A crescente prevalência de distúrbios cardíacos nos EUA, especialmente arritmias como a fibrilhação auricular, o bloqueio cardíaco e a taquicardia ventricular, é um fator chave para o mercado de equipamentos de eletrofisiologia. Com o aumento das taxas de condições relacionadas com o estilo de vida, como a hipertensão, a diabetes e a obesidade, a incidência de distúrbios do ritmo cardíaco tem aumentado nos últimos anos. Esta crescente carga sobre o sistema de saúde exige a utilização de ferramentas de diagnóstico e terapêuticas avançadas, como dispositivos de eletrofisiologia, para diagnosticar, monitorizar e tratar estas condições de forma eficaz. À medida que mais doentes procuram tratamentos como a ablação por cateter e outros procedimentos de eletrofisiologia para controlar as suas condições, a procura por equipamentos de EP de alto desempenho aumenta, impulsionando tanto a inovação como a adoção no mercado. Este aumento da procura por opções de tratamento eficazes impulsiona diretamente o crescimento do mercado, tornando a crescente prevalência de distúrbios cardíacos um fator crucial para o setor de equipamentos de eletrofisiologia.
Aceda ao relatório completo em https://www.databridgemarketresearch.com/reports/us-electrophysiology-equipment-market
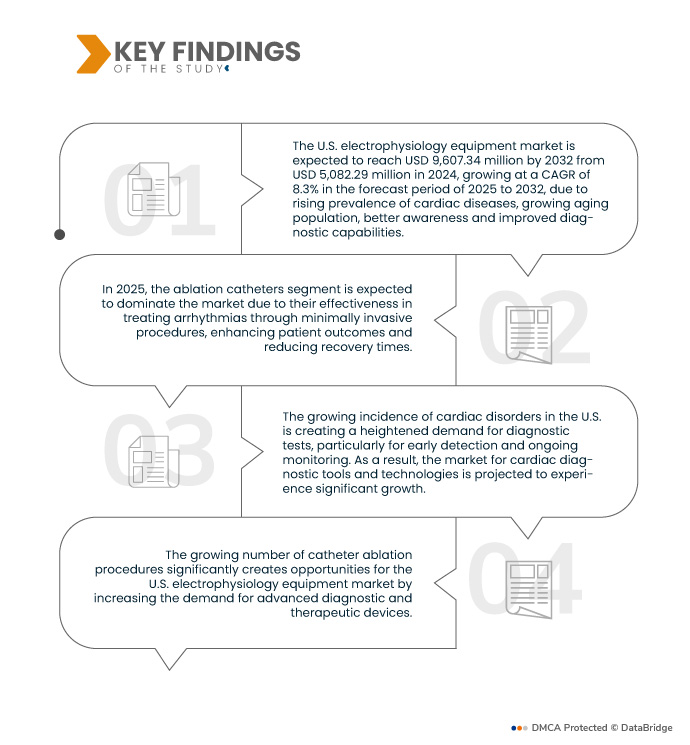
A Data Bridge Market Research analisa que o mercado de equipamentos de eletrofisiologia dos EUA deverá atingir os 9,60 mil milhões de dólares até 2032, face aos 5,08 mil milhões de dólares em 2024, crescendo a um CAGR de 8,3% no período previsto de 2025 a 2031.
Principais conclusões do estudo
Número crescente de procedimentos de ablação por cateter
O número crescente de procedimentos de ablação por cateter cria oportunidades significativas para o mercado de equipamentos de eletrofisiologia dos EUA, aumentando a procura de dispositivos de diagnóstico e terapia avançados. A ablação por cateter, utilizada principalmente para tratar arritmias, como a fibrilhação auricular e a taquicardia ventricular, é um procedimento minimamente invasivo que oferece vantagens em relação aos métodos tradicionais, como o tratamento medicamentoso ou as intervenções cirúrgicas. À medida que a consciencialização sobre os benefícios da ablação por cateter aumenta entre os doentes e os profissionais de saúde, e à medida que mais unidades de saúde adotam estas tecnologias, a procura por equipamentos de eletrofisiologia, como cateteres, sistemas de mapeamento e analisadores de eletrofisiologia, aumenta. Esta tendência é ainda reforçada pelo envelhecimento da população, que está mais propensa a doenças cardiovasculares que exijam tais intervenções.
Âmbito do Relatório e Segmentação de Mercado
Métrica de Reporte
|
Detalhes
|
Período de previsão
|
2025 a 2032
|
Ano base
|
2024
|
Ano Histórico
|
2023 (Personalizável 2013-2017)
|
Unidades quantitativas
|
Receita em biliões de dólares americanos
|
Segmentos abrangidos
|
Tipo (cateteres de ablação, equipamento de laboratório de electrofisiologia, cateteres de diagnóstico, dispositivos de acesso e outros), indicação ( fibrilhação auricular , flutter auricular , taquicardia de reentrada nodal auriculoventricular (AVNRT), síndrome de Wolff-Parkinson-White (WPW) e outros), função (avançada, convencional, crioablação e outros), procedimento (fibrilhação ventricular, ablação tumoral , mapeamento sem contacto de posição única, mapeamento electroanatómico multiponto para arritmias simples, varizes e outras), utilizador final (hospitais, centros de cirurgia ambulatória, centros de diagnóstico, laboratórios de cateterismo cardíaco, clínicas especializadas e outros), canal de distribuição (licitação directa e venda a retalho)
|
Participantes do mercado abrangidos
|
Johnsons & Johnsons (EUA), Abbott (EUA), Medtronic (Dublin, Irlanda), Boston Scientific Corporation (EUA) e Koninklijke Philips NV (EUA), entre outros
|
Pontos de dados abordados no relatório
|
Para além dos insights sobre os cenários de mercado, tais como o valor de mercado, a taxa de crescimento, a segmentação, a cobertura geográfica e os principais participantes, os relatórios de mercado selecionados pela Data Bridge Market Research incluem também análises aprofundadas de especialistas, epidemiologia dos doentes, análise de pipeline, análise de preços e estrutura regulamentar.
|
Análise de Segmentos
O mercado de equipamentos de eletrofisiologia dos EUA está segmentado em seis segmentos notáveis com base no tipo, na indicação, na função, no procedimento, no utilizador final e no canal de distribuição.
- Com base no tipo, o mercado está segmentado em cateteres de ablação, equipamentos de laboratório de eletrofisiologia, cateteres de diagnóstico, dispositivos de acesso e outros
Em 2025, prevê-se que o segmento dos cateteres de ablação domine o mercado com uma quota de mercado de 46,73%
Em 2025, prevê-se que o segmento dos cateteres de ablação domine o mercado com uma quota de mercado de 46,73% devido à sua eficácia no tratamento de arritmias através de procedimentos minimamente invasivos, melhorando os resultados dos doentes e reduzindo os tempos de recuperação.
- Com base na indicação, o mercado está segmentado em fibrilhação auricular, flutter auricular, taquicardia de reentrada nodal atrioventricular (AVNRT), síndrome de Wolff-Parkinson-White (WPW) e outros.
Em 2025, prevê-se que o segmento da fibrilhação auricular domine o mercado com uma quota de mercado de 46,19%
Em 2025, prevê-se que o segmento da fibrilhação auricular domine o mercado com uma quota de mercado de 46,19% devido à sua elevada prevalência entre a população idosa e à crescente procura de opções de tratamento eficazes para controlar esta arritmia cardíaca comum.
- Com base na função, o mercado está segmentado em avançado, convencional, crioablação e outros. Em 2025, prevê-se que o segmento avançado domine o mercado com uma quota de mercado de 42,11%
- Com base no procedimento, o mercado está segmentado em fibrilhação ventricular, ablação de tumores, mapeamento sem contacto de posição única, mapeamento eletroanatómico multiponto para arritmias simples, varizes e outros. Em 2025, prevê-se que o segmento da fibrilhação ventricular domine o mercado com uma quota de mercado de 30,76%
- Com base no utilizador final, o mercado está segmentado em hospitais, centros de cirurgia ambulatória, centros de diagnóstico, laboratórios de cateterismo cardíaco, clínicas especializadas e outros. Em 2025, prevê-se que o segmento dos Hospitais domine o mercado com uma quota de mercado de 53,23%
- Com base no canal de distribuição, o mercado está segmentado em licitação direta e vendas a retalho. Em 2025, prevê-se que o segmento de licitação direta domine o mercado com uma quota de mercado de 61,51%
Principais jogadores
A Data Bridge Market Research analisa a Johnsons & Johnsons (EUA), Abbott (EUA), Medtronic (Dublin, Irlanda), Boston Scientific Corporation (EUA), Koninklijke Philips NV (EUA) como principais participantes do mercado.
Desenvolvimento de Mercado
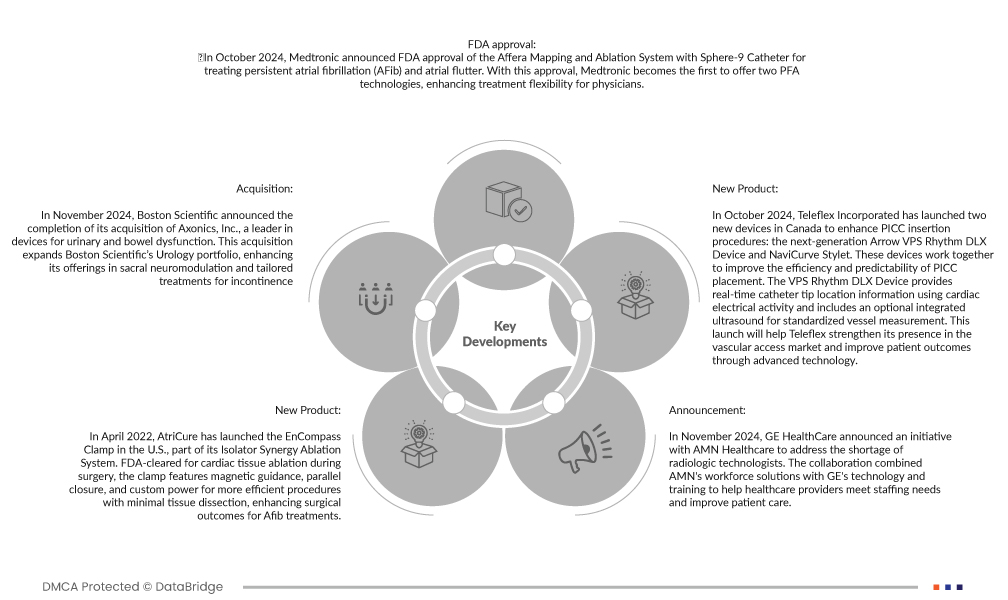
- Em novembro de 2024, a GE HealthCare anunciou uma iniciativa com a AMN Healthcare para lidar com a escassez de tecnólogos radiológicos. A colaboração combinou as soluções de força de trabalho da AMN com a tecnologia e formação da GE para ajudar os prestadores de cuidados de saúde a satisfazer as necessidades de pessoal e melhorar os cuidados prestados aos doentes.
- Em novembro de 2024, a Boston Scientific anunciou a conclusão da aquisição da Axonics, Inc., líder em dispositivos para disfunção urinária e intestinal. Esta aquisição expande o portefólio de Urologia da Boston Scientific, melhorando as suas ofertas em neuromodulação sacral e tratamentos personalizados para incontinência
- Em outubro de 2024, a Teleflex Incorporated lançou dois novos dispositivos no Canadá para melhorar os procedimentos de inserção do PICC: o dispositivo Arrow VPS Rhythm DLX de última geração e o estilete NaviCurve. Estes dispositivos trabalham em conjunto para melhorar a eficiência e a previsibilidade do posicionamento do PICC. O dispositivo VPS Rhythm DLX fornece informações de localização da ponta do cateter em tempo real, utilizando a atividade elétrica cardíaca, e inclui um ultrassom integrado opcional para a medição padronizada dos vasos. Este lançamento ajudará a Teleflex a reforçar a sua presença no mercado de acesso vascular e a melhorar os resultados dos doentes através de tecnologia avançada.
- Em outubro de 2024, a Medtronic anunciou a aprovação da FDA para o Sistema de Mapeamento e Ablação Affera com Cateter Sphere-9 para o tratamento da fibrilhação auricular persistente (FA) e flutter auricular. Com esta aprovação, a Medtronic torna-se a primeira a oferecer duas tecnologias PFA, aumentando a flexibilidade do tratamento para os médicos
- Em abril de 2022, a AtriCure lançou o EnCompass Clamp nos EUA, parte do seu Sistema de Ablação Isolator Synergy. Aprovado pela FDA para a ablação de tecido cardíaco durante a cirurgia, o grampo possui orientação magnética, fecho paralelo e alimentação personalizada para procedimentos mais eficientes com dissecção mínima do tecido, melhorando os resultados cirúrgicos para tratamentos de fibrilhação auricular.
De acordo com a análise de pesquisa de mercado da Data Bridge:
Para obter informações mais detalhadas sobre o relatório de mercado de equipamentos de eletrofisiologia dos EUA, clique aqui – https://www.databridgemarketresearch.com/reports/us-electrophysiology-equipment-market