Asia Pacific Exosome Therapeutic Market
Tamanho do mercado em biliões de dólares
CAGR :
% 
 USD
122.59 Thousand
USD
404.38 Thousand
2021
2029
USD
122.59 Thousand
USD
404.38 Thousand
2021
2029
| 2022 –2029 | |
| USD 122.59 Thousand | |
| USD 404.38 Thousand | |
|
|
|
Asia-Pacific Exosome Therapeutics Market, By Type (Natural Exosome, Hybrid Exosome), Source (Mesenchymal Stem Cells, Blood, Body Fluids, Urine, Dendritic Cells, Saliva, Milk, and Others), Therapy (Immunotherapy, Chemotherapy, and Gene Therapy), Transporting Capacity (Bio Macromolecules and Small Molecules), Application (Metabolic Disorders, Oncology, Cardiac Disorders, Neurology, Inflammatory Disorders, Organ Transplantation, Gynecology Disorders, Blood Disorders, and Others), Route of administration (Parenteral and Oral), End User (Research and Academic Institutes, Hospitals and Diagnostic Centers), Country (South Korea, Australia, Hong-Kong, Rest of Asia-Pacific) Industry Trends and Forecast To 2029
 Market Analysis and Insights: Asia Pacific Exosome Therapeutics Market
Market Analysis and Insights: Asia Pacific Exosome Therapeutics Market
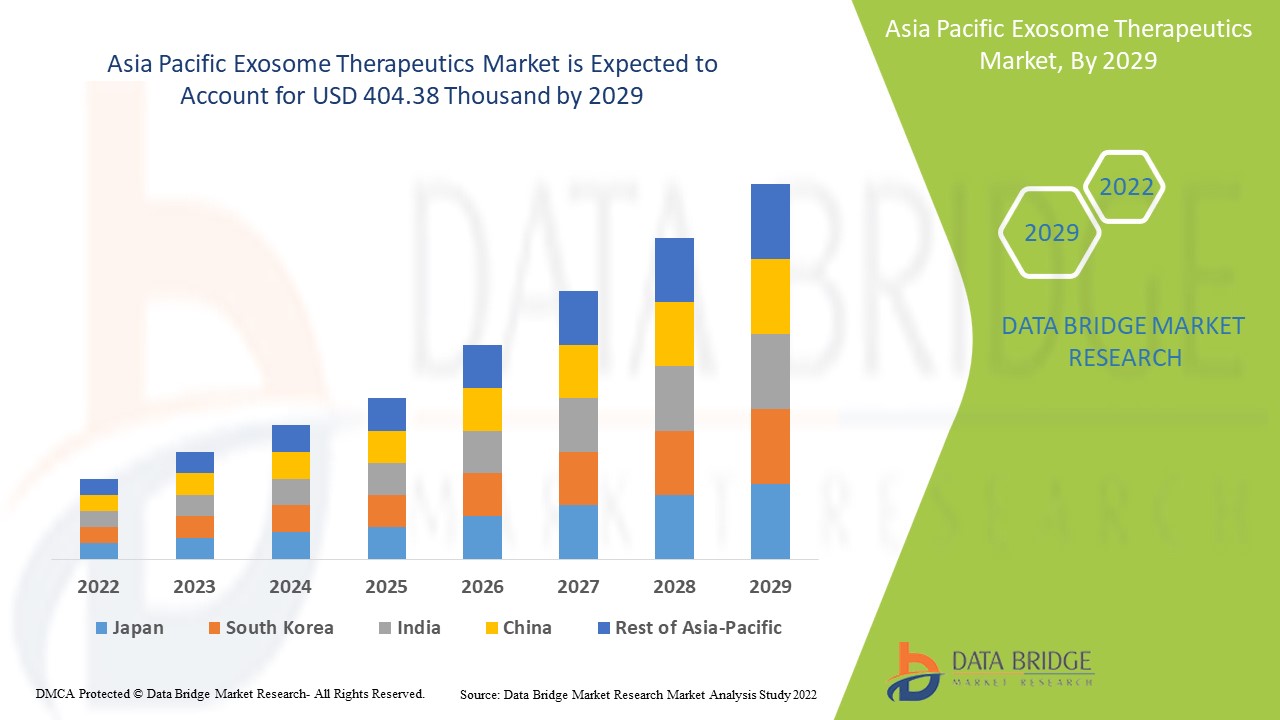
Asia Pacific exosome therapeutics market is expected to gain market growth in the forecast period of 2022 to 2029. Data Bridge Market Research analyses that the market is growing with a CAGR of 16.4% in the forecast period of 2022 to 2029 and is expected to reach USD 404.38 thousand by 2029 from USD 122.59 thousand in 2021. The rising prevalence of chronic inflammatory autoimmune diseases and technological developments in exosome therapeutics are likely to act as the major drivers for the demand of the market in the forecast period.
The exosomes are a specific class of cell-derived extracellular vesicles composed of endosomes and are typically 30-150 nm in diameter – the smallest type of extracellular vesicle. Protected by a lipid bilayer, the exosomes are pushed into the extracellular environment, which contains a complex cargo of contents derived from the original cell. The contents present in the cargo are proteins, lipids, messenger ribonucleic acid (mRNA), microsomal ribonucleic acid (miRNA), and deoxyribonucleic acid (DNA). The exosomes are distinct by how they are formed – through the fusion and exocytosis of multivesicular bodies into the extracellular space. The exosomes have been connected to treat various chronic conditions such as autoimmune disorders. Nanotechnology has shown novel insights for the prior detection of cancer-based on nanocarriers such as exosomes. Since the exosomes provide strong potential for applicability in therapeutic interventions, the exosomes have been regarded as potential drug carriers.
The exosomes consist of two types, the natural exosomes, and the hybrid exosomes. The natural exosomes are further sub-segmented into exogenous exosomes and autologous exosomes. The autologous exosomes are safe and operative vehicles for the targeted delivery of drugs for the treatment of cancer, autoimmune diseases, and chronic inflammatory diseases. The exogenous exosomes are tiny extracellular membrane vesicles released from endosomes of various cells and can be found in most of the body fluids, such as the synovial fluid, amniotic fluid, and semen. In cancer, exosomes have vital roles in the metastatic spread, drug resistance, and the formation of new blood vessels.
The driving factors responsible for the growth of the Asia-Pacific exosome therapeutics market are the increased incidence of chronic inflammatory diseases, the rise in research and development activities for exosome therapeutics, and government funding for the development and production of exosome therapeutics. Moreover, the growth potential in the emerging economies for exosome therapeutics and increased use of anti-aging therapy bolsters the exosome therapeutics market growth. However, the rise in cost, the stringent regulations imposed, and the risks observed while using the exosome therapeutics are the restraints that may hinder the market growth. An increase in healthcare expenditure is expected to provide a lucrative opportunity for market growth. On the other hand, rising investments, coupled with a lack of standardized procedures for isolating exosomes as well as non-availability of required expertise, are some of the significant challenges that are expected to affect the market growth.
The Asia-Pacific exosome therapeutics market report provides details of market share, new developments, and impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, product approvals, strategic decisions, product launches, geographic expansions, and technological innovations in the market. To understand the analysis and the market scenario, contact us for an Analyst Brief. Our team will help you create a revenue impact solution to achieve your desired goal.
 Asia- Pacific Exosome Therapeutics Market Scope and Market Size
Asia- Pacific Exosome Therapeutics Market Scope and Market Size
Asia-Pacific exosome therapeutics market is categorized into seven notable segments which are based on type, source, therapy, transporting capacity, application, route of administration, and end user.
- On the basis of type, the Asia-Pacific exosome therapeutics market is segmented into natural exosomes and hybrid exosomes. In 2022, the natural exosomes segment is expected to dominate the Asia-Pacific exosome therapeutics market due to the presence of natural material of the exosomes and the benefits in drug delivery.
- On the basis of source, the Asia-Pacific exosome therapeutics market is segmented into mesenchymal stem cells, blood, body fluids, urine, dendritic cells, saliva, milk, and others. In 2022, the mesenchymal stem cell segment is expected to dominate the Asia-Pacific exosome therapeutics market due to emerging applications of mesenchymal stem cells and expansion of pipeline, availability, and ease of harvesting.
- On the basis of therapy, the Asia-Pacific exosome therapeutics market is segmented into immunotherapy, gene therapy, and chemotherapy. In 2022, the immunotherapy segment is expected to dominate the Asia-Pacific exosome therapeutics market due to ease of convenience, high accuracy, and improvement in the long-term survival rate.
- On the basis of transporting capacity, the Asia-Pacific exosome therapeutics market is segmented into bio macromolecules and small molecules. In 2022, the bio macromolecules segment is expected to dominate the Asia-Pacific exosome therapeutics market due to the presence of high sensitivity, increased use of protein therapeutics to cure inflammatory disorders, and boosting the natural defenses in the body to combat inflammatory diseases.
- On the basis of application, the Asia-Pacific exosome therapeutics market is segmented into metabolic disorders, oncology, cardiac disorders, neurology, inflammatory disorders, organ transplantation, gynecology disorders, blood disorders, and others. In 2022, the metabolic disorders segment is expected to dominate the Asia-Pacific exosome therapeutics market due to the increase in cases of metabolic disorders in China and India and the availability of exosome therapeutics in diagnostic laboratories.
- On the basis of route of administration, the Asia-Pacific exosome therapeutics market is segmented into parenteral and oral. In 2022, the parenteral segment is expected to dominate the Asia-Pacific exosome therapeutics market due to enhanced bioavailability and immediate onset of action.
- On the basis of end user, the Asia-Pacific exosome therapeutics market is segmented into research and academic institutes, hospitals, and diagnostics centers. In 2022, the research and academic institutes segment is expected to dominate the Asia-Pacific exosome therapeutics market due to the rise in research and development of exosomes in Japan and support from the government in funding.
Asia-Pacific Exosome Therapeutics Market Country Level Analysis
Asia-Pacific exosome therapeutics market is analyzed, and market size information is provided by type, source, therapy, transporting capacity, application, route of administration, and end user.
The countries covered in the exosome therapeutics market report are Japan, China, India, South Korea, Australia, Singapore, Indonesia, the Philippines, and rest of Asia-Pacific.
Asia-Pacific is expected to grow with the highest CAGR in the forecast periods as in the Asia-Pacific's countries demand exosome therapeutics products is increasing very rapidly with the urbanization and laboratory automation. China is expected to dominate in the market in the Asia-Pacific market as China is one of the leading countries to inculcate the exosome therapeutics market.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impact the current and future trends of the market. Data points such as new sales, replacement sales, country demographics, regulatory acts, and import-export tariffs are some of the major pointers used to forecast the market scenario for individual countries. Also, the presence and availability of Asia Pacific brands and their challenges faced due to large or scarce competition from local and domestic brands, the impact of sales channels are considered while providing forecast analysis of the country data.
The Growth Potential For Exosome Therapeutics In Emerging Economies And The Strategic Initiatives By Market Players Are Creating New Opportunities In The Asia-Pacific Exosome Therapeutics Market
Asia Pacific exosome therapeutics market also provides you with detailed market analysis for every country's growth in a particular industry with exosome therapeutics sales, the impact of advancement in the exosome therapeutics, and changes in regulatory scenarios with their support for the exosome therapeutics market. The data is available for the historic period 2011 to 2020.
Competitive Landscape and Asia Pacific Exosome Therapeutics Market Share Analysis
Asia Pacific exosome therapeutics market competitive landscape provides details by a competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, production sites and facilities, company strengths and weaknesses, product launch, product trials pipelines, product approvals, patents, product width, and breadth, application dominance, technology lifeline curve. The above data points provided are only related to the company's focus related to the exosome therapeutics market.
The major company providing Asia- Pacific exosome therapeutics is Exopharm.
DBMR analysts understand competitive strengths and provide competitive analysis for each competitor separately.
The strategic initiatives by market players along with new technological advancements for exosome therapeutics are bridging the gap for the treatment of chronic autoimmune diseases.
For instance,
- In September 2021, Exopharm collaborated with Japan's Showa Denko Materials for the use of LEAP technology in exosome therapeutics. The LEAP technology reduces the major problem faced during the production of exosome s, which are used as regenerative medicine for diseases such as cancer. The collaboration would evaluate Exopharm's LEAP – Ligand-based Exosome Affinity Purification – technology platform within its Yokohama regenerative medicine business unit. It would result in Showa Denko Materials leveraging the exosome technology of Exopharm
Collaboration, joint ventures, and other strategies by the market players are enhancing the company market in the exosome therapeutics market, which also provides the benefit for the organization to improve their offering for the exosome therapeutics market.
SKU-
Obtenha acesso online ao relatório sobre a primeira nuvem de inteligência de mercado do mundo
- Painel interativo de análise de dados
- Painel de análise da empresa para oportunidades de elevado potencial de crescimento
- Acesso de analista de pesquisa para personalização e customização. consultas
- Análise da concorrência com painel interativo
- Últimas notícias, atualizações e atualizações Análise de tendências
- Aproveite o poder da análise de benchmark para um rastreio abrangente da concorrência
Índice
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF THE ASIA PACIFIC EXOSOME THERAPEUTICS MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATIONS
1.6 MARKETS COVERED
2 ASIA PACIFIC EXOSOME THERAPEUTICS MARKET: SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 DBMR TRIPOD DATA VALIDATION MODEL
2.5 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.6 MULTIVARIATE MODELLING
2.7 TYPE SEGMENT LIFELINE CURVE
2.8 DBMR MARKET POSITION GRID
2.9 VENDOR SHARE ANALYSIS
2.1 MARKET END USER COVERAGE GRID
2.11 SECONDARY SOURCES
2.12 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
5 PIPELINE ANALYSIS
6 ASIA PACIFIC EXOSOME THERAPEUTICS MARKET: REGULATIONS
7 MARKET OVERVIEW
7.1 DRIVERS
7.1.1 RISING PREVALENCE OF CHRONIC INFLAMMATION, AUTOIMMUNE DISEASE, LYME DISEASE, AND OTHER CHRONIC DEGENERATIVE DISEASES
7.1.2 RISE IN INCIDENCE OF ONCOLOGY DISEASES
7.1.3 TECHNOLOGICAL ADVANCEMENTS IN EXOSOME THERAPEUTICS
7.1.4 RISE IN RESEARCH AND DEVELOPMENT ACTIVITIES, INVOLVED IN EXOSOME THERAPEUTICS
7.1.5 GOVERNMENT FUNDING FOR THE DEVELOPMENT AND PRODUCTION OF EXOSOME THERAPEUTICS
7.2 RESTRAINTS
7.2.1 HIGH COST ASSOCIATED WITH THE EXOSOME THERAPEUTICS
7.2.2 LACK OF AUTHENTICATION REQUIREMENTS FOR ISOLATION OF EXOSOMES
7.2.3 RISKS OBSERVED WHILE USING EXOSOME THERAPEUTICS
7.2.4 UNMET MEDICAL NEEDS
7.3 OPPORTUNITIES
7.3.1 INCREASE USE OF ANTI-AGING THERAPY
7.3.2 STRATEGIC INITIATIVES BY MARKET PLAYERS
7.3.3 RISE IN HEALTHCARE EXPENDITURE
7.3.4 AVAILABILITY OF VARIOUS EXOSOME ISOLATION AND PURIFICATION TECHNIQUES
7.3.5 PROGRESSING THERAPEUTIC VALUE OF EXOSOME
7.4 CHALLENGES
7.4.1 THE SHORTAGE OF SKILLED PROFESSIONALS REQUIRED FOR THE ISOLATION OF EXOSOME
7.4.2 LATE APPROVAL ASSOCIATED WITH PRODUCT LAUNCHES
8 IMPACT OF COVID-19 ON ASIA PACIFIC EXOSOME THERAPEUTICS MARKET
8.1 IMPACT ON PRICE
8.2 IMPACT ON DEMAND
8.3 IMPACT ON SUPPLY CHAIN
8.4 STRATEGIC DECISIONS BY MANUFACTURERS
8.5 CONCLUSION
9 ASIA PACIFIC EXOSOME THERAPEUTICS MARKET, BY TYPE
9.1 OVERVIEW
9.2 NATURAL EXOSOMES
9.2.1 AUTOLOGOUS EXOSOMES
9.2.2 EXOGENOUS EXOSOMES
9.3 HYBRID EXOSOMES
10 ASIA PACIFIC EXOSOME THERAPEUTICS MARKET, BY SOURCE
10.1 OVERVIEW
10.2 MESENCHYMAL STEM CELLS
10.3 BLOOD
10.3.1 T-LYMPHOCYTES
10.3.2 OTHERS
10.4 BODY FLUIDS
10.4.1 AMNIOTIC FLUID
10.4.2 SEMEN
10.4.3 SYNOVIAL FLUID
10.4.4 OTHERS
10.5 URINE
10.6 DENDRITIC CELLS
10.7 SALIVA
10.8 MILK
10.9 OTHERS
11 ASIA PACIFIC EXOSOME THERAPEUTICS MARKET, BY THERAPY
11.1 OVERVIEW
11.2 IMMUNOTHERAPY
11.3 GENE THERAPY
11.4 CHEMOTHERAPY
12 ASIA PACIFIC EXOSOME THERAPEUTICS MARKET, BY TRANSPORTING CAPACITY
12.1 OVERVIEW
12.2 BIO MACROMOLECULES
12.2.1 NUCLEIC ACIDS
12.2.2 PROTEINS
12.2.3 PEPTIDES
12.3 SMALL MOLECULES
13 ASIA PACIFIC EXOSOME THERAPEUTICS MARKET, BY APPLICATION
13.1 OVERVIEW
13.2 METABOLIC DISORDERS
13.3 ONCOLOGY
13.3.1 NON-SMALL CELL LUNG CANCER
13.3.2 BREAST CANCER
13.3.3 GASTRIC CANCER
13.3.4 HEAD AND NECK CANCER
13.3.5 OTHERS
13.4 CARDIAC DISORDERS
13.5 NEUROLOGY
13.5.1 ALZHEIMER'S DISEASE
13.5.2 PARKINSON'S DISEASE
13.5.3 OTHERS
13.6 INFLAMMATORY DISORDERS
13.7 ORGAN TRANSPLANTATION
13.8 GYNECOLOGY DISORDERS
13.9 BLOOD DISORDERS
13.1 OTHERS
14 ASIA PACIFIC EXOSOME THERAPEUTICS MARKET, BY ROUTE OF ADMINISTRATION
14.1 OVERVIEW
14.2 PARENTERAL
14.3 ORAL
15 ASIA PACIFIC EXOSOME THERAPEUTICS MARKET, BY END USER
15.1 OVERVIEW
15.2 RESEARCH AND ACADEMIC INSTITUTES
15.3 HOSPITALS
15.4 DIAGNOSTIC CENTERS
16 ASIA PACIFIC EXOSOME THERAPEUTICS MARKET, BY REGION
16.1 ASIA-PACIFIC
16.1.1 SOUTH KOREA
16.1.2 AUSTRALIA
16.1.3 HONG-KONG
16.1.4 REST OF ASIA-PACIFIC
17 ASIA PACIFIC EXOSOME THERAPEUTICS MARKET: COMPANY LANDSCAPE
17.1 COMPANY SHARE ANALYSIS: ASIA PACIFIC
18 COMPANY PROFILE
18.1 KIMERA LABS
18.1.1 COMPANY SNAPSHOT
18.1.2 COMPANY SHARE ANALYSIS
18.1.3 PRODUCT PORTFOLIO
18.1.4 RECENT DEVELOPMENTS
18.2 STEM CELLS GROUP
18.2.1 COMPANY SNAPSHOT
18.2.2 COMPANY SHARE ANALYSIS
18.2.3 PRODUCT PORTFOLIO
18.2.4 RECENT DEVELOPMENTS
18.3 AEGLE THERAPEUTICS
18.3.1 COMPANY SNAPSHOT
18.3.2 PRODUCT PORTFOLIO
18.3.3 RECENT DEVELOPMENTS
18.4 AVALON GLOBOCARE CORP.(2021)
18.4.1 COMPANY SNAPSHOT
18.4.2 REVENUE ANALYSIS
18.4.3 PRODUCT PORTFOLIO
18.4.4 RECENT DEVELOPMENTS
18.5 CAPRICOR THERAPEUTICS (2021)
18.5.1 COMPANY SNAPSHOT
18.5.2 REVENUE ANALYSIS
18.5.3 PRODUCT PORTFOLIO
18.5.4 RECENT DEVELOPMENTS
18.6 CODIAK (2021)
18.6.1 COMPANY SNAPSHOT
18.6.2 REVENUE ANALYSIS
18.6.3 PRODUCT PORTFOLIO
18.6.4 RECENT DEVELOPMENTS
18.7 EXOSOME SCIENCES (A SUBSIDIARY OF AETHLON MEDICAL) (2021)
18.7.1 COMPANY SNAPSHOT
18.7.2 REVENUE ANALYSIS
18.7.3 PRODUCT PORTFOLIO
18.7.4 RECENT DEVELOPMENTS NO RECENT DEVELOPMENTS
18.8 EXOPHARM
18.8.1 COMPANY SNAPSHOT
18.8.2 PRODUCT PORTFOLIO
18.8.3 RECENT DEVELOPMENTS
18.9 EVOX THERAPEUTICS
18.9.1 COMPANY SNAPSHOT
18.9.2 PRODUCT PORTFOLIO
18.9.3 RECENT DEVELOPMENTS
18.1 EV THERAPEUTICS
18.10.1 COMPANY SNAPSHOT
18.10.2 PRODUCT PORTFOLIO
18.10.3 RECENT DEVELOPMENTS
18.11 JAZZ PHARMACEUTICALS, INC
18.11.1 COMPANY SNAPSHOT
18.11.2 REVENUE ANALYSIS
18.11.3 PRODUCT PORTFOLIO
18.11.4 RECENT DEVELOPMENTS
18.12 RENEURON GROUP PLC
18.12.1 COMPANY SNAPSHOT
18.12.2 REVENUE ANALYSIS
18.12.3 PRODUCT PORTFOLIO
18.12.4 RECENT DEVELOPMENTS
18.13 STEM CELL MEDICINE
18.13.1 COMPANY SNAPSHOT
18.13.2 PRODUCT PORTFOLIO
18.13.3 RECENT DEVELOPMENTS
19 QUESTIONNAIRE
20 RELATED REPORTS
Lista de Tabela
TABLE 1 ASIA PACIFIC EXOSOME THERAPEUTICSMARKET, PIPELINE ANALYSIS
TABLE 2 ASIA PACIFIC EXOSOME THERAPEUTICS MARKET, BY TYPE, 2020-2029 (USD THOUSAND)
TABLE 3 ASIA PACIFIC NATURAL EXOSOMES IN EXOSOME THERAPEUTICS MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 4 ASIA PACIFIC NATURAL EXOSOMES IN EXOSOME THERAPEUTICS MARKET, BY TYPE, 2020-2029 (USD THOUSAND)
TABLE 5 ASIA PACIFIC HYBRID EXOSOMES IN EXOSOME THERAPEUTICS MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 6 ASIA PACIFIC EXOSOME THERAPEUTICS MARKET, BY SOURCE, 2020-2029 (USD THOUSAND)
TABLE 7 ASIA PACIFIC MESENCHYMAL STEM CELLS IN EXOSOME THERAPEUTICS MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 8 ASIA PACIFIC BLOOD IN EXOSOME THERAPEUTICS MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 9 ASIA PACIFIC BLOOD IN EXOSOME THERAPEUTICS MARKET, BY SOURCE, 2020-2029 (USD THOUSAND)
TABLE 10 ASIA PACIFIC BODY FLUIDS IN EXOSOME THERAPEUTICS MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 11 ASIA PACIFIC BLOOD IN EXOSOME THERAPEUTICS MARKET, BY SOURCE, 2020-2029 (USD THOUSAND)
TABLE 12 ASIA PACIFIC URINE IN EXOSOME THERAPEUTICS MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 13 ASIA PACIFIC DENDRITIC CELLS IN EXOSOME THERAPEUTICS MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 14 ASIA PACIFIC SALIVA IN EXOSOME THERAPEUTICS MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 15 ASIA PACIFIC MILK IN EXOSOME THERAPEUTICS MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 16 ASIA PACIFIC OTHERS IN EXOSOME THERAPEUTICS MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 17 ASIA PACIFIC EXOSOME THERAPEUTICS MARKET, BY THERAPY, 2020-2029 (USD THOUSAND)
TABLE 18 ASIA PACIFIC IMMUNOTHERAPY IN EXOSOME THERAPEUTICS MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 19 ASIA PACIFIC GENE THERAPY IN EXOSOME THERAPEUTICS MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 20 ASIA PACIFIC CHEMOTHERAPY IN EXOSOME THERAPEUTICS MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 21 ASIA PACIFIC EXOSOME THERAPEUTICS MARKET, BY TRANSPORTING CAPACITY, 2020-2029 (USD THOUSAND)
TABLE 22 ASIA PACIFIC BIO MACROMOLECULES IN EXOSOME THERAPEUTICS MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 23 ASIA PACIFIC BIO MACROMOLECULES IN EXOSOME THERAPEUTICS MARKET, BY TRANSPORTING CAPACITY, 2020-2029 (USD THOUSAND)
TABLE 24 ASIA PACIFIC SMALL MOLECULES IN EXOSOME THERAPEUTICS MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 25 ASIA PACIFIC EXOSOME THERAPEUTICS MARKET, BY APPLICATION, 2020-2029 (USD THOUSAND)
TABLE 26 ASIA PACIFIC METABOLIC DISORDERS IN EXOSOME THERAPEUTICS MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 27 ASIA PACIFIC ONCOLOGY IN EXOSOME THERAPEUTICS MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 28 ASIA PACIFIC ONCOLOGY IN ASIA PACIFIC EXOSOME THERAPEUTICS MARKET, BY APPLICATION, 2020-2029 (USD THOUSAND)
TABLE 29 ASIA PACIFIC CARDIAC DISORDERS IN EXOSOME THERAPEUTICS MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 30 ASIA PACIFIC NEUROLOGY IN EXOSOME THERAPEUTICS MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 31 ASIA PACIFIC NEUROLOGY IN EXOSOME THERAPEUTICS MARKET, BY APPLICATION, 2020-2029 (USD THOUSAND)
TABLE 32 ASIA PACIFIC INFLAMMATORY DISORDERS IN EXOSOME THERAPEUTICS MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 33 ASIA PACIFIC ORGAN TRANSPLANTATION IN EXOSOME THERAPEUTICS MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 34 ASIA PACIFIC GYNECOLOGY DISORDERS IN EXOSOME THERAPEUTICS MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 35 ASIA PACIFIC BLOOD DISORDERS IN EXOSOME THERAPEUTICS MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 36 ASIA PACIFIC OTHERS IN EXOSOME THERAPEUTICS MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 37 ASIA PACIFIC EXOSOME THERAPEUTICS MARKET, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD THOUSAND)
TABLE 38 ASIA PACIFIC PARENTERAL IN EXOSOME THERAPEUTICS MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 39 ASIA PACIFIC ORAL IN EXOSOME THERAPEUTICS MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 40 ASIA PACIFIC EXOSOME THERAPEUTICS MARKET, BY END USER, 2020-2029 (USD THOUSAND)
TABLE 41 ASIA PACIFIC RESEARCH AND ACADEMIC INSTITUTES IN EXOSOME THERAPEUTICS MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 42 ASIA PACIFIC HOSPITALS IN EXOSOME THERAPEUTICS MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 43 ASIA PACIFIC DIAGNOSTIC CENTERS IN EXOSOME THERAPEUTICS MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 44 ASIA-PACIFIC EXOSOME THERAPEUTICS MARKET, BY COUNTRY, 2019-2029 (USD THOUSAND)
TABLE 45 ASIA-PACIFIC EXOSOME THERAPEUTICS MARKET, BY TYPE, 2020-2029 (USD THOUSAND)
TABLE 46 ASIA-PACIFIC NATURAL EXOSOME IN EXOSOME THERAPEUTICS MARKET, BY TYPE, 2020-2029 (USD THOUSAND)
TABLE 47 ASIA-PACIFIC EXOSOME THERAPEUTICS MARKET, BY SOURCE, 2020-2029 (USD THOUSAND)
TABLE 48 ASIA-PACIFIC BLOOD IN EXOSOME THERAPEUTICS MARKET, BY SOURCE, 2020-2029 (USD THOUSAND)
TABLE 49 ASIA-PACIFIC BODY FLUIDS IN EXOSOME THERAPEUTICS MARKET, BY SOURCE, 2020-2029 (USD THOUSAND)
TABLE 50 ASIA-PACIFIC EXOSOME THERAPEUTICS MARKET, BY THERAPY, 2020-2029 (USD THOUSAND)
TABLE 51 ASIA-PACIFIC EXOSOME THERAPEUTICS MARKET, BY TRANSPORTING CAPACITY, 2020-2029 (USD THOUSAND)
TABLE 52 ASIA-PACIFIC BIO MACROMOLECULES IN EXOSOME THERAPEUTICS MARKET, BY TRANSPORTING CAPACITY, 2020-2029 (USD THOUSAND)
TABLE 53 ASIA-PACIFIC EXOSOME THERAPEUTICS MARKET, BY APPLICATION, 2020-2029 (USD THOUSAND)
TABLE 54 ASIA-PACIFIC ONCOLOGY IN EXOSOME THERAPEUTICS MARKET, BY APPLICATION, 2020-2029 (USD THOUSAND)
TABLE 55 ASIA-PACIFIC NEUROLOGY IN EXOSOME THERAPEUTICS MARKET, BY APPLICATION, 2020-2029 (USD THOUSAND)
TABLE 56 ASIA-PACIFIC EXOSOME THERAPEUTICS MARKET, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD THOUSAND)
TABLE 57 ASIA-PACIFIC EXOSOME THERAPEUTICS MARKET, BY END USER, 2020-2029 (USD THOUSAND)
TABLE 58 SOUTH KOREA EXOSOME THERAPEUTICS MARKET, BY TYPE, 2020-2029 (USD THOUSAND)
TABLE 59 SOUTH KOREA NATURAL EXOSOME IN EXOSOME THERAPEUTICS MARKET, BY TYPE, 2020-2029 (USD THOUSAND)
TABLE 60 SOUTH KOREA EXOSOME THERAPEUTICS MARKET, BY SOURCE, 2020-2029 (USD THOUSAND)
TABLE 61 SOUTH KOREA BLOOD IN EXOSOME THERAPEUTICS MARKET, BY SOURCE, 2020-2029 (USD THOUSAND)
TABLE 62 SOUTH KOREA BODY FLUIDS IN EXOSOME THERAPEUTICS MARKET, BY SOURCE, 2020-2029 (USD THOUSAND)
TABLE 63 SOUTH KOREA EXOSOME THERAPEUTICS MARKET, BY THERAPY, 2020-2029 (USD THOUSAND)
TABLE 64 SOUTH KOREA EXOSOME THERAPEUTICS MARKET, BY TRANSPORTING CAPACITY, 2020-2029 (USD THOUSAND)
TABLE 65 SOUTH KOREA BIO MACROMOLECULES IN EXOSOME THERAPEUTICS MARKET, BY TRANSPORTING CAPACITY, 2020-2029 (USD THOUSAND)
TABLE 66 SOUTH KOREA EXOSOME THERAPEUTICS MARKET, BY APPLICATION, 2020-2029 (USD THOUSAND)
TABLE 67 SOUTH KOREA ONCOLOGY IN EXOSOME THERAPEUTICS MARKET, BY APPLICATION, 2020-2029 (USD THOUSAND)
TABLE 68 SOUTH KOREA NEUROLOGY IN EXOSOME THERAPEUTICS MARKET, BY APPLICATION, 2020-2029 (USD THOUSAND)
TABLE 69 SOUTH KOREA EXOSOME THERAPEUTICS MARKET, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD THOUSAND)
TABLE 70 SOUTH KOREA EXOSOME THERAPEUTICS MARKET, BY END USER, 2020-2029 (USD THOUSAND)
TABLE 71 AUSTRALIA EXOSOME THERAPEUTICS MARKET, BY TYPE, 2020-2029 (USD THOUSAND)
TABLE 72 AUSTRALIA NATURAL EXOSOME IN EXOSOME THERAPEUTICS MARKET, BY TYPE, 2020-2029 (USD THOUSAND)
TABLE 73 AUSTRALIA EXOSOME THERAPEUTICS MARKET, BY SOURCE, 2020-2029 (USD THOUSAND)
TABLE 74 AUSTRALIA BLOOD IN EXOSOME THERAPEUTICS MARKET, BY SOURCE, 2020-2029 (USD THOUSAND)
TABLE 75 AUSTRALIA BODY FLUIDS IN EXOSOME THERAPEUTICS MARKET, BY SOURCE, 2020-2029 (USD THOUSAND)
TABLE 76 AUSTRALIA EXOSOME THERAPEUTICS MARKET, BY THERAPY, 2020-2029 (USD THOUSAND)
TABLE 77 AUSTRALIA EXOSOME THERAPEUTICS MARKET, BY TRANSPORTING CAPACITY, 2020-2029 (USD THOUSAND)
TABLE 78 AUSTRALIA BIO MACROMOLECULES IN EXOSOME THERAPEUTICS MARKET, BY TRANSPORTING CAPACITY, 2020-2029 (USD THOUSAND)
TABLE 79 AUSTRALIA EXOSOME THERAPEUTICS MARKET, BY APPLICATION, 2020-2029 (USD THOUSAND)
TABLE 80 AUSTRALIA ONCOLOGY IN EXOSOME THERAPEUTICS MARKET, BY APPLICATION, 2020-2029 (USD THOUSAND)
TABLE 81 AUSTRALIA NEUROLOGY IN EXOSOME THERAPEUTICS MARKET, BY APPLICATION, 2020-2029 (USD THOUSAND)
TABLE 82 AUSTRALIA EXOSOME THERAPEUTICS MARKET, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD THOUSAND)
TABLE 83 AUSTRALIA EXOSOME THERAPEUTICS MARKET, BY END USER, 2020-2029 (USD THOUSAND)
TABLE 84 HONG-KONG EXOSOME THERAPEUTICS MARKET, BY TYPE, 2020-2029 (USD THOUSAND)
TABLE 85 HONG-KONG NATURAL EXOSOME IN EXOSOME THERAPEUTICS MARKET, BY TYPE, 2020-2029 (USD THOUSAND)
TABLE 86 HONG-KONG EXOSOME THERAPEUTICS MARKET, BY SOURCE, 2020-2029 (USD THOUSAND)
TABLE 87 HONG-KONG BLOOD IN EXOSOME THERAPEUTICS MARKET, BY SOURCE, 2020-2029 (USD THOUSAND)
TABLE 88 HONG-KONG BODY FLUIDS IN EXOSOME THERAPEUTICS MARKET, BY SOURCE, 2020-2029 (USD THOUSAND)
TABLE 89 HONG-KONG EXOSOME THERAPEUTICS MARKET, BY THERAPY, 2020-2029 (USD THOUSAND)
TABLE 90 HONG-KONG EXOSOME THERAPEUTICS MARKET, BY TRANSPORTING CAPACITY, 2020-2029 (USD THOUSAND)
TABLE 91 HONG-KONG BIO MACROMOLECULES IN EXOSOME THERAPEUTICS MARKET, BY TRANSPORTING CAPACITY, 2020-2029 (USD THOUSAND)
TABLE 92 HONG-KONG EXOSOME THERAPEUTICS MARKET, BY APPLICATION, 2020-2029 (USD THOUSAND)
TABLE 93 HONG-KONG ONCOLOGY IN EXOSOME THERAPEUTICS MARKET, BY APPLICATION, 2020-2029 (USD THOUSAND)
TABLE 94 HONG-KONG NEUROLOGY IN EXOSOME THERAPEUTICS MARKET, BY APPLICATION, 2020-2029 (USD THOUSAND)
TABLE 95 HONG-KONG EXOSOME THERAPEUTICS MARKET, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD THOUSAND)
TABLE 96 HONG-KONG EXOSOME THERAPEUTICS MARKET, BY END USER, 2020-2029 (USD THOUSAND)
TABLE 97 REST OF ASIA-PACIFIC EXOSOME THERAPEUTICS MARKET, BY TYPE, 2020-2029 (USD THOUSAND)
Lista de Figura
FIGURE 1 ASIA PACIFIC EXOSOME THERAPEUTICS MARKET: SEGMENTATION
FIGURE 2 ASIA PACIFIC EXOSOME THERAPEUTICS MARKET: DATA TRIANGULATION
FIGURE 3 ASIA PACIFIC EXOSOME THERAPEUTICS MARKET: DROC ANALYSIS
FIGURE 4 ASIA PACIFIC EXOSOME THERAPEUTICS MARKET: ASIA PACIFIC VS REGIONAL MARKET ANALYSIS
FIGURE 5 ASIA PACIFIC EXOSOME THERAPEUTICS MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 ASIA PACIFIC EXOSOME THERAPEUTICS MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 ASIA PACIFIC EXOSOME THERAPEUTICS MARKET: DBMR POSITION GRID
FIGURE 8 ASIA PACIFIC EXOSOME THERAPEUTICS MARKET: VENDOR SHARE ANALYSIS
FIGURE 9 ASIA PACIFIC EXOSOME THERAPEUTICS MARKET: END USER COVERAGE GRID
FIGURE 10 ASIA PACIFIC EXOSOME THERAPEUTICS MARKET: SEGMENTATION
FIGURE 11 THE INCREASED PREVALENCE OF AUTOIMMUNE DISORDERS, LYME DISEASES, INCIDENCE OF CANCER AND RISE IN TECHNOLOGICAL ADVANCEMENTS IS EXPECTED TO DRIVE THE ASIA PACIFIC EXOSOME THERAPEUTICS MARKET FROM 2022 TO 2029
FIGURE 12 TYPE SEGMENT IS EXPECTED TO HAVE THE LARGEST SHARE OF THE ASIA PACIFIC EXOSOME THERAPEUTICS MARKET IN 2022 & 2029
FIGURE 13 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF ASIA PACIFIC EXOSOME THERAPEUTICS MARKET
FIGURE 14 THE PREVALENCE OF MULTIPLE SCLEROSIS IN WORLD HEALTH ORGANISATION (WHO) REGIONS IN 2020.
FIGURE 15 INCIDENCE RATE OF CANCER IN AUSTRALIA AND OTHER COUNTRIES (2020)
FIGURE 16 ASIA PACIFIC EXOSOME THERAPEUTICS MARKET: BY TYPE, 2021
FIGURE 17 ASIA PACIFIC EXOSOME THERAPEUTICS MARKET: BY TYPE, 2020-2029 (USD THOUSAND)
FIGURE 18 ASIA PACIFIC EXOSOME THERAPEUTICS MARKET: BY TYPE, CAGR (2022-2029)
FIGURE 19 ASIA PACIFIC EXOSOME THERAPEUTICS MARKET: BY TYPE, LIFELINE CURVE
FIGURE 20 ASIA PACIFIC EXOSOME THERAPEUTICS MARKET: BY SOURCE, 2021
FIGURE 21 ASIA PACIFIC EXOSOME THERAPEUTICS MARKET: BY SOURCE, 2020-2029 (USD THOUSAND)
FIGURE 22 ASIA PACIFIC EXOSOME THERAPEUTICS MARKET: BY SOURCE, CAGR (2022-2029)
FIGURE 23 ASIA PACIFIC EXOSOME THERAPEUTICS MARKET: BY SOURCE, LIFELINE CURVE
FIGURE 24 ASIA PACIFIC EXOSOME THERAPEUTICS MARKET: BY THERAPY, 2021
FIGURE 25 ASIA PACIFIC EXOSOME THERAPEUTICS MARKET: BY THERAPY, 2020-2029 (USD THOUSAND)
FIGURE 26 ASIA PACIFIC EXOSOME THERAPEUTICS MARKET: BY THERAPY, CAGR (2022-2029)
FIGURE 27 ASIA PACIFIC EXOSOME THERAPEUTICS MARKET: BY THERAPY, LIFELINE CURVE
FIGURE 28 ASIA PACIFIC EXOSOME THERAPEUTICS MARKET: BY TRANSPORTING CAPACITY, 2021
FIGURE 29 ASIA PACIFIC EXOSOME THERAPEUTICS MARKET: BY TRANSPORTING CAPACITY, 2020-2029 (USD THOUSAND)
FIGURE 30 ASIA PACIFIC EXOSOME THERAPEUTICS MARKET: BY TRANSPORTING CAPACITY, CAGR (2022-2029)
FIGURE 31 ASIA PACIFIC EXOSOME THERAPEUTICS MARKET: BY TRANSPORTING CAPACITY, LIFELINE CURVE
FIGURE 32 ASIA PACIFIC EXOSOME THERAPEUTICS MARKET: BY APPLICATION, 2021
FIGURE 33 ASIA PACIFIC EXOSOME THERAPEUTICS MARKET: BY APPLICATION, 2020-2029 (USD THOUSAND)
FIGURE 34 ASIA PACIFIC EXOSOME THERAPEUTICS MARKET: BY APPLICATION, CAGR (2022-2029)
FIGURE 35 ASIA PACIFIC EXOSOME THERAPEUTICS MARKET: BY APPLICATION, LIFELINE CURVE
FIGURE 36 ASIA PACIFIC EXOSOME THERAPEUTICS MARKET: BY ROUTE OF ADMINISTRATION, 2021
FIGURE 37 ASIA PACIFIC EXOSOME THERAPEUTICS MARKET: BY ROUTE OF ADMINISTRATION, 2020-2029 (USD THOUSAND)
FIGURE 38 ASIA PACIFIC EXOSOME THERAPEUTICS MARKET: BY ROUTE OF ADMINISTRATION, CAGR (2022-2029)
FIGURE 39 ASIA PACIFIC EXOSOME THERAPEUTICS MARKET: BY ROUTE OF ADMINISTRATION, LIFELINE CURVE
FIGURE 40 ASIA PACIFIC EXOSOME THERAPEUTICS MARKET: BY END USER, 2021
FIGURE 41 ASIA PACIFIC EXOSOME THERAPEUTICS MARKET: BY END USER, 2020-2029 (USD THOUSAND)
FIGURE 42 ASIA PACIFIC EXOSOME THERAPEUTICS MARKET: BY END USER, CAGR (2022-2029)
FIGURE 43 ASIA PACIFIC EXOSOME THERAPEUTICS MARKET: BY END USER, LIFELINE CURVE
FIGURE 44 ASIA-PACIFIC EXOSOME THERAPEUTICS MARKET: SNAPSHOT (2021)
FIGURE 45 ASIA-PACIFIC EXOSOME THERAPEUTICS MARKET: BY COUNTRY (2021)
FIGURE 46 ASIA-PACIFIC EXOSOME THERAPEUTICS MARKET: BY COUNTRY (2022 & 2029)
FIGURE 47 ASIA-PACIFIC EXOSOME THERAPEUTICS MARKET: BY COUNTRY (2021 & 2029)
FIGURE 48 ASIA-PACIFIC EXOSOME THERAPEUTICS MARKET: BY TYPE (2022-2029)
FIGURE 49 ASIA PACIFIC EXOSOME THERAPEUTICS MARKET: COMPANY SHARE 2021 (%)

Metodologia de Investigação
A recolha de dados e a análise do ano base são feitas através de módulos de recolha de dados com amostras grandes. A etapa inclui a obtenção de informações de mercado ou dados relacionados através de diversas fontes e estratégias. Inclui examinar e planear antecipadamente todos os dados adquiridos no passado. Da mesma forma, envolve o exame de inconsistências de informação observadas em diferentes fontes de informação. Os dados de mercado são analisados e estimados utilizando modelos estatísticos e coerentes de mercado. Além disso, a análise da quota de mercado e a análise das principais tendências são os principais fatores de sucesso no relatório de mercado. Para saber mais, solicite uma chamada de analista ou abra a sua consulta.
A principal metodologia de investigação utilizada pela equipa de investigação do DBMR é a triangulação de dados que envolve a mineração de dados, a análise do impacto das variáveis de dados no mercado e a validação primária (especialista do setor). Os modelos de dados incluem grelha de posicionamento de fornecedores, análise da linha de tempo do mercado, visão geral e guia de mercado, grelha de posicionamento da empresa, análise de patentes, análise de preços, análise da quota de mercado da empresa, normas de medição, análise global versus regional e de participação dos fornecedores. Para saber mais sobre a metodologia de investigação, faça uma consulta para falar com os nossos especialistas do setor.
Personalização disponível
A Data Bridge Market Research é líder em investigação formativa avançada. Orgulhamo-nos de servir os nossos clientes novos e existentes com dados e análises que correspondem e atendem aos seus objetivos. O relatório pode ser personalizado para incluir análise de tendências de preços de marcas-alvo, compreensão do mercado para países adicionais (solicite a lista de países), dados de resultados de ensaios clínicos, revisão de literatura, mercado remodelado e análise de base de produtos . A análise de mercado dos concorrentes-alvo pode ser analisada desde análises baseadas em tecnologia até estratégias de carteira de mercado. Podemos adicionar quantos concorrentes necessitar de dados no formato e estilo de dados que procura. A nossa equipa de analistas também pode fornecer dados em tabelas dinâmicas de ficheiros Excel em bruto (livro de factos) ou pode ajudá-lo a criar apresentações a partir dos conjuntos de dados disponíveis no relatório.













