Muitas tendências e avanços revolucionários foram testemunhados pelas indústrias farmacêuticas e de dispositivos médicos que melhoraram dramaticamente os medicamentos disponíveis para pacientes em todo o mundo na última década. Em apenas dez anos, foi possível testemunhar o efeito da inteligência artificial e do big data no diagnóstico e tratamento de doenças, e um movimento no sentido de evitar doenças potencialmente fatais em vez de medicá-las.
Além disso, o surgimento da pandemia da COVID-19 aumentou a procura de produtos farmacêuticos e dispositivos médicos em todo o mundo, desde sistemas de ventilação, kits descartáveis e vários medicamentos, entre outros. Várias empresas reformularam seus sistemas para atender à demanda por dispositivos médicos. A pesquisa e o desenvolvimento de várias empresas farmacêuticas aumentaram em todo o mundo para atender à necessidade urgente da vacina contra a COVID-19, levando ao aumento da demanda por testes de controle de qualidade de endotoxinas e pirogênios.
A prosperidade e a expansão dessas empresas aumentam proporcionalmente a necessidade de monitoramento de endotoxinas e pirogênios para a gestão da qualidade de seus produtos, porque se a endotoxina estiver presente em medicamentos injetáveis prescritos ou em dispositivos implantáveis e entrar na corrente sanguínea ou na medula espinhal, os incidentes começam com choque séptico, febre , danos a órgãos e possivelmente morte. Portanto, espera-se que o rápido crescimento dos dispositivos farmacêuticos e médicos impulsione o crescimento do mercado.
Acesse o relatório completo @https://www.databridgemarketresearch.com/reports/asia-pacific-endotoxin-and-pyrogen-testing-market
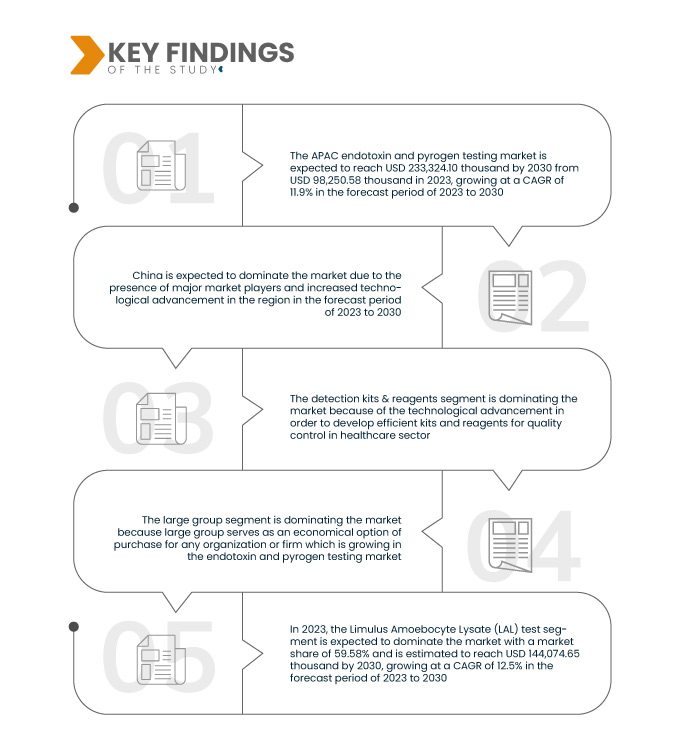
A Data Bridge Market Research analisa que o Mercado Ásia-Pacífico de testes de endotoxinas e pirogênios deverá crescer com um CAGR de 11,9% no período de previsão de 2023 a 2030, e deverá atingir US$ 233.324,10 mil até 2030.
Principais conclusões do estudo
O aumento dos investimentos em P&D e das aprovações de medicamentos pode impulsionar o crescimento do mercado
As indústrias associadas ao controle de qualidade realizam extensa pesquisa e desenvolvimento não apenas para gerar receitas, mas muitas vezes trazem resultados em vidas, salvando ou melhorando a vida de um paciente, fornecendo medicamentos parenterais estéreis ou livres de contaminação e dispositivos médicos implantáveis. A I&D é crucial em todas as indústrias, incluindo as indústrias que lidam com o controlo de qualidade, como os testes de endotoxinas.
A P&D no mercado de testes de endotoxinas realiza pesquisas para fornecer um método de teste eficiente com resultados aprimorados para minimizar os erros que levam ao recall de produtos entre diversas indústrias. Além disso, os dispositivos são desenvolvidos para atender aos padrões regulatórios de testes de endotoxinas. Hoje em dia, as empresas associadas ao controle de qualidade têm aumentado o foco em P&D, o que aumentou a demanda por testes de endotoxinas.
Além disso, prevê-se que o crescimento promissor no pipeline de produtos e o aumento da aprovação de medicamentos impulsionem o crescimento do mercado. O surgimento da COVID-19 também levou a um aumentotestes clínicos para o desenvolvimento de vacinas para a COVID-19. Isto aumentou ainda mais a demanda por produtos e serviços de testes de endotoxinas e pirogênios.
Escopo do relatório e segmentação de mercado
|
Métrica de relatório
|
Detalhes
|
|
Período de previsão
|
2023 a 2030
|
|
Ano base
|
2022 (personalizável para 2015-2020)
|
|
Anos históricos
|
2021
|
|
Unidades Quantitativas
|
Receita em milhares de dólares e preços em dólares
|
|
Segmentos cobertos
|
Tipo de produto (kits de detecção e reagentes, instrumentos e sistemas, serviços de teste de endotoxinas e pirogênios, consumíveis e acessórios), tipo de teste (teste de lisado de amebócitos de Limulus (LAL), testes TAL, teste de ativação de monócitos (MAT), C recombinante (RFC) Ensaio e teste de pirogênio em coelho), aplicação (fabricação farmacêutica, fabricação de dispositivos médicos, produção de matérias-primas e fabricação de embalagens), método (teste de endotoxina e pirogênio em coágulo de gel, teste de endotoxina e pirogênio cromogênico e teste turbidimétrico de endotoxina e pirogênio), modo de Compra (Grupo Grande, Grupo Médio e Pequeno e Individual), Produto Final (Vacinas e/ou CGT, biológicos, injetáveis e outros), usuário final (empresas farmacêuticas, empresas de biotecnologia, empresas biomédicas, empresas de dispositivos médicos, organização de pesquisa contratada (CRO), organização de fabricação contratada (CMO) e outras)
|
|
Países abrangidos
|
China, Japão, Índia, Coreia do Sul, Austrália, Singapura, Nova Zelândia, Indonésia, Filipinas, Tailândia, Malásia, Vietname e resto da Ásia-Pacífico
|
|
Participantes do mercado cobertos
|
Pall Corporation (EUA), Thermo Fisher Scientific Inc. (EUA), Charles River Laboratories (EUA), Eurofins Scientific (Luxemburgo) e SGS Société Générale de Surveillance SA. (Suíça), entre outros.
|
|
Pontos de dados abordados no relatório
|
Além dos insights sobre cenários de mercado, como valor de mercado, taxa de crescimento, segmentação, cobertura geográfica e grandes players, os relatórios de mercado com curadoria da Data Bridge Market Research também incluem análises especializadas aprofundadas, epidemiologia de pacientes, análise de pipeline, análise de preços, e quadro regulamentar.
|
Análise de Segmento
O mercado de testes de endotoxinas e pirogênios da Ásia-Pacífico é segmentado em sete segmentos notáveis com base no tipo de produto, tipo de teste, aplicação, método, modo de compra, produto final e usuário final.
- Com base no tipo de produto, o mercado é segmentado em kits de detecção e reagentes, instrumentos e sistemas, serviços de testes de endotoxinas e pirogênios e consumíveis e acessórios
Em 2023, espera-se que o segmento de kits de detecção e reagentes domine o mercado.
Em 2023, espera-se que o segmento de kits de detecção e reagentes domine o mercado com uma participação de mercado de 62,81% devido ao avanço tecnológico para desenvolver kits e reagentes eficientes para controle de qualidade no setor de saúde.
- Com base no tipo de teste, o mercado é segmentado em teste de lisado de amebócito limulus (LAL), teste TAL, teste de ativação de monócitos (MAT), teste de pirogênio de coelho e ensaio C recombinante (RFC).
Em 2023, espera-se que o segmento de teste Limulus Amoebocyte Lysate (LAL) domine o mercado.
Em 2023, espera-se que o segmento de testes de Lisado de Amebócitos Limulus (LAL) domine o mercado com uma participação de mercado de 59,58% devido ao aumento da eficiência e confiabilidade de vários consumidores e órgãos reguladores no tipo de teste específico para testes de endotoxinas.
- Com base na aplicação, o mercado é segmentado em fabricação farmacêutica, fabricação de dispositivos médicos, produção de matérias-primas e fabricação de embalagens. Em 2023, espera-se que o segmento de fabricação farmacêutica domine o mercado com uma participação de mercado de 39,19%
- Com base no método, o mercado é segmentado em endotoxina de coágulo de gel e teste de pirogênio, endotoxina cromogênica e teste de pirogênio e endotoxina turbidimétrica e teste de pirogênio. Em 2023, espera-se que o segmento de testes de endotoxina de coágulo de gel e pirogênio domine o mercado com uma participação de mercado de 59,55%
- Com base no modo de compra, o mercado é segmentado em grupos grandes, grupos médios e pequenos e individuais. Em 2023, espera-se que o segmento de grandes grupos domine o mercado com uma participação de mercado de 63,64%
- Com base no produto final, o mercado é segmentado em vacinas e/ou CGT, biológicos, injetáveis, entre outros. Em 2023, espera-se que o segmento de Vacinas e/ou CGT domine o mercado com uma participação de mercado de 62,33%
- Com base no usuário final, o mercado é segmentado em empresas farmacêuticas, empresas de biotecnologia, empresas biomédicas, empresas de dispositivos médicos, Organização de Pesquisa Contratual (CRO), Organização de Fabricação de Contrato (CMO), entre outras. Em 2023, espera-se que o segmento de empresas farmacêuticas domine o mercado com uma participação de mercado de 39,84%
Jogadores principais
A Data Bridge Market Research analisa Pall Corporation (EUA), Thermo Fisher Scientific Inc. (EUA), Charles River Laboratories (EUA), Eurofins Scientific (Luxemburgo) e SGS Société Générale de Surveillance SA. (Suíça) como os principais players do mercado.
Desenvolvimentos de mercado
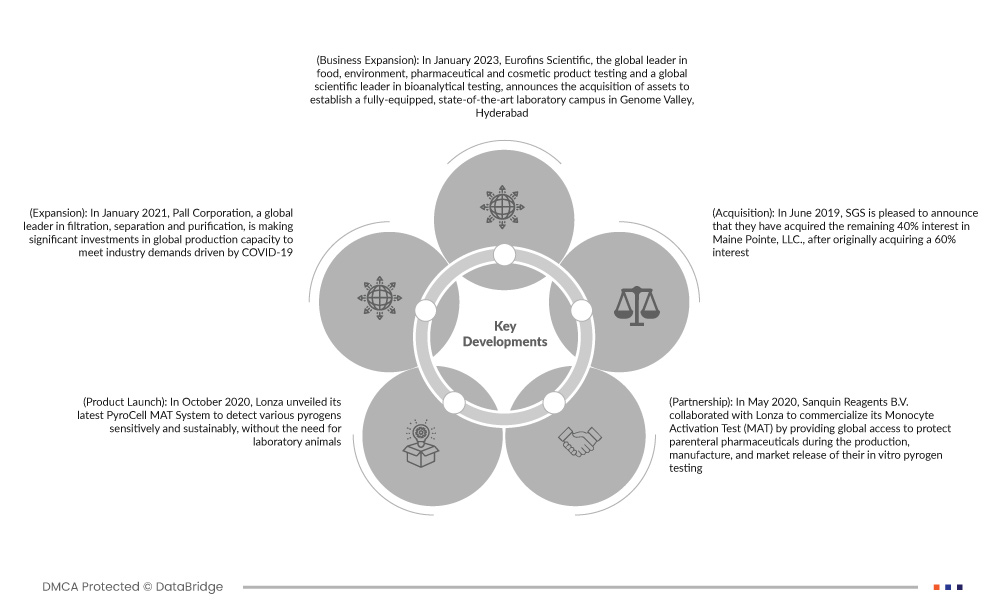
- Em janeiro de 2023, a Eurofins Scientific, líder global em testes de produtos alimentícios, ambientais, farmacêuticos e cosméticos e líder científica global em testes bioanalíticos, anuncia a aquisição de ativos para estabelecer um laboratório totalmente equipado e de última geração campus em Genome Valley, Hyderabad
- Em janeiro de 2021, a Pall Corporation, líder global em filtração, separação e purificação, está fazendo investimentos significativos na capacidade de produção global para atender às demandas da indústria impulsionadas pela COVID-19. Estes investimentos incluem capacidade de produção adicional em seis instalações de produção existentes na Europa e nos Estados Unidos, bem como uma nova unidade de produção nos Estados Unidos. Isso ajuda a organização no desenvolvimento da receita geral
- Em outubro de 2020, a Lonza revelou seu mais recente sistema PyroCell MAT para detectar vários pirogênios de forma sensível e sustentável, sem a necessidade de animais de laboratório. O produto foi lançado com uma solução completa com reagentes MAT otimizados da Sanquin e meios e acessórios da Lonza. A introdução deste novo produto pela empresa aumentou sua credibilidade e demanda no mercado
- Em maio de 2020, a Sanquin Reagents BV colaborou com a Lonza para comercializar seu teste de ativação de monócitos (MAT), fornecendo acesso global para proteger produtos farmacêuticos parenterais durante a produção, fabricação e lançamento no mercado de testes de pirogênios in vitro. O relacionamento da empresa ampliou sua gama de produtos de gestão de qualidade
- Em junho de 2019, a SGS teve o prazer de anunciar que havia adquirido os 40% restantes de participação na Maine Pointe, LLC., após adquirir originalmente uma participação de 60%. Isso ajudará a organização no desenvolvimento da receita geral
Análise Regional
Geograficamente, os países abrangidos no relatório de mercado são China, Japão, Índia, Coreia do Sul, Austrália, Singapura, Nova Zelândia, Indonésia, Filipinas, Tailândia, Malásia, Vietname e resto da Ásia-Pacífico.
De acordo com a análise da pesquisa de mercado da Data Bridge:
A China é o país dominante e de crescimento mais rápido no mercado durante o período de previsão 2023-2030
Em 2023, espera-se que a China domine o mercado devido ao maior nível de investimentos de diversos fabricantes e ao aumento dos avanços tecnológicos na região. A China continuará a dominar o mercado em termos de quota de mercado e receitas de mercado e continuará a florescer o seu domínio durante o período de previsão. Estima-se que a China seja o país que mais cresce no mercado durante o período de previsão de 2023 a 2030 devido à crescente adoção de tecnologia avançada e ao lançamento de novos produtos nesta região.
Para obter informações mais detalhadas sobre o relatório de mercado de testes de endotoxinas e pirogênios da Ásia-Pacífico, clique aqui –https://www.databridgemarketresearch.com/reports/asia-pacific-endotoxin-and-pyrogen-testing-market