACIFIC COMPARATIVE GENOMIC HYBRIDIZATION IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 46 ASIA PACIFIC IMMUNOHISTOCHEMICAL IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 47 ASIA PACIFIC OTHERS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 48 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 49 ASIA PACIFIC SCREENING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 50 ASIA PACIFIC SCREENING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 51 ASIA PACIFIC DIAGNOSTIC AND PREDICTIVE IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 52 ASIA PACIFIC DIAGNOSTIC AND PREDICTIVE IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 53 ASIA PACIFIC PROGNOSTIC IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 54 ASIA PACIFIC PROGNOSTIC IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 55 ASIA PACIFIC RESEARCH IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 56 ASIA PACIFIC RESEARCH IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 57 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 58 ASIA PACIFIC HOSPITALS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 59 ASIA PACIFIC DIAGNOSTIC CENTERS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 60 ASIA PACIFIC CANCER RESEARCH CENTERS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 61 ASIA PACIFIC ACADEMIC INSTITUTES IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 62 ASIA PACIFIC AMBULATORY SURGICAL CENTERS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 63 ASIA PACIFIC OTHERS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 64 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 65 ASIA PACIFIC DIRECT TENDER IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 66 ASIA PACIFIC RETAIL SALES IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 67 ASIA PACIFIC OTHERS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 68 ASIA-PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET, BY COUNTRY, 2021-2030 (USD MILLION)
TABLE 69 ASIA-PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 70 ASIA-PACIFIC IMAGING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 71 ASIA-PACIFIC BIOPSY IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 72 ASIA-PACIFIC BIOMARKER TEST IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 73 ASIA-PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 74 ASIA-PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 75 ASIA-PACIFIC RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 76 ASIA-PACIFIC CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 77 ASIA-PACIFIC NON CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 78 ASIA-PACIFIC PAPILLARY RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 79 ASIA-PACIFIC CHROMOPHOBE RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 80 ASIA-PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 81 ASIA-PACIFIC INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 82 ASIA-PACIFIC INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 83 ASIA-PACIFIC INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 84 ASIA-PACIFIC PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 85 ASIA-PACIFIC PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 86 ASIA-PACIFIC PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 87 ASIA-PACIFIC KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 88 ASIA-PACIFIC KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 89 ASIA-PACIFIC KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 90 ASIA-PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 91 ASIA-PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 92 ASIA-PACIFIC SCREENING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 93 ASIA-PACIFIC DIAGNOSTIC AND PREDICTIVE IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 94 ASIA-PACIFIC PROGNOSTIC IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 95 ASIA-PACIFIC RESEARCH IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 96 ASIA-PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 97 ASIA-PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 98 CHINA KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 99 CHINA IMAGING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 100 CHINA BIOPSY IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 101 CHINA BIOMARKER TEST IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 102 CHINA KIDNEY CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 103 CHINA KIDNEY CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 104 CHINA RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 105 CHINA CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 106 CHINA NON CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 107 CHINA PAPILLARY RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 108 CHINA CHROMOPHOBE RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 109 CHINA KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 110 CHINA INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 111 CHINA INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 112 CHINA INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 113 CHINA PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 114 CHINA PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 115 CHINA PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 116 CHINA KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 117 CHINA KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 118 CHINA KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 119 CHINA KIDNEY CANCER DIAGNOSTICS MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 120 CHINA KIDNEY CANCER DIAGNOSTICS MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 121 CHINA SCREENING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 122 CHINA DIAGNOSTIC AND PREDICTIVE IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 123 CHINA PROGNOSTIC IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 124 CHINA RESEARCH IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 125 CHINA KIDNEY CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 126 CHINA KIDNEY CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 127 JAPAN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 128 JAPAN IMAGING TEST IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 129 JAPAN BIOPSY TEST IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 130 JAPAN BIOMARKER TEST IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 131 JAPAN KIDNEY CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 132 JAPAN KIDNEY CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 133 JAPAN RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 134 JAPAN CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 135 JAPAN NON CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 136 JAPAN PAPILLARY RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 137 JAPAN CHROMOPHOBE RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 138 JAPAN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 139 JAPAN INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 140 JAPAN INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 141 JAPAN INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 142 JAPAN PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 143 JAPAN PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 144 JAPAN PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 145 JAPAN KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 146 JAPAN KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 147 JAPAN KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 148 JAPAN KIDNEY CANCER DIAGNOSTICS MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 149 JAPAN KIDNEY CANCER DIAGNOSTICS MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 150 JAPAN SCREENING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 151 JAPAN DIAGNOSTIC AND PREDICTIVE IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 152 JAPAN PROGNOSTIC IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 153 JAPAN RESEARCH IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 154 JAPAN KIDNEY CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 155 JAPAN KIDNEY CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 156 INDIA KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 157 INDIA IMAGING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 158 INDIA BIOPSY IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 159 INDIA BIOMARKER TEST IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 160 INDIA KIDNEY CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 161 INDIA KIDNEY CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 162 INDIA RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 163 INDIA CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 164 INDIA NON CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 165 INDIA PAPILLARY RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 166 INDIA CHROMOPHOBE RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 167 INDIA KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 168 INDIA INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 169 INDIA INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 170 INDIA INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 171 INDIA PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 172 INDIA PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 173 INDIA PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 174 INDIA KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 175 INDIA KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 176 INDIA KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 177 INDIA KIDNEY CANCER DIAGNOSTICS MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 178 INDIA KIDNEY CANCER DIAGNOSTICS MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 179 INDIA SCREENING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 180 INDIA DIAGNOSTIC AND PREDICTIVE IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 181 INDIA PROGNOSTIC IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 182 INDIA RESEARCH IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 183 INDIA KIDNEY CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 184 INDIA KIDNEY CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 185 SOUTH KOREA KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 186 SOUTH KOREA IMAGING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 187 SOUTH KOREA BIOPSY IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 188 SOUTH KOREA BIOMARKER TEST IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 189 SOUTH KOREA KIDNEY CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 190 SOUTH KOREA KIDNEY CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 191 SOUTH KOREA RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 192 SOUTH KOREA CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 193 SOUTH KOREA NON CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 194 SOUTH KOREA PAPILLARY RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 195 SOUTH KOREA CHROMOPHOBE RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 196 SOUTH KOREA KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 197 SOUTH KOREA INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 198 SOUTH KOREA INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 199 SOUTH KOREA INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 200 SOUTH KOREA PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 201 SOUTH KOREA PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 202 SOUTH KOREA PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 203 SOUTH KOREA KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 204 SOUTH KOREA KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 205 SOUTH KOREA KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 206 SOUTH KOREA KIDNEY CANCER DIAGNOSTICS MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 207 SOUTH KOREA KIDNEY CANCER DIAGNOSTICS MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 208 SOUTH KOREA SCREENING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 209 SOUTH KOREA DIAGNOSTIC AND PREDICTIVE IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 210 SOUTH KOREA PROGNOSTIC IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 211 SOUTH KOREA RESEARCH IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 212 SOUTH KOREA KIDNEY CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 213 SOUTH KOREA KIDNEY CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 214 AUSTRALIA KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 215 AUSTRALIA IMAGING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 216 AUSTRALIA BIOPSY IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 217 AUSTRALIA BIOMAKER IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 218 AUSTRALIA KIDNEY CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 219 AUSTRALIA KIDNEY CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 220 AUSTRALIA RENAL CELL CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 221 AUSTRALIA CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 222 AUSTRALIA NON CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 223 AUSTRALIA PAPILLARY RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 224 AUSTRALIA CHROMOPHOBE RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 225 AUSTRALIA KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 226 AUSTRALIA INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 227 AUSTRALIA INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 228 AUSTRALIA INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 229 AUSTRALIA PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 230 AUSTRALIA PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 231 AUSTRALIA PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 232 AUSTRALIA KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 233 AUSTRALIA KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 234 AUSTRALIA KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 235 AUSTRALIA KIDNEY CANCER DIAGNOSTICS MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 236 AUSTRALIA KIDNEY CANCER DIAGNOSTICS MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 237 AUSTRALIA SCREENING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 238 AUSTRALIA DIAGNOSTIC AND PREDICTIVE IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 239 AUSTRALIA PROGNOSTIC IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 240 AUSTRALIA RESEARCH IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 241 AUSTRALIA KIDNEY CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 242 AUSTRALIA KIDNEY CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 243 TAIWAN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 244 TAIWAN IMAGING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 245 TAIWAN BIOPSY IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 246 TAIWAN BIOMARKER TEST IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 247 TAIWAN KIDNEY CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 248 TAIWAN KIDNEY CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 249 TAIWAN RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 250 TAIWAN CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 251 TAIWAN NON CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 252 TAIWAN PAPILLARY RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 253 TAIWAN CHROMOPHOBE RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 254 TAIWAN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 255 TAIWAN INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 256 TAIWAN INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 257 TAIWAN INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 258 TAIWAN PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 259 TAIWAN PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 260 TAIWAN PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 261 TAIWAN KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 262 TAIWAN KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 263 TAIWAN KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 264 TAIWAN KIDNEY CANCER DIAGNOSTICS MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 265 TAIWAN KIDNEY CANCER DIAGNOSTICS MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 266 TAIWAN SCREENING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 267 TAIWAN DIAGNOSTIC AND PREDICTIVE IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 268 TAIWAN PROGNOSTIC IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 269 TAIWAN RESEARCH IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 270 TAIWAN KIDNEY CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 271 TAIWAN KIDNEY CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 272 NEW ZEALAND KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 273 NEW ZEALAND IMAGING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 274 NEW ZEALAND BIOPSY IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 275 NEW ZEALAND BIOMARKER TEST IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 276 NEW ZEALAND KIDNEY CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 277 NEW ZEALAND KIDNEY CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 278 NEW ZEALAND RENAL CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 279 NEW ZEALAND CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 280 NEW ZEALAND NON CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 281 NEW ZEALAND PAPILLARY RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 282 NEW ZEALAND CHROMOPHOBE RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 283 NEW ZEALAND KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 284 NEW ZEALAND INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 285 NEW ZEALAND INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 286 NEW ZEALAND INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 287 NEW ZEALAND PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 288 NEW ZEALAND PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 289 NEW ZEALAND PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 290 NEW ZEALAND KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 291 NEW ZEALAND KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 292 NEW ZEALAND KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 293 NEW ZEALAND KIDNEY CANCER DIAGNOSTICS MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 294 NEW ZEALAND KIDNEY CANCER DIAGNOSTICS MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 295 NEW ZEALAND SCREENING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 296 NEW ZEALAND DIAGNOSTIC AND PREDICTIVE IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 297 NEW ZEALAND PROGNOSTIC IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 298 NEW ZEALAND RESEARCH IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 299 NEW ZEALAND KIDNEY CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 300 NEW ZEALAND KIDNEY CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 301 INDONESIA KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 302 INDONESIA IMAGING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 303 INDONESIA BIOPSY IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 304 INDONESIA BIOMARKER TEST IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 305 INDONESIA KIDNEY CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 306 INDONESIA KIDNEY CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 307 INDONESIA RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 308 INDONESIA CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 309 INDONESIA NON CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 310 INDONESIA PAPILLARY RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 311 INDONESIA CHROMOPHOBE RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 312 INDONESIA KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 313 INDONESIA INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 314 INDONESIA INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 315 INDONESIA INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 316 INDONESIA PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 317 INDONESIA PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 318 INDONESIA PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 319 INDONESIA KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 320 INDONESIA KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 321 INDONESIA KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 322 INDONESIA KIDNEY CANCER DIAGNOSTICS MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 323 INDONESIA KIDNEY CANCER DIAGNOSTICS MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 324 INDONESIA SCREENING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 325 INDONESIA DIAGNOSTIC AND PREDICTIVE IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 326 INDONESIA PROGNOSTIC IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 327 INDONESIA RESEARCH IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 328 INDONESIA KIDNEY CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 329 INDONESIA KIDNEY CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 330 THAILAND KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 331 THAILAND IMAGING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 332 THAILAND BIOPSY IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 333 THAILAND BIOMARKER TEST IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 334 THAILAND KIDNEY CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 335 THAILAND KIDNEY CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 336 THAILAND RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 337 THAILAND CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 338 THAILAND NON CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 339 THAILAND PAPILLARY RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 340 THAILAND CHROMOPHOBE RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 341 THAILAND KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 342 THAILAND INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 343 THAILAND INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 344 THAILAND INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 345 THAILAND PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 346 THAILAND PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 347 THAILAND PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 348 THAILAND KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 349 THAILAND KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 350 THAILAND KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 351 THAILAND KIDNEY CANCER DIAGNOSTICS MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 352 THAILAND KIDNEY CANCER DIAGNOSTICS MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 353 THAILAND SCREENING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 354 THAILAND DIAGNOSTIC AND PREDICTIVE IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 355 THAILAND PROGNOSTIC IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 356 THAILAND RESEARCH IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 357 THAILAND KIDNEY CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 358 THAILAND KIDNEY CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 359 PHILIPPINES KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 360 PHILIPPINES IMAGING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 361 PHILIPPINES BIOPSY IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 362 PHILIPPINES BIOMARKER TEST IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 363 PHILIPPINES KIDNEY CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 364 PHILIPPINES KIDNEY CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 365 PHILIPPINES RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 366 PHILIPPINES CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 367 PHILIPPINES NON CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 368 PHILIPPINES PAPILLARY RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 369 PHILIPPINES CHROMOPHOBE RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 370 PHILIPPINES KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 371 PHILIPPINES INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 372 PHILIPPINES INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 373 PHILIPPINES INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 374 PHILIPPINES PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 375 PHILIPPINES PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 376 PHILIPPINES PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 377 PHILIPPINES KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 378 PHILIPPINES KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 379 PHILIPPINES KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 380 PHILIPPINES KIDNEY CANCER DIAGNOSTICS MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 381 PHILIPPINES KIDNEY CANCER DIAGNOSTICS MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 382 PHILIPPINES SCREENING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 383 PHILIPPINES DIAGNOSTIC AND PREDICTIVE IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 384 PHILIPPINES PROGNOSTIC IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 385 PHILIPPINES RESEARCH IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 386 PHILIPPINES KIDNEY CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 387 PHILIPPINES KIDNEY CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 388 MALAYSIA KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 389 MALAYSIA IMAGING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 390 MALAYSIA BIOPSY TEST IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 391 MALAYSIA BIOMARKER TEST IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 392 MALAYSIA KIDNEY CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 393 MALAYSIA KIDNEY CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 394 MALAYSIA RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 395 MALAYSIA CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 396 MALAYSIA NON CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 397 MALAYSIA PAPILLARY RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 398 MALAYSIA CHROMOPHOBE RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 399 MALAYSIA KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 400 MALAYSIA INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 401 MALAYSIA INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 402 MALAYSIA INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 403 MALAYSIA PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 404 MALAYSIA PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 405 MALAYSIA PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 406 MALAYSIA KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 407 MALAYSIA KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 408 MALAYSIA KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 409 MALAYSIA KIDNEY CANCER DIAGNOSTICS MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 410 MALAYSIA KIDNEY CANCER DIAGNOSTICS MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 411 MALAYSIA SCREENING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 412 MALAYSIA DIAGNOSTIC AND PREDICTIVE IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 413 MALAYSIA PROGNOSTIC IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 414 MALAYSIA RESEARCH IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 415 MALAYSIA KIDNEY CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 416 MALAYSIA KIDNEY CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 417 VIETNAM KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 418 VIETNAM IMAGING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 419 VIETNAM BIOPSY IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 420 VIETNAM BIOMARKER TEST IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 421 VIETNAM KIDNEY CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 422 VIETNAM KIDNEY CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 423 VIETNAM RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 424 VIETNAM CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 425 VIETNAM NON CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 426 VIETNAM PAPILLARY RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 427 VIETNAM CHROMOPHOBE RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 428 VIETNAM KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 429 VIETNAM INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 430 VIETNAM INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 431 VIETNAM INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 432 VIETNAM PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 433 VIETNAM PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 434 VIETNAM PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 435 VIETNAM KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 436 VIETNAM KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 437 VIETNAM KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 438 VIETNAM KIDNEY CANCER DIAGNOSTICS MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 439 VIETNAM KIDNEY CANCER DIAGNOSTICS MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 440 VIETNAM SCREENING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 441 VIETNAM DIAGNOSTIC AND PREDICTIVE IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 442 VIETNAM PROGNOSTIC IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 443 VIETNAM RESEARCH IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 444 VIETNAM KIDNEY CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 445 VIETNAM KIDNEY CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 446 SINGAPORE KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 447 SINGAPORE IMAGING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 448 SINGAPORE BIOPSY IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 449 SINGAPORE BIOMARKER TEST IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 450 SINGAPORE KIDNEY CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 451 SINGAPORE KIDNEY CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 452 SINGAPORE RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 453 SINGAPORE CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 454 SINGAPORE NON CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 455 SINGAPORE PAPILLARY RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 456 SINGAPORE CHROMOPHOBE RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 457 SINGAPORE KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 458 SINGAPORE INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 459 SINGAPORE INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 460 SINGAPORE INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 461 SINGAPORE PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 462 SINGAPORE PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 463 SINGAPORE PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 464 SINGAPORE KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 465 SINGAPORE KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 466 SINGAPORE KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 467 SINGAPORE KIDNEY CANCER DIAGNOSTICS MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 468 SINGAPORE KIDNEY CANCER DIAGNOSTICS MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 469 SINGAPORE SCREENING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 470 SINGAPORE DIAGNOSTIC AND PREDICTIVE IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 471 SINGAPORE PROGNOSTIC IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 472 SINGAPORE RESEARCH IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 473 SINGAPORE KIDNEY CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 474 SINGAPORE KIDNEY CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 475 REST OF ASIA-PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
Lista de Figura
FIGURE 1 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET: SEGMENTATION
FIGURE 2 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET: DATA TRIANGULATION
FIGURE 3 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET: DROC ANALYSIS
FIGURE 4 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET: ASIA PACIFIC VS REGIONAL MARKET ANALYSIS
FIGURE 5 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET: MARKET APPLICATION COVERAGE GRID
FIGURE 8 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET: DBMR MARKET POSITION GRID
FIGURE 9 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET: SEGMENTATION
FIGURE 11 GROWING AWARENESS OF KIDNEY CANCER AND INCREASING HEALTHCARE EXPENDITURE ARE EXPECTED TO DRIVE THE GROWTH OF THE ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET FROM 2023 TO 2030
FIGURE 12 THE IMAGING TEST SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET IN 2023 & 2030
FIGURE 13 DRIVERS, RESTRAINTS, OPPORTUNITIES AND CHALLENGES OF ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET
FIGURE 14 KIDNEY CANCER INCIDENCE, BOTH SEXES, BY REGION (2020)
FIGURE 15 KIDNEY CANCER MORTALITY, BOTH SEXES, BY REGION (2020)
FIGURE 16 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET : BY TEST TYPE, 2022
FIGURE 17 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET : BY TEST TYPE, 2023-2030 (USD MILLION)
FIGURE 18 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET : BY TEST TYPE, CAGR (2023-2030)
FIGURE 19 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET : BY TEST TYPE, LIFELINE CURVE
FIGURE 20 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET : BY CANCER STAGE , 2022
FIGURE 21 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET : BY CANCER STAGE, 2023-2030 (USD MILLION)
FIGURE 22 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET : BY CANCER STAGE, CAGR (2023-2030)
FIGURE 23 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET : BY CANCER STAGE, LIFELINE CURVE
FIGURE 24 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET : BY TUMOR TYPE, 2022
FIGURE 25 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET : BY TUMOR TYPE, 2023-2030 (USD MILLION)
FIGURE 26 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET : BY TUMOR TYPE, CAGR (2023-2030)
FIGURE 27 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET : BY TUMOR TYPE, LIFELINE CURVE
FIGURE 28 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET : BY PRODUCT, 2022
FIGURE 29 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET : BY PRODUCT, 2023-2030 (USD MILLION)
FIGURE 30 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET : BY PRODUCT, CAGR (2023-2030)
FIGURE 31 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET : BY PRODUCT, LIFELINE CURVE
FIGURE 32 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET : BY TECHNOLOGY, 2022
FIGURE 33 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET : BY TECHNOLOGY, 2023-2030 (USD MILLION)
FIGURE 34 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET : BY TECHNOLOGY, CAGR (2023-2030)
FIGURE 35 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET : BY TECHNOLOGY, LIFELINE CURVE
FIGURE 36 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET : BY APPLICATION, 2022
FIGURE 37 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET : BY APPLICATION, 2023-2030 (USD MILLION)
FIGURE 38 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET : BY APPLICATION, CAGR (2023-2030)
FIGURE 39 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET : BY APPLICATION, LIFELINE CURVE
FIGURE 40 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET : BY END USER, 2022
FIGURE 41 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET : BY END USER, 2023-2030 (USD MILLION)
FIGURE 42 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET : BY END USER, CAGR (2023-2030)
FIGURE 43 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET : BY END USER, LIFELINE CURVE
FIGURE 44 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET : BY DISTRIBUTION CHANNEL, 2022
FIGURE 45 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET : BY DISTRIBUTION CHANNEL, 2023-2030 (USD MILLION)
FIGURE 46 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET : BY DISTRIBUTION CHANNEL, CAGR (2023-2030)
FIGURE 47 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET : BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 48 ASIA-PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET: SNAPSHOT (2022)
FIGURE 49 ASIA-PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET: BY COUNTRY (2022)
FIGURE 50 ASIA-PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET: BY COUNTRY (2023 & 2030)
FIGURE 51 ASIA-PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET: BY COUNTRY (2022 & 2030)
FIGURE 52 ASIA-PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET: TEST TYPE (2023-2030)
FIGURE 53 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET: COMPANY SHARE 2022 (%)

Metodologia de Investigação
A recolha de dados e a análise do ano base são feitas através de módulos de recolha de dados com amostras grandes. A etapa inclui a obtenção de informações de mercado ou dados relacionados através de diversas fontes e estratégias. Inclui examinar e planear antecipadamente todos os dados adquiridos no passado. Da mesma forma, envolve o exame de inconsistências de informação observadas em diferentes fontes de informação. Os dados de mercado são analisados e estimados utilizando modelos estatísticos e coerentes de mercado. Além disso, a análise da quota de mercado e a análise das principais tendências são os principais fatores de sucesso no relatório de mercado. Para saber mais, solicite uma chamada de analista ou abra a sua consulta.
A principal metodologia de investigação utilizada pela equipa de investigação do DBMR é a triangulação de dados que envolve a mineração de dados, a análise do impacto das variáveis de dados no mercado e a validação primária (especialista do setor). Os modelos de dados incluem grelha de posicionamento de fornecedores, análise da linha de tempo do mercado, visão geral e guia de mercado, grelha de posicionamento da empresa, análise de patentes, análise de preços, análise da quota de mercado da empresa, normas de medição, análise global versus regional e de participação dos fornecedores. Para saber mais sobre a metodologia de investigação, faça uma consulta para falar com os nossos especialistas do setor.
Personalização disponível
A Data Bridge Market Research é líder em investigação formativa avançada. Orgulhamo-nos de servir os nossos clientes novos e existentes com dados e análises que correspondem e atendem aos seus objetivos. O relatório pode ser personalizado para incluir análise de tendências de preços de marcas-alvo, compreensão do mercado para países adicionais (solicite a lista de países), dados de resultados de ensaios clínicos, revisão de literatura, mercado remodelado e análise de base de produtos . A análise de mercado dos concorrentes-alvo pode ser analisada desde análises baseadas em tecnologia até estratégias de carteira de mercado. Podemos adicionar quantos concorrentes necessitar de dados no formato e estilo de dados que procura. A nossa equipa de analistas também pode fornecer dados em tabelas dinâmicas de ficheiros Excel em bruto (livro de factos) ou pode ajudá-lo a criar apresentações a partir dos conjuntos de dados disponíveis no relatório.