Asia Pacific Anti Nuclear Antibody Test Market
Tamanho do mercado em biliões de dólares
CAGR :
% 
 USD
558.77 Million
USD
1,639.25 Million
2024
2032
USD
558.77 Million
USD
1,639.25 Million
2024
2032
| 2025 –2032 | |
| USD 558.77 Million | |
| USD 1,639.25 Million | |
|
|
|
|
Segmentação do mercado de testes de anticorpos antinucleares da Ásia-Pacífico, por tipo de anticorpo (antígenos nucleares extraíveis (ENA), anti-DSDNA e histonas, anticorpos anti-DFS70, anti-PM-SCL, anticorpos anticentrômero, anti-SP100 e outros), produto (instrumentos, consumíveis e reagentes e serviços), técnica (ELISA, imunofluorescência indireta (IFI), teste de blotting, microarray de antígeno, técnicas baseadas em gel, ensaio multiplex, citometria de fluxo, hemaglutinação passiva (PHA) e outros), aplicação (doenças autoimunes e doenças infecciosas), por usuário final (hospitais, laboratórios, centros de diagnóstico, institutos de pesquisa e outros), canal de distribuição (licitação direta, vendas no varejo e distribuidor terceirizado e outros) - tendências do setor e previsão para 2032
Tamanho do mercado de testes de anticorpos antinucleares da Ásia-Pacífico
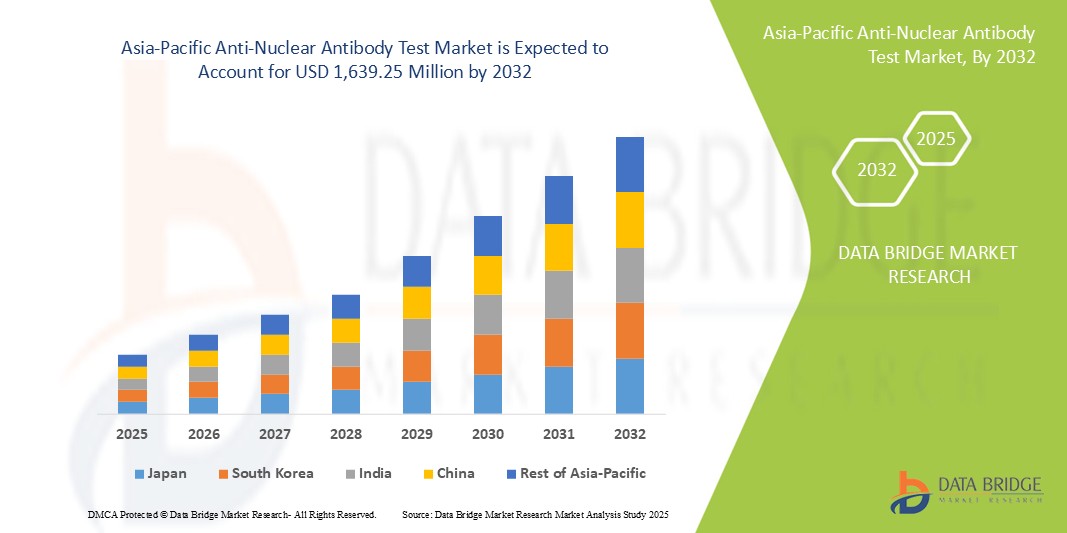
- O tamanho do mercado de testes de anticorpos antinucleares da Ásia-Pacífico foi avaliado em US$ 558,77 milhões em 2024 e deve atingir US$ 1.639,25 milhões até 2032 , com um CAGR de 14,40% durante o período previsto.
- Este crescimento é impulsionado por fatores como a crescente prevalência de doenças autoimunes, avanços tecnológicos em diagnósticos e aumento da conscientização sobre cuidados de saúde.
Análise do Mercado de Testes de Anticorpos Antinucleares da Ásia-Pacífico
- Os testes de anticorpos antinucleares (ANA) são ferramentas diagnósticas essenciais usadas para detectar autoanticorpos no sangue, auxiliando no diagnóstico de doenças autoimunes, como lúpus eritematoso sistêmico (LES), artrite reumatoide e síndrome de Sjögren. Esses testes desempenham um papel vital na detecção precoce e no tratamento dessas doenças.
- A crescente incidência de doenças autoimunes, como lúpus eritematoso sistêmico (LES) e artrite reumatoide, está impulsionando a demanda por testes de ANA em toda a região
- Espera-se que a China domine o mercado de testes de anticorpos antinucleares da Ásia-Pacífico com 32,4% de participação de mercado, impulsionada por sua grande base populacional, prevalência crescente de doenças autoimunes e investimentos significativos em infraestrutura de saúde.
- Espera-se que a Índia seja o país com crescimento mais rápido, com um CAGR de 14,6% no mercado de testes de anticorpos antinucleares da Ásia-Pacífico, impulsionado pela crescente conscientização sobre saúde e pelo aumento dos gastos com saúde.
- Espera-se que o ELISA (Enzyme-Linked Immunosorbent Assay) domine o mercado com uma participação de mercado de 52,60% devido à sua alta sensibilidade, custo-efetividade e ampla disponibilidade, tornando-o a escolha preferida para triagem em larga escala em laboratórios clínicos.
Escopo do Relatório e Segmentação do Mercado de Testes de Anticorpos Antinucleares da Ásia-Pacífico
|
Atributos |
Principais insights de mercado sobre o teste de anticorpos antinucleares da Ásia-Pacífico |
|
Segmentos abrangidos |
|
|
Países abrangidos |
Ásia-Pacífico
|
|
Principais participantes do mercado |
|
|
Oportunidades de mercado |
|
|
Conjuntos de informações de dados de valor agregado |
Além dos insights sobre cenários de mercado, como valor de mercado, taxa de crescimento, segmentação, cobertura geográfica e principais participantes, os relatórios de mercado selecionados pela Data Bridge Market Research também incluem análise de importação e exportação, visão geral da capacidade de produção, análise de consumo de produção, análise de tendência de preço, cenário de mudança climática, análise da cadeia de suprimentos, análise da cadeia de valor, visão geral de matéria-prima/consumíveis, critérios de seleção de fornecedores, análise PESTLE, análise de Porter e estrutura regulatória. |
Tendências do mercado de testes de anticorpos antinucleares na Ásia-Pacífico
“Avanços em Testes de Anticorpos Antinucleares (ANA) e Automação para Diagnóstico Autoimune na Ásia-Pacífico”
- Uma tendência notável na evolução dos testes ANA na região da Ásia-Pacífico é o uso crescente de plataformas de testes automatizados e tecnologias de diagnóstico aprimoradas
- Essas inovações melhoram significativamente a velocidade, a precisão e a eficiência dos testes de ANA, proporcionando maior sensibilidade e confiabilidade na detecção de doenças autoimunes.
- Por exemplo, os sistemas automatizados de ensaio de imunofluorescência (IFA) estão a simplificar o processo de teste, permitindo que os laboratórios manipulem maiores volumes de amostras com menos erros humanos e tempos de resposta mais rápidos, o que é crucial para o diagnóstico preciso de doenças autoimunes.
- O aumento no desenvolvimento e na adoção de dispositivos de teste no local de atendimento contribui ainda mais para o crescimento do mercado. Esses dispositivos fornecem resultados mais rápidos, facilitando decisões de tratamento oportunas, especialmente em áreas remotas com acesso limitado a cuidados de saúde especializados.
- Esses avanços estão transformando os diagnósticos autoimunes na Ásia-Pacífico, melhorando a precisão do diagnóstico e aprimorando a detecção precoce de doenças autoimunes, impulsionando assim a demanda por soluções de teste de ANA mais eficientes e acessíveis.
Dinâmica do mercado de testes de anticorpos antinucleares da Ásia-Pacífico
Motorista
“Crescente prevalência de doenças autoimunes”
- A prevalência crescente de doenças autoimunes, como lúpus eritematoso sistêmico (LES), artrite reumatoide e distúrbios autoimunes da tireoide, está aumentando significativamente a demanda por testes de ANA na região da Ásia-Pacífico.
- À medida que a população em muitos países da Ásia-Pacífico envelhece e os fatores ambientais contribuem para as alterações do sistema imunológico, há um aumento notável na incidência de doenças autoimunes, particularmente entre as mulheres.
- Com mais indivíduos sendo diagnosticados com essas condições, a demanda por testes ANA está aumentando, garantindo um diagnóstico preciso e melhores resultados no gerenciamento da doença.
Por exemplo,
- Em outubro de 2023, a Organização Mundial da Saúde (OMS) relatou que a prevalência de doenças autoimunes na Ásia está aumentando, especialmente em países como China e Índia, que têm grandes populações envelhecidas e crescente conscientização sobre os cuidados de saúde.
- Como resultado, a crescente incidência de doenças autoimunes criou uma necessidade urgente de testes de ANA, expandindo assim o mercado de ferramentas de diagnóstico.
Oportunidade
“Avanços tecnológicos em testes de ANA”
- O desenvolvimento de plataformas de diagnóstico automatizadas e de alto rendimento para testes ANA oferece uma oportunidade significativa para aumentar a eficiência e a precisão dos testes
- A automação pode reduzir o risco de erro humano, aumentar a produtividade nos laboratórios e acelerar o processo de diagnóstico, tornando-o mais acessível a populações maiores, especialmente em regiões rurais ou carentes
- Além disso, a adoção de dispositivos de teste no ponto de atendimento (POC) para testes de ANA representa uma oportunidade para melhorar o diagnóstico em locais com acesso limitado a laboratórios especializados
Por exemplo,
- Em março de 2024, um estudo publicado no Asian Journal of Clinical Immunology destacou que os sistemas automatizados de IFA (ensaio de imunofluorescência) estão se tornando cada vez mais populares em países como Japão e Coreia do Sul, melhorando a precisão e a eficiência geral dos testes em ambientes clínicos movimentados.
- Ao aproveitar a automação e a tecnologia POC, os testes ANA podem ser realizados mais rapidamente, fornecendo resultados diagnósticos mais rápidos e permitindo intervenções médicas oportunas para doenças autoimunes.
Restrição/Desafio
“Alto custo e acesso limitado à infraestrutura de diagnóstico”
- O custo dos testes ANA, juntamente com a disponibilidade limitada de equipamentos de diagnóstico avançados em áreas rurais ou em desenvolvimento, representa um desafio para o crescimento do mercado na região da Ásia-Pacífico
- Embora os testes ANA sejam cruciais para o diagnóstico de doenças autoimunes, o custo de manutenção de sistemas de diagnóstico avançados pode ser uma barreira para os profissionais de saúde em ambientes com poucos recursos
- Além disso, a necessidade de técnicos de laboratório altamente qualificados e a natureza complexa dos testes de ANA limitam o acesso a esses serviços de diagnóstico em certas regiões.
Por exemplo,
- Em agosto de 2023, um artigo publicado pela Associação Médica Indiana enfatizou o desafio da acessibilidade e da infraestrutura, especialmente nas áreas rurais da Índia, onde o acesso a serviços de diagnóstico avançados é limitado devido a restrições financeiras e à falta de profissionais treinados.
- Como resultado, os sistemas de saúde nessas regiões podem ter dificuldades para fornecer testes de ANA oportunos e precisos, dificultando o crescimento geral do mercado na região.
Escopo do mercado de testes de anticorpos antinucleares da Ásia-Pacífico
O mercado é segmentado com base no tipo de anticorpo, produto, técnica, aplicação, usuário final e canal de distribuição.
|
Segmentação |
Sub-segmentação |
|
Por tipo de anticorpo |
|
|
Por produto |
|
|
Por Técnica |
|
|
Por aplicação
|
|
|
Por usuário final |
|
|
Por canal de distribuição |
|
Em 2025, prevê-se que o ELISA domine o mercado com a maior participação no segmento de técnicas
Espera-se que o segmento ELISA (Enzyme-Linked Immunosorbent Assay) domine o mercado de testes de anticorpos antinucleares da Ásia-Pacífico, com a maior participação, de 52,60%, em 2025. Essa dominância se deve principalmente à sua alta sensibilidade, custo-efetividade e ampla disponibilidade, tornando-o a escolha preferencial para triagem em larga escala em laboratórios clínicos. A confiabilidade e a eficiência do ELISA na detecção de doenças autoimunes contribuem ainda mais para sua liderança de mercado.
Espera-se que os antígenos nucleares extraíveis (ENA) representem a maior fatia durante o período previsto no mercado de tipos de anticorpos
Em 2025, espera-se que o segmento de antígenos nucleares extraíveis (ENA) domine o mercado, com a maior participação, de 29,35%. Essa dominância se deve principalmente ao papel crucial dos testes de ENA no diagnóstico de doenças autoimunes específicas, como lúpus eritematoso sistêmico (LES) e síndrome de Sjögren. A precisão e a especificidade dos testes de ENA os tornam um componente vital do diagnóstico de doenças autoimunes, impulsionando sua liderança de mercado.
Análise regional do mercado de testes de anticorpos antinucleares da Ásia-Pacífico
- A China detém uma fatia dominante do mercado de testes ANA da Ásia-Pacífico, com 32,4% de participação de mercado, impulsionada por sua grande base populacional, prevalência crescente de doenças autoimunes e investimentos significativos em infraestrutura de saúde.
- O Japão é um país líder na região Ásia-Pacífico, conhecido por suas tecnologias avançadas em saúde, altos gastos com saúde e forte foco na detecção precoce de doenças. O Japão contribui com cerca de 20% para o mercado regional de testes de ANA, refletindo seu sistema de saúde bem estabelecido e o envelhecimento da população.
- A Índia deverá apresentar a maior taxa de crescimento anual composta (CAGR) do mercado, com uma participação de mercado de 14,6%, impulsionada pela crescente conscientização sobre os cuidados de saúde e pelo aumento dos gastos com saúde.
- Os países do Sudeste Asiático, incluindo Indonésia, Malásia e Filipinas, estão experimentando um rápido crescimento de mercado devido ao aumento dos investimentos em saúde, à conscientização sobre doenças autoimunes e à expansão das capacidades de diagnóstico.
- A Austrália e a Nova Zelândia também estão testemunhando um crescimento constante do mercado, apoiado por altos padrões de saúde, ampla cobertura de seguros e uma crescente população idosa.
- A tendência crescente de diagnósticos no local de atendimento (POC) na Ásia-Pacífico, especialmente em regiões remotas e rurais, está impulsionando a demanda por testes de FAN. Soluções de diagnóstico POC na região
Teste de anticorpos antinucleares da Ásia-Pacífico
O cenário competitivo do mercado fornece detalhes por concorrente. Os detalhes incluem visão geral da empresa, finanças da empresa, receita gerada, potencial de mercado, investimento em pesquisa e desenvolvimento, novas iniciativas de mercado, presença global, locais e instalações de produção, capacidades de produção, pontos fortes e fracos da empresa, lançamento de produto, amplitude e abrangência do produto e domínio da aplicação. Os pontos de dados fornecidos acima referem-se apenas ao foco das empresas em relação ao mercado.
Os principais líderes de mercado que operam no mercado são:
- Thermo Fisher Scientific Inc. (EUA)
- Bio-Rad Laboratories, Inc. (EUA)
- Abbott (EUA)
- Euroimmun Medizinische Labordiagnostika AG (Alemanha)
- Revvity Inc. (EUA)
- Trinity Biotech (Irlanda)
- LIFESPAN BIOSCIENCES, INC (EUA)
- ORIGENE TECHNOLOGIES, INC. (EUA)
- Abnova Corporation (Taiwan)
- CUSABIO TECHNOLOGY LLC (EUA)
- Biorbyt Ltd. (Inglaterra)
Últimos desenvolvimentos no mercado de testes de anticorpos antinucleares da Ásia-Pacífico
- Em março de 2025, a Shenzhen Mindray Bio-Medical Electronics Co., Ltd. anunciou o lançamento de sua plataforma de testes ANA de última geração na região Ásia-Pacífico, projetada para melhorar a precisão e a produtividade dos testes. O novo sistema integra automação avançada e inteligência artificial para fornecer resultados rápidos e confiáveis para o diagnóstico de doenças autoimunes, auxiliando na tomada de decisões clínicas mais rápidas.
- Em fevereiro de 2025, a Sysmex Corporation, empresa japonesa líder em diagnósticos, revelou sua plataforma ELISA aprimorada para testes de ANA, com maior sensibilidade e precisão. Este desenvolvimento visa atender à crescente demanda por diagnósticos autoimunes precisos no mercado da Ásia-Pacífico, impulsionada pela crescente conscientização sobre doenças autoimunes e pela expansão da infraestrutura de saúde.
- Em janeiro de 2025, a Bio-Rad Laboratories, Inc. expandiu seu portfólio de testes de ANA na região da Ásia-Pacífico com a introdução de um novo sistema de ensaio multiplex, oferecendo perfil abrangente de autoanticorpos para um diagnóstico mais preciso de doenças. Este lançamento está alinhado com a estratégia da empresa de fortalecer sua presença de mercado no setor de diagnóstico em rápido crescimento da Ásia-Pacífico.
- Em dezembro de 2024, a MBL (Medical & Biological Laboratories Co., Ltd.) lançou sua mais recente linha de reagentes de diagnóstico para testes de ANA no mercado da Ásia-Pacífico. Esses reagentes foram desenvolvidos para fornecer resultados altamente específicos e precisos, auxiliando no diagnóstico precoce e no manejo eficaz de doenças autoimunes, como lúpus eritematoso sistêmico (LES) e artrite reumatoide.
SKU-
Obtenha acesso online ao relatório sobre a primeira nuvem de inteligência de mercado do mundo
- Painel interativo de análise de dados
- Painel de análise da empresa para oportunidades de elevado potencial de crescimento
- Acesso de analista de pesquisa para personalização e customização. consultas
- Análise da concorrência com painel interativo
- Últimas notícias, atualizações e atualizações Análise de tendências
- Aproveite o poder da análise de benchmark para um rastreio abrangente da concorrência
Metodologia de Investigação
A recolha de dados e a análise do ano base são feitas através de módulos de recolha de dados com amostras grandes. A etapa inclui a obtenção de informações de mercado ou dados relacionados através de diversas fontes e estratégias. Inclui examinar e planear antecipadamente todos os dados adquiridos no passado. Da mesma forma, envolve o exame de inconsistências de informação observadas em diferentes fontes de informação. Os dados de mercado são analisados e estimados utilizando modelos estatísticos e coerentes de mercado. Além disso, a análise da quota de mercado e a análise das principais tendências são os principais fatores de sucesso no relatório de mercado. Para saber mais, solicite uma chamada de analista ou abra a sua consulta.
A principal metodologia de investigação utilizada pela equipa de investigação do DBMR é a triangulação de dados que envolve a mineração de dados, a análise do impacto das variáveis de dados no mercado e a validação primária (especialista do setor). Os modelos de dados incluem grelha de posicionamento de fornecedores, análise da linha de tempo do mercado, visão geral e guia de mercado, grelha de posicionamento da empresa, análise de patentes, análise de preços, análise da quota de mercado da empresa, normas de medição, análise global versus regional e de participação dos fornecedores. Para saber mais sobre a metodologia de investigação, faça uma consulta para falar com os nossos especialistas do setor.
Personalização disponível
A Data Bridge Market Research é líder em investigação formativa avançada. Orgulhamo-nos de servir os nossos clientes novos e existentes com dados e análises que correspondem e atendem aos seus objetivos. O relatório pode ser personalizado para incluir análise de tendências de preços de marcas-alvo, compreensão do mercado para países adicionais (solicite a lista de países), dados de resultados de ensaios clínicos, revisão de literatura, mercado remodelado e análise de base de produtos . A análise de mercado dos concorrentes-alvo pode ser analisada desde análises baseadas em tecnologia até estratégias de carteira de mercado. Podemos adicionar quantos concorrentes necessitar de dados no formato e estilo de dados que procura. A nossa equipa de analistas também pode fornecer dados em tabelas dinâmicas de ficheiros Excel em bruto (livro de factos) ou pode ajudá-lo a criar apresentações a partir dos conjuntos de dados disponíveis no relatório.