Os distúrbios da coluna vertebral abrangem uma vasta gama de condições que afetam a coluna vertebral, a medula espinhal e as estruturas associadas. Vários fatores contribuem para o aumento da incidência de distúrbios da coluna vertebral e, consequentemente, para a procura de materiais de enxerto da coluna vertebral. Os estilos de vida modernos, que podem envolver estar sentado durante muito tempo, redução da atividade física e má postura, podem contribuir para o desenvolvimento de distúrbios da coluna vertebral. Estes fatores podem acelerar o desgaste das estruturas da coluna vertebral e aumentar o risco de condições como a hérnia discal e a degeneração lombar. As lesões traumáticas, como acidentes de viação, quedas e lesões desportivas, podem resultar em fraturas da coluna vertebral, luxações e outros distúrbios graves da coluna vertebral. Estas lesões necessitam geralmente de intervenções cirúrgicas e do uso de materiais de enxerto para reconstrução da coluna vertebral.
A crescente incidência de distúrbios da coluna vertebral sublinha a importância de opções de tratamento eficazes, incluindo os enxertos espinhais, para tratar estas condições e melhorar a qualidade de vida dos doentes.
Aceda ao relatório completo em https://www.databridgemarketresearch.com/reports/europe-spinal-allografts-and-xenograft-market
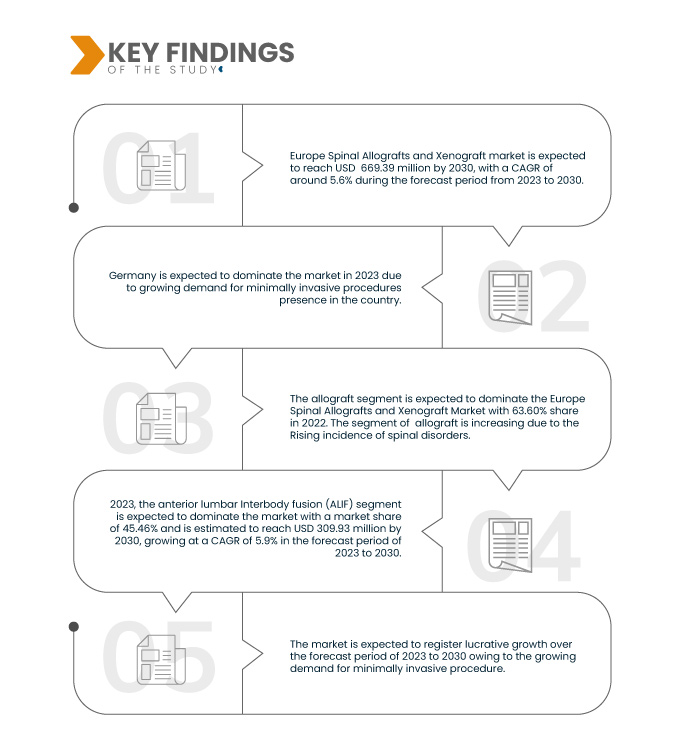
A Data Bridge Market Research analisa que o mercado europeu de aloenxertos espinhais e xenoenxertos deverá crescer com um CAGR de 5,6% no período previsto de 2023 a 2030 e deverá atingir os 669,39 milhões de dólares até 2030. O segmento dos aloenxertos deverá impulsionar o crescimento do mercado devido aos avanços nas técnicas cirúrgicas utilizadas nos enxertos espinhais.
Principais conclusões do estudo
Avanços nas Medicinas Regenerativas
A medicina regenerativa é um campo em rápida evolução que se concentra em aproveitar a capacidade natural do corpo para reparar e regenerar tecidos. Os enxertos alogénicos e xenogénicos desempenham um papel crucial na medicina regenerativa, servindo como materiais de enxerto para vários procedimentos de reparação de tecidos e órgãos. As técnicas de medicina regenerativa, como a engenharia de tecidos e a terapia com células estaminais , estão a fazer avançar as capacidades de regeneração de tecidos. Os aloenxertos e xenoenxertos são frequentemente utilizados como andaimes ou matrizes em aplicações de engenharia de tecidos. A sua compatibilidade com abordagens regenerativas torna-os valiosos para alcançar uma regeneração tecidular melhorada, aumentando a procura por estes materiais de enxerto. As terapias com células estaminais, incluindo os tratamentos com células estaminais mesenquimais (MSC), estão a ser utilizadas para promover a reparação e regeneração de tecidos. Os aloenxertos e xenoenxertos podem servir como transportadores de células estaminais, auxiliando a sua entrega e integração em tecidos danificados. Esta sinergia entre os materiais de enxerto e as terapias com células estaminais oferece oportunidades para tratamentos regenerativos inovadores.
Os avanços nos biomateriais e nas tecnologias de processamento de enxertos levaram ao desenvolvimento de materiais de enxerto mais biocompatíveis e amigos dos tecidos. A biocompatibilidade melhorada garante que os materiais de enxerto são bem tolerados pelo organismo, reduzindo o risco de complicações e promovendo uma regeneração bem-sucedida dos tecidos.
Assim sendo, espera-se que os avanços na medicina regenerativa proporcionem uma oportunidade de crescimento do mercado.
Âmbito do Relatório e Segmentação de Mercado
Métrica de Reporte
|
Detalhes
|
Período de previsão
|
2023 a 2030
|
Ano base
|
2022
|
Anos Históricos
|
2021 (personalizável para 2015 - 2020)
|
Unidades quantitativas
|
Receita em milhões de dólares americanos
|
Segmentos abrangidos
|
Tipo de produto (aloenxerto, xenoenxerto, suplementos de enxerto ósseo), abordagens ( fusão intersomática lombar anterior (ALIF), fusão intersomática lombar transforaminal (TLIF), fusão intersomática lombar posterior (PLIF), tipo de cirurgia (cirurgia de coluna aberta e cirurgia de coluna minimamente invasiva), indicação (doenças degenerativas, traumas ou fraturas da coluna, tumores da coluna, cirurgias de revisão, infeções da coluna (osteomielite ou discite), deformidades da coluna, anormalidades congénitas da coluna e outras), faixa etária (adulto, geriátrico e pediátrico), utente final (hospital, clínica especializada, centros de cirurgia de ambulatório e outros)
|
Países abrangidos
|
Alemanha, França, Reino Unido, Itália, Espanha, Rússia, Suíça, Países Baixos, Turquia, Polónia, Suécia, Bélgica, Dinamarca, Finlândia, Noruega e resto da Europa
|
Participantes do mercado abrangidos
|
Medtronic (Irlanda), Arthrex, Inc. (EUA), Stryker (EUA), ZimVie Inc. (EUA) e Medical Devices Business Services, Inc. (EUA), RTI Surgical (EUA), Integra LifeSciences (EUA), Orthofix US LLC. (EUA), ATEC Spine, Inc (EUA), Globus Medical (EUA), Exactech, Inc. (EUA), Regenity (EUA), Cerapedics.Inc (EUA), Bioventus (EUA) e entre outros
|
Pontos de dados abordados no relatório
|
Para além dos insights sobre os cenários de mercado, tais como o valor de mercado, a taxa de crescimento, a segmentação, a cobertura geográfica e os principais participantes, os relatórios de mercado selecionados pela Data Bridge Market Research incluem também análises aprofundadas de especialistas, epidemiologia dos doentes, análise de pipeline, análise de preços e estrutura regulamentar.
|
Análise de Segmentos:
O mercado europeu de aloenxertos espinais e xenoenxertos está segmentado em seis segmentos notáveis com base no tipo de produto, abordagens, tipo de cirurgia, indicação, faixa etária e utilizador final.
- Com base no tipo de produto, o mercado está segmentado em aloenxertos, xenoenxertos e suplementos para enxerto ósseo.
Em 2023, prevê-se que o segmento dos aloenxertos domine o mercado europeu de aloenxertos espinhais e xenoenxertos
Em 2023, prevê-se que o segmento dos aloenxertos domine o mercado com uma quota de mercado de 63,67% devido à crescente incidência de perturbações da coluna vertebral na Europa.
- Com base nas abordagens, o mercado está segmentado em fusão intersomática lombar anterior (ALIF), fusão intersomática lombar transforaminal (TLIF) e fusão intersomática lombar posterior (PLIF).
Em 2023, prevê-se que o segmento da fusão intersomática lombar anterior (ALIF) domine o mercado europeu de aloenxertos espinais e xenoenxertos
Em 2023, prevê-se que o segmento da fusão intersomática lombar anterior (ALIF) domine o mercado com uma quota de mercado de 45,46% devido aos avanços nas técnicas cirúrgicas utilizadas no enxerto espinal.
- Com base no tipo de cirurgia, o mercado está segmentado em cirurgia de coluna aberta e cirurgia de coluna minimamente invasiva. Em 2023, prevê-se que o segmento da cirurgia da coluna aberta domine o mercado com uma quota de mercado de 57,42%.
- Com base na indicação, o mercado está segmentado em doenças degenerativas, traumatismos ou fraturas da coluna vertebral, tumores da coluna vertebral, cirurgias de revisão, infeções da coluna vertebral (osteomielite ou discite), deformidades da coluna vertebral, anomalias congénitas da coluna vertebral e outras. Em 2023, prevê-se que o segmento das doenças degenerativas domine o mercado, com uma quota de mercado de 38,14%.
- Com base na faixa etária, o mercado está segmentado em geriátrico, adulto e pediátrico. Em 2023, prevê-se que o segmento adulto domine o mercado com uma quota de mercado de 50,40%.
Com base no utilizador final, o mercado está segmentado em hospitais, clínicas especializadas, centros de cirurgia ambulatória e outros. Em 2023, prevê-se que o segmento hospitalar domine o mercado com uma quota de mercado de 48,80%.
Principais jogadores
A Data Bridge Market Research reconhece as seguintes empresas como participantes do mercado europeu de aloenxertos espinais e xenoenxertos: Medtronic (Irlanda), Arthrex, Inc. (EUA), Stryker (EUA), ZimVie Inc. (EUA), Medical Devices Business Services, Inc. (EUA) e, entre outras.
Desenvolvimento de Mercado
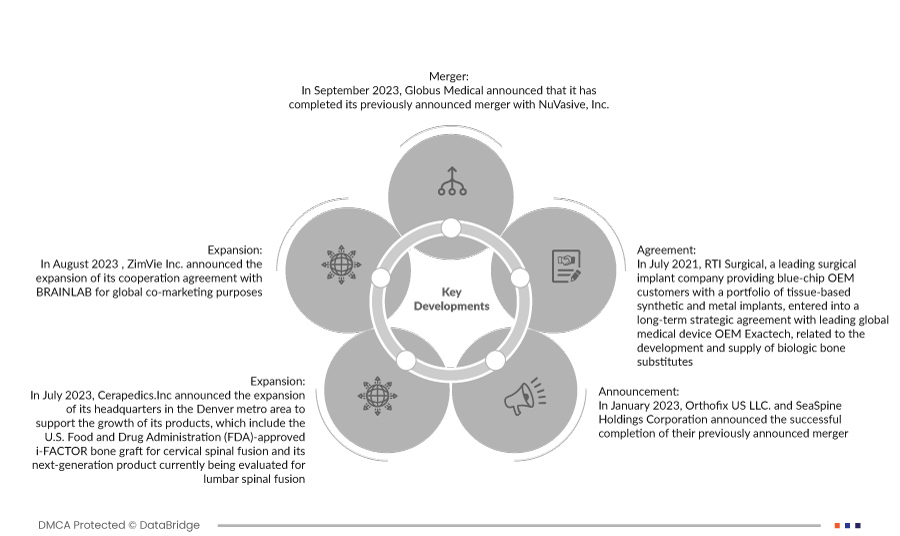
- Em setembro de 2023, a Globus Medical anunciou que tinha concluído a sua fusão previamente anunciada com a NuVasive, Inc. A empresa combinada irá fornecer aos cirurgiões e aos pacientes uma das ofertas mais abrangentes de soluções de procedimentos musculoesqueléticos e tecnologias capacitadoras para impactar o continuum de cuidados.
- Em agosto de 2023, a ZimVie Inc. anunciou a expansão do seu acordo de cooperação com a BRAINLAB para fins de co-marketing global. Isto ajudará a organização a desenvolver a receita anual. Em julho de 2022, a AbbVie e a iSTAR Medical SA anunciaram uma transação estratégica para desenvolver e comercializar ainda mais o dispositivo MINIject da iSTAR Medical, um dispositivo cirúrgico minimamente invasivo para o glaucoma (MIGS) para doentes com glaucoma. O acordo reforça a sua capacidade de desenvolver e fabricar produtos para cirurgia de glaucoma para os seus clientes, para utilização em procedimentos minimamente invasivos.
- Em julho de 2023, a Cerapedics.Inc. anunciou a expansão da sua sede na área metropolitana de Denver para apoiar o crescimento dos seus produtos, que incluem o enxerto ósseo i-FACTOR aprovado pela Food and Drug Administration (FDA) dos EUA para a fusão espinhal cervical e o seu produto de última geração está atualmente a ser avaliado para a fusão espinhal lombar. Isto aumentará a receita anual da empresa.
- Em janeiro de 2023, a Orthofix US LLC. e a SeaSpine Holdings Corporation anunciaram a conclusão com sucesso da sua fusão anteriormente anunciada. Isto ajudará a organização a desenvolver uma imagem de marca entre outras.
- Em julho de 2021, a RTI Surgical, empresa líder em implantes cirúrgicos que fornece aos clientes OEM de primeira linha um portefólio de implantes sintéticos e metálicos baseados em tecidos, assinou um acordo estratégico de longo prazo com a Exactech, o principal fabricante global de dispositivos médicos, relacionado com o desenvolvimento e fornecimento de substitutos ósseos biológicos.
Análise Regional
Geograficamente, os países abrangidos pelo relatório de mercado de enxertos espinais e xenoenxertos da Europa são a Alemanha, França, Reino Unido, Itália, Espanha, Rússia, Suíça, Países Baixos, Turquia, Polónia, Suécia, Bélgica, Dinamarca, Finlândia, Noruega e Resto da Europa.
De acordo com a análise de pesquisa de mercado da Data Bridge:
A Alemanha é o país dominante e mais rápido na Europa no mercado de enxertos espinais e xenoenxertos durante o período previsto de 2023 a 2030.
Espera-se que a Alemanha domine e seja o país com crescimento mais rápido devido ao elevado avanço tecnológico e à crescente presença de participantes no mercado no país.
Para obter informações mais detalhadas sobre o relatório de mercado de aloenxertos espinais e xenoenxertos na Europa, clique aqui – https://www.databridgemarketresearch.com/reports/europe-spinal-allografts-and-xenograft-market