O mercado global de identificação de parasitologia abrange uma gama diversificada de ferramentas e tecnologias de diagnóstico concebidas para identificar e analisar infeções parasitárias em humanos e animais. Com a crescente prevalência de doenças parasitárias em todo o mundo, este mercado tem assistido a um crescimento substancial. Técnicas moleculares avançadas, como a PCR e a sequenciação de ADN, juntamente com a microscopia tradicional, contribuem para uma identificação precisa e atempada. À medida que os sistemas de saúde globalmente priorizam a gestão de doenças infeciosas , a procura por ferramentas fiáveis e eficientes de identificação de parasitologia deverá continuar a crescer, moldando o cenário do mercado global de identificação de parasitologia.
Aceda ao relatório completo em https://www.databridgemarketresearch.com/reports/global-parasitology-identification-market
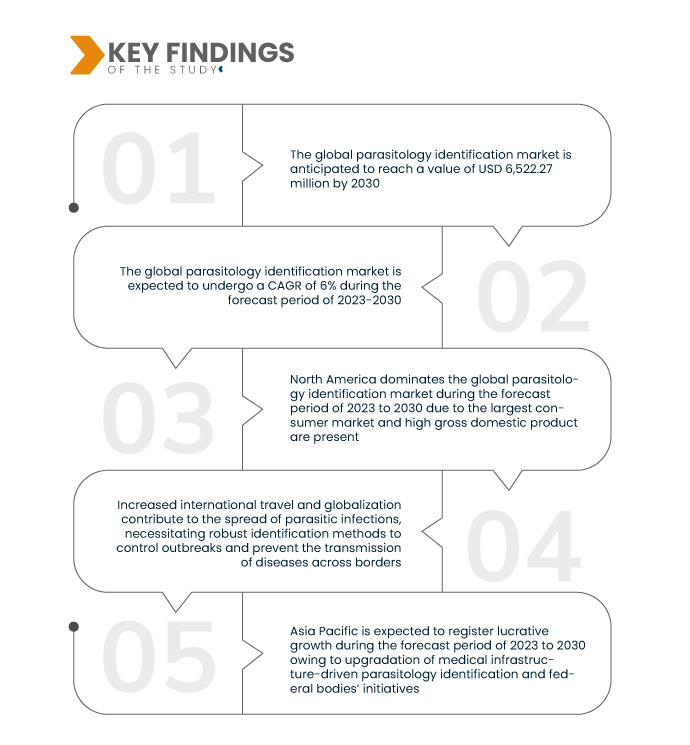
A Data Bridge Market Research analisa que o mercado global de identificação de parasitologia , avaliado em 4.092,15 milhões de dólares em 2022, atingirá os 6.522,27 milhões de dólares até 2030, crescendo a um CAGR de 6% durante o período previsto de 2023 a 2030. As colaborações entre instituições de investigação, empresas farmacêuticas e fabricantes de ferramentas de diagnóstico impulsionam a criação de soluções inovadoras de identificação de parasitologia, alavancando a experiência e os recursos combinados para avanços no diagnóstico e tratamento.
Principais conclusões do estudo
Espera-se que a integração da inteligência artificial (IA) impulsione a taxa de crescimento do mercado
A infusão de inteligência artificial (IA) e aprendizagem automática na identificação de parasitologia acelera significativamente os diagnósticos com maior precisão. Esta integração atrai os profissionais de saúde e os laboratórios, uma vez que não só melhora a eficiência, como também representa um salto tecnológico na procura de uma identificação mais precisa e atempada das infeções parasitárias, contribuindo, em última análise, para melhorar os cuidados prestados aos doentes e a gestão das doenças parasitológicas.
Âmbito do Relatório e Segmentação de Mercado
Métrica de Reporte
|
Detalhes
|
Período de previsão
|
2023 a 2030
|
Ano base
|
2022
|
Anos Históricos
|
2021 (personalizável para 2015-2020)
|
Unidades quantitativas
|
Receita em milhões de dólares americanos, volumes em unidades, preços em dólares americanos
|
Segmentos abrangidos
|
Produtos (Consumíveis e Acessórios, Dispositivos), Métodos (Identificação Microscópica, Identificação Fecal, Técnicas Imunológicas, Identificação Morfológica, Técnicas Moleculares, Testes de Diagnóstico Rápido (RDTs), Maldi-TOF MS, Outros), Tipo de Patógeno (Protozoários, Helmintos, Artrópodes, Tênia, Ectoparasita), Amostra (Fezes, Sangue, Urina, Líquido Cefalorraquidiano (LCR), Soro e Plasma, Outros), Concentração e Preparação de Fezes (Técnica de Concentração, Técnica de Desconcentração), Utente Final (Centros de Diagnóstico, Hospitais, Clínicas, Centros Cirúrgicos de Ambulatório , Outros)
|
Países abrangidos
|
EUA, Canadá e México na América do Norte, Alemanha, França, Reino Unido, Holanda, Suíça, Bélgica, Rússia, Itália, Espanha, Turquia, Resto da Europa na Europa, China, Japão, Índia, Coreia do Sul, Singapura, Malásia, Austrália, Tailândia, Indonésia, Filipinas, Resto da Ásia-Pacífico (APAC) na Ásia-Pacífico (APAC), Arábia Saudita, Emirados Árabes Unidos, África do Sul, Egito, Israel, Resto do Médio Oriente e África (MEA) como parte do Médio Oriente e África (MEA), Brasil, Argentina e Resto da América do Sul como parte da América do Sul
|
Participantes do mercado abrangidos
|
Abbott (EUA), BD (EUA), bioMérieux, Inc (França), Bruker (EUA), Quest Diagnostics (EUA), Akron Biotech (EUA), Thermo Fisher Scientific Inc (EUA), altona Diagnostics GmbH (Alemanha), Biomerica (EUA), Bio-Rad Laboratories, Inc. (EUA), Creative Diagnostics (EUA), Luminex Corporation (EUA), Meridian Bioscience, Inc. (EUA), R-Biopharm AG (Alemanha), Shimadzu Corporation (Japão), Institut Virion/Serion (Alemanha), VWR International, LLC. (EUA), Hardy Diagnostics (EUA), TECHLAB, Inc. (EUA), Alpha-Tec (EUA), Medical Chemical Corporation (MCC). (NÓS)
|
Pontos de dados abordados no relatório
|
Para além dos insights sobre os cenários de mercado, tais como o valor de mercado, a taxa de crescimento, a segmentação, a cobertura geográfica e os principais participantes, os relatórios de mercado selecionados pela Data Bridge Market Research incluem também análises aprofundadas de especialistas, epidemiologia dos doentes, análise de pipeline, análise de preços e estrutura regulamentar.
|
Análise de Segmentos:
O mercado global de identificação de parasitologia está segmentado com base no produto, método, tipo de agente patogénico, amostra, concentração e preparação de fezes e utilizador final.
- Com base nos produtos, o mercado global de identificação de parasitologia está segmentado em consumíveis, acessórios e dispositivos
- Com base nos métodos, o mercado global de identificação de parasitologia está segmentado em identificação fecal, técnicas imunológicas, identificação morfológica, técnicas moleculares, testes de diagnóstico rápido (RDTS), maldi-TOF MS e outros.
- Com base no tipo de agente patogénico, o mercado global de identificação de parasitologia está segmentado em protozoários, helmintos e ectoparasitas
- Com base na amostra, o mercado global de identificação de parasitologia está segmentado em fezes, sangue, urina, soro e plasma, e outros
- Com base na concentração e preparação das fezes, o mercado global de identificação de parasitologia está segmentado em técnica de concentração e técnica de desconcentração.
- Com base no utilizador final, o mercado global de identificação de parasitologia está segmentado em centros de diagnóstico, hospitais, clínicas e outros
Principais jogadores
A Data Bridge Market Research reconhece as seguintes empresas como os principais participantes do mercado global de identificação de parasitologia: Luminex Corporation (EUA), Meridian Bioscience, Inc. (EUA), R-Biopharm AG (Alemanha), Shimadzu Corporation (Japão), Institut Virion/Serion (Alemanha), VWR International, LLC. (EUA), Hardy Diagnostics (EUA), TECHLAB, Inc. (EUA), Alpha-Tec (EUA), Medical Chemical Corporation (MCC). (NÓS)
Desenvolvimentos de mercado
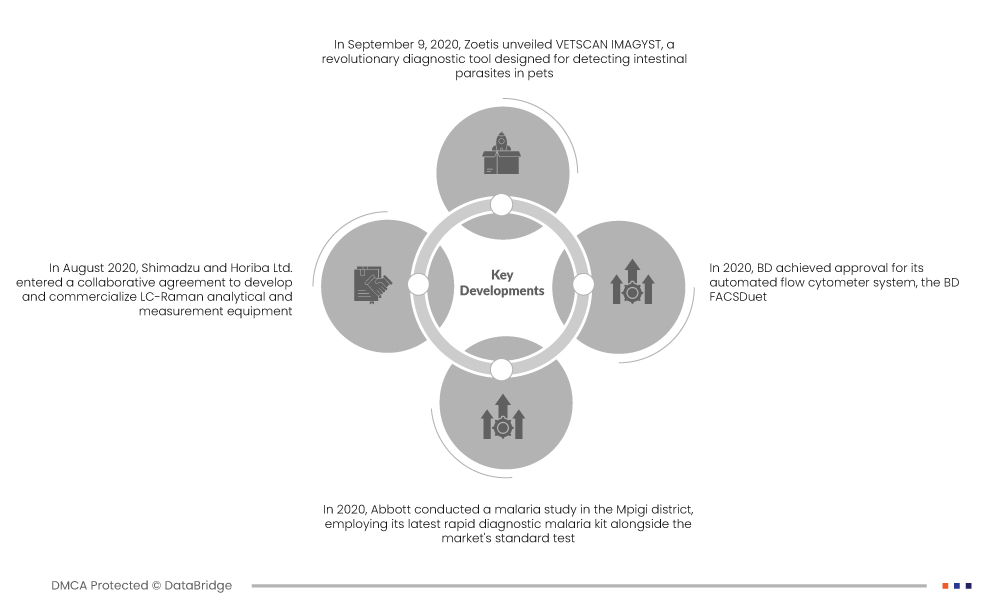
- Em setembro de 2020, a Zoetis revelou o VETSCAN IMAGYST, uma ferramenta de diagnóstico revolucionária concebida para detetar parasitas intestinais em animais de estimação. Esta ferramenta inovadora utiliza uma combinação de tecnologia de reconhecimento de imagem e inteligência artificial baseada na nuvem para fornecer resultados de testes precisos. Ao automatizar o exame fecal, o VETSCAN IMAGYST garante um diagnóstico rápido e preciso, identificando ovos de parasitas internos, quistos e outros indicadores. O lançamento da Zoetis marca um passo significativo na alavancagem da tecnologia para diagnósticos veterinários melhorados e eficientes
- Em agosto de 2020, a Shimadzu e a Horiba Ltd. assinaram um acordo de colaboração para desenvolver e comercializar equipamentos analíticos e de medição LC-Raman. Esta tecnologia inovadora combina os cromatógrafos líquidos (LCs) de alto desempenho da Shimadzu com os espectrómetros Raman da Horiba. Com quotas de mercado líderes no Japão, a abordagem sinérgica visa revolucionar a medicina, a saúde, as ciências biológicas, a investigação de materiais e os estudos ambientais. Os sistemas LC-Raman integram a separação eficiente de amostras por LCs com medições químicas qualitativas e quantitativas pelos espectrómetros Raman da Horiba, oferecendo avanços em vários setores
- Em 2020, a Abbott realizou um estudo sobre a malária no distrito de Mpigi, empregando o seu mais recente kit de diagnóstico rápido da malária juntamente com o teste padrão do mercado. Surpreendentemente, o teste de diagnóstico rápido de Abbott revelou uma taxa de positividade duplicada em comparação com o teste padrão, sinalizando um avanço. Este resultado não só demonstra a eficácia da tecnologia da Abbott, como também aumenta significativamente o valor da marca da empresa no mercado, consolidando a sua posição como líder em soluções de diagnóstico inovadoras e fiáveis para a malária.
- Em 2020, a BD obteve aprovação para o seu sistema de citómetro de fluxo automatizado, o BD FACSDuet. Esta inovação aumenta a precisão e a padronização entre instrumentos e operadores. As soluções de automação, exemplificadas pelo BD FACSDuetTM e BD FACSLyricTM Complete Sample-to-Answer Solution, elevam a produtividade e proporcionam resultados consistentes. Estes avanços não só aumentam a eficiência, como também fornecem aos médicos dados fiáveis, cruciais para orientar os cuidados prestados aos doentes, marcando um avanço significativo no campo da citometria de fluxo.
Análise Regional
Geograficamente, os países abrangidos pelo relatório do mercado global de identificação de parasitologia são
os EUA, Canadá e México na América do Norte, Alemanha, França, Reino Unido, Holanda, Suíça, Bélgica, Rússia, Itália, Espanha, Turquia, Resto da Europa na Europa, China, Japão, Índia, Coreia do Sul, Singapura, Malásia, Austrália, Tailândia, Indonésia, Filipinas, Resto da Ásia-Pacífico (APAC) na Ásia-Pacífico (APAC), Arábia Saudita, Emirados Árabes Unidos, África do Sul, Egito, Israel, Resto do Oriente Médio e África (MEA) como parte do Médio Oriente e África (MEA), Brasil, Argentina e Resto da América do Sul como parte da América do Sul
De acordo com a análise de pesquisa de mercado da Data Bridge:
A América do Norte é a região dominante no mercado global de identificação de parasitologia durante o período previsto de 2023-2030
A América do Norte domina o mercado global de identificação de parasitologia, impulsionada pelo seu estatuto de maior mercado consumidor e pelo elevado produto interno bruto. Espera-se que os EUA, um ator importante na região, mantenham este domínio, registando um crescimento no mercado de identificação de parasitologia durante o período previsto. Fatores como o aumento dos custos com os cuidados de saúde, as iniciativas intensificadas de investigação e desenvolvimento relacionadas com a parasitologia e os avanços contínuos nas ferramentas de diagnóstico contribuem para o crescimento previsto, solidificando a posição de liderança da América do Norte.
Estima-se que a Ásia-Pacífico seja a região de crescimento mais rápido no mercado global de identificação de parasitologia durante o período previsto de 2023-2030
Espera-se que a Ásia-Pacífico domine o mercado global de identificação de parasitologia, registando a maior taxa de crescimento. Este domínio é alimentado pela atualização da infraestrutura médica e por iniciativas proativas de organismos federais. Na região, a China está pronta para ser líder de mercado, apresentando uma rápida adoção de produtos e avanços notáveis na identificação de parasitologia. Os contributos significativos do país posicionam-no como pioneiro no panorama global de identificação de parasitologia.
Para obter informações mais detalhadas sobre o relatório do mercado global de identificação de parasitologia, clique aqui – https://www.databridgemarketresearch.com/reports/global-parasitology-identification-market