Um dos principais fatores que impulsionam o crescimento do mercado é a crescente prevalência de falência de órgãos. Além disso, a crescente popularidade dos métodos de diagnóstico não invasivos em todo o mundo é outro fator-chave para o crescimento do mercado. Os avanços nas técnicas de preservação de órgãos também influenciam o mercado. Porém, a escassez de profissionais com expertise em diagnóstico de transplantes está restringindo o crescimento do mercado.
Por outro lado, a iniciação estratégica e o lançamento de tecnologia pelos players do mercado funcionam como uma oportunidade de crescimento do mercado. No entanto, os desafios éticos enfrentados durante o transplante de órgãos podem criar um desafio ao crescimento do mercado.
A demanda por testes de diagnóstico aumentará devido ao aumento dos avanços tecnológicos. Assim, diversas empresas estão tomando iniciativas que levam gradativamente ao crescimento do mercado.
Acesse o relatório completo @https://www.databridgemarketresearch.com/reports/global-pcr-based-transplant-diagnostics-market
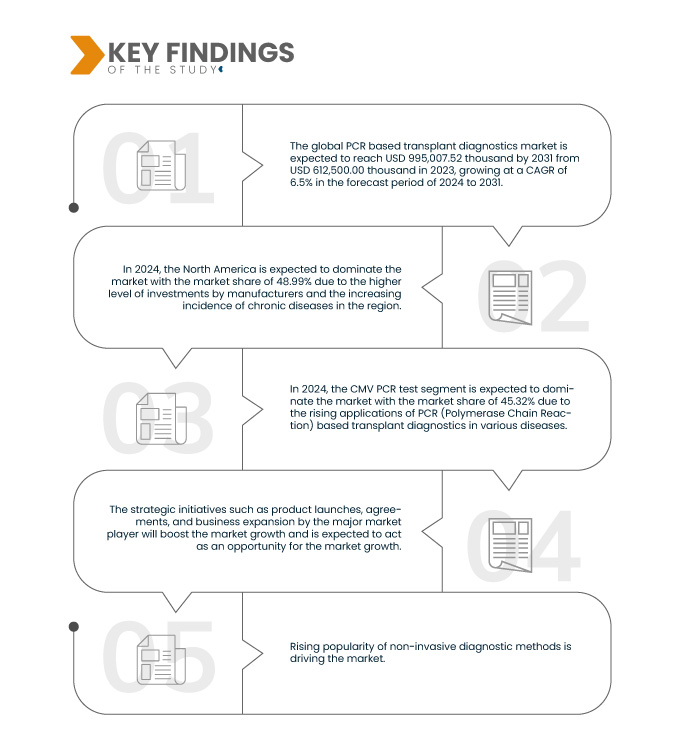
A Data Bridge Market Research analisa que o Mercado global de diagnóstico de transplante baseado em PCR deverá atingir US$ 995.007,52 mil até 2031, de US$ 612.500,00 mil em 2023, crescendo com um CAGR de 6,5% no período previsto de 2024 a 2031. O segmento de teste CMV PCR é projetado para impulsionar o crescimento do mercado e aumentar as aplicações de global Diagnóstico de transplante baseado em PCR em todo o mundo.
Principais conclusões do estudo
Iniciação Estratégica e Lançamentos de Tecnologia por Participantes do Mercado
O crescente crescimento do mercado de diagnóstico de transplantes baseado em PCR, impulsionado pelo aumento da demanda por órgãos de doadores, enfatiza iniciativas estratégicas como parcerias, expansões de negócios e esforços de desenvolvimento. Estas iniciativas, guiadas por estratégias bem pensadas, incluem a introdução de kits de diagnóstico de transplante, como o teste PCR de Aspergillus Spp, teste PCR, teste PCR CMV, teste PCR EBV, teste PCR BKV e teste PCR VZV. A implementação destes quadros estratégicos permite que os participantes no mercado se harmonizem com as suas atividades organizacionais, aumentando a eficiência operacional e alinhando esforços com o cenário dinâmico do diagnóstico de transplantes.
Assim, pode-se esperar que essas iniciativas estratégicas dos participantes do mercado funcionem como uma oportunidade para o crescimento do mercado.
Escopo do relatório e segmentação de mercado
|
Métrica de relatório
|
Detalhes
|
|
Período de previsão
|
2024 a 2031
|
|
Ano base
|
2023
|
|
Anos históricos
|
2022 (personalizável para 2016-2021)
|
|
Unidades Quantitativas
|
Receita em mil dólares
|
|
Segmentos cobertos
|
Tipo de teste (teste PCR de CMV, teste PCR de EBV, teste PCR BKV, teste PCR VZV, teste PCR HSV1, teste PCR HSV2, teste PCR de parvovírus B19, teste PCR de P. Jirovecii, teste PCR JCV, teste PCR de adenovírus e PCR de Aspergillus Spp Teste), Tipo de Transplante (Transplante de Rim, Transplante de Fígado, Transplante de Coração, Transplante de Pulmão, Transplante de Pâncreas e Outros Transplantes), Aplicação (Aplicações de Diagnóstico e Aplicações de Pesquisa), Usuário Final (Hospitais e Centros de Transplante, Provedores de Serviços Comerciais, Laboratórios de Pesquisa e Institutos Acadêmicos)
|
|
Países abrangidos
|
EUA, Canadá, México, Alemanha, França, Reino Unido, Itália, Espanha, Rússia, Turquia, Bélgica, Holanda, Suíça, Resto da Europa, China, Japão, Índia, Coreia do Sul, Austrália, Singapura, Tailândia, Malásia, Indonésia, Filipinas , Resto da Ásia-Pacífico, Brasil, Argentina, Resto da América do Sul, África do Sul, Arábia Saudita, Emirados Árabes Unidos, Egito, Israel e Resto do Oriente Médio e África
|
|
Participantes do mercado cobertos
|
Quest Diagnostics Incorporada. (EUA), Laboratory Corporation of America Holdings (EUA), EUROFINS VIRACOR (EUA), Sonic Healthcare Limited (EUA) e Dr Lal PathLabs (Índia), entre outros
|
|
Pontos de dados abordados no relatório
|
Além dos insights sobre cenários de mercado, como valor de mercado, taxa de crescimento, segmentação, cobertura geográfica e grandes players, os relatórios de mercado com curadoria da Data Bridge Market Research também incluem análises especializadas aprofundadas, epidemiologia de pacientes, análise de pipeline, análise de preços, e quadro regulamentar
|
Análise de Segmento
O mercado global de diagnóstico de transplante baseado em PCR é segmentado em quatro segmentos notáveis com base no tipo de teste, tipo de transplante, aplicação e usuário final.
- Com base no tipo de teste, o mercado global de diagnóstico de transplante baseado em PCR é segmentado em teste PCR CMV, teste PCR EBV, teste PCR BKV, teste PCR VZV, teste PCR HSV1, teste PCR HSV2, teste PCR Parvovirus B19, P. Jirovecii PCR teste, teste PCR JCV, teste PCR de adenovírus e teste PCR de Aspergillus Spp
Em 2024, espera-se que o segmento de testes CMV PCR domine o mercado global de diagnósticos de transplantes baseados em PCR
Em 2024, espera-se que o segmento de testes PCR CMV domine o mercado com uma participação de mercado de 45,32% devido à crescente demanda por transplante de órgãos.
- Com base no tipo de transplante, o mercado global de diagnóstico de transplante baseado em PCR é segmentado em transplante de rim, transplante de fígado, transplante de coração, transplante de pulmão, transplante de pâncreas e outros transplantes
Em 2024, espera-se que o segmento de transplante renal domine o Mercado global de diagnóstico de transplante baseado em PCR
Em 2024, espera-se que o segmento de transplante renal domine o mercado com uma participação de mercado de 65,28% devido à crescente prevalência de insuficiência renal em todo o mundo.
- Com base na aplicação, o mercado global de diagnóstico de transplante baseado em PCR é segmentado em aplicações de diagnóstico e aplicações de pesquisa. Em 2024, espera-se que o segmento de aplicações de diagnóstico domine o mercado com uma participação de mercado de 96,44%
- Com base no usuário final, o mercado global de diagnóstico de transplante baseado em PCR é segmentado em hospitais e centros de transplante, prestadores de serviços comerciais e laboratórios de pesquisa e institutos acadêmicos. Em 2024, espera-se que o segmento de hospitais e centros de transplante domine o mercado com uma participação de mercado de 78,38%
Jogadores principais
A Data Bridge Market Research analisa a Quest Diagnostics Incorporated. (EUA), Laboratory Corporation of America Holdings (EUA). EUROFINS VIRACOR (EUA), Sonic Healthcare Limited (EUA) e Dr Lal PathLabs (Índia) como os principais players do mercado global PCR mercado de diagnóstico de transplante baseado.
Desenvolvimento de mercado
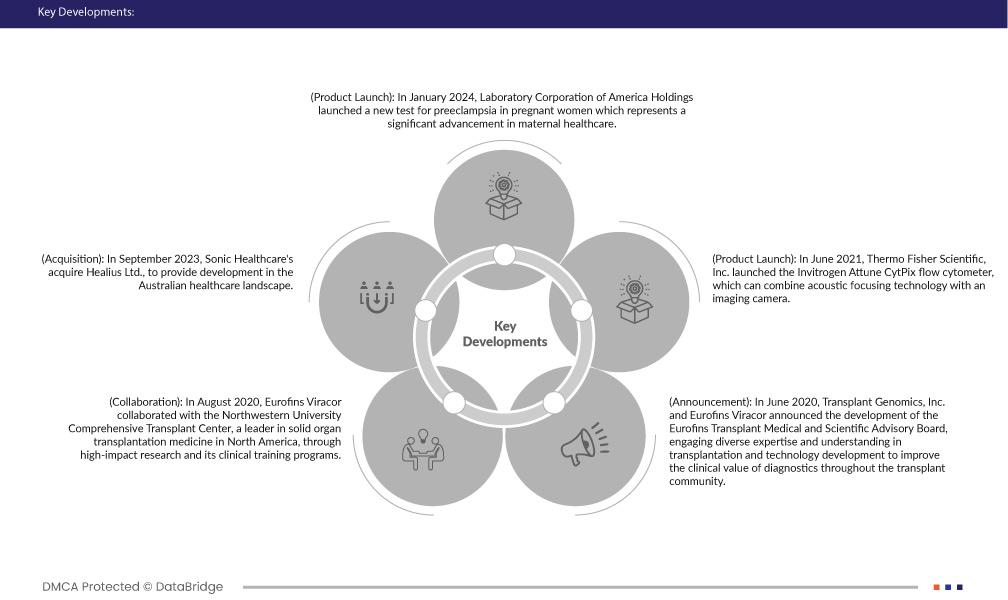
- Em janeiro de 2024, o Laboratory Corporation of America Holdings lançou um novo teste para pré-eclâmpsia em mulheres grávidas, o que representa um avanço significativo na saúde materna. Com essas ferramentas de diagnóstico avançadas, os profissionais de saúde podem identificar mulheres com maior risco de pré-eclâmpsia grave no início da gravidez. Isto permite à empresa um monitoramento mais vigilante e uma intervenção médica proativa, reduzindo potencialmente o risco de complicações e melhorando os resultados maternos e fetais
- Em setembro de 2023, a Sonic Healthcare adquiriu a Healius Ltd., para fornecer desenvolvimento no cenário de saúde australiano. Esta aquisição solidifica ainda mais a posição da Sonic como líder global em patologia, fortalecendo a sua vantagem competitiva contra outros players internacionais. Significa o compromisso da empresa com a expansão e consolidação dentro da indústria, potencialmente abrindo caminho para fusões semelhantes em outras regiões.
- Em outubro de 2023, o investimento da Sonic Healthcare na PaigeAI, uma empresa de patologia alimentada por IA, sublinha o seu compromisso em aproveitar tecnologia de ponta para melhorar a precisão e eficiência do diagnóstico no campo da patologia. Este movimento tem implicações significativas para o futuro dos diagnósticos médicos. Esta abordagem de precisão pode levar a tratamentos mais eficazes e potencialmente reduzir o risco de efeitos colaterais de medicamentos desnecessários
- Em agosto de 2020, a Eurofins Viracor colaborou com o Comprehensive Transplant Center da Northwestern University, líder em medicina de transplante de órgãos sólidos na América do Norte, através de pesquisas de alto impacto e seus programas de treinamento clínico. Esta colaboração de pesquisa verificou ainda mais o novo teste obtido através da recente aquisição da Transplant Genomics Inc. (“TGI”) pela Eurofins e seu teste de expressão genética no sangue, TruGraf. O TruGraf pode descartar a rejeição renal subclínica “silenciosa” em pacientes transplantados renais com função renal estável. A TGI recebeu recentemente um projeto de decisão de Determinação de Cobertura Local (LCD) dos Centros de Serviços Medicare e Medicaid (CMS) por meio do Programa de Serviços de Diagnóstico Molecular (MolDX) do Palmetto GBA. Isso ajudou a empresa a validar sua pesquisa
- Em junho de 2020, a Transplant Genomics, Inc. e a Eurofins Viracor anunciaram o desenvolvimento do Conselho Consultivo Médico e Científico da Eurofins Transplant, envolvendo diversos conhecimentos e compreensão em transplantes e desenvolvimento de tecnologia para melhorar o valor clínico do diagnóstico em toda a comunidade de transplantes. Os associados do conselho estão se concentrando na melhoria e no desenvolvimento de produtos, utilizando suas perspectivas únicas para ajudar a TGI e a Viracor a se expandirem para atender às necessidades não atendidas em transplantes e orientar produtos futuros. Isso ajudou a empresa a expandir seu portfólio de produtos
Análise Regional
Geograficamente, os países cobertos pelo relatório global de mercado de diagnóstico de transplante baseado em PCR são EUA, Canadá, México, Alemanha, França, Reino Unido, Itália, Espanha, Rússia, Turquia, Bélgica, Holanda, Suíça, resto da Europa, China, Japão, Índia , Coreia do Sul, Austrália, Singapura, Tailândia, Malásia, Indonésia, Filipinas, resto da Ásia-Pacífico, Brasil, Argentina, resto da América do Sul, África do Sul, Arábia Saudita, Emirados Árabes Unidos, Egito, Israel e resto do Médio Oriente e África .
De acordo com a análise da pesquisa de mercado da Data Bridge:
Espera-se que a América do Norte domine o Mercado global de diagnóstico de transplante baseado em PCR
Espera-se que a América do Norte domine o mercado devido ao maior nível de investimentos dos fabricantes e ao aumento da aplicação de diagnósticos de transplante baseados em PCR (Reação em Cadeia da Polimerase) para diversas doenças.
Espera-se que a Ásia-Pacífico seja a região que mais cresce no mercado global de diagnósticos de transplantes baseados em PCR
Espera-se que a Ásia-Pacífico cresça durante o período de previsão devido à presença de grandes players do mercado e ao rápido desenvolvimento das indústrias farmacêuticas e biotecnológicas nesta região.
Para obter informações mais detalhadas sobre o relatório global do mercado de diagnóstico de transplante baseado em PCR, clique aqui – https://www.databridgemarketresearch.com/reports/global-pcr-based-transplant-diagnostics-market