Adult somatic cells can be used to make induced Pluripotent Stem Cells (iPSCs)—a form of stem cell (such as skin cells or blood cells). These cells have been rewired to become pluripotent, or more such as embryonic stem cells, in the past. They are useful for various research applications, disease modeling, regenerative therapy, and drug discovery because iPSCs can differentiate into multiple bodily cell types. Typically, the process of creating iPSCs entails inserting particular genes or factors into adult cells, which causes them to undergo a developmental clock reset and take on traits akin to embryonic stem cells.
Access Full Report @ https://www.databridgemarketresearch.com/reports/us-induced-pluripotent-stem-cells-market
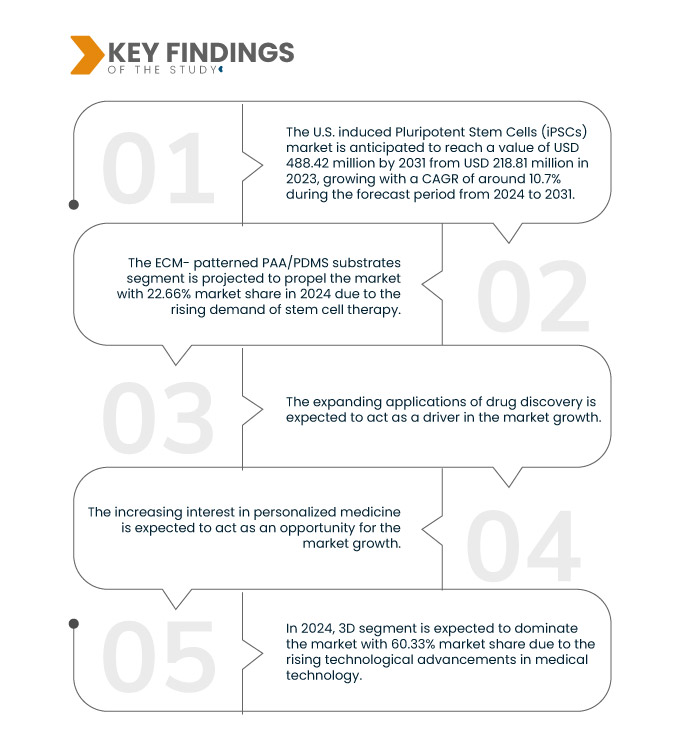
Data Bridge Market Research analyzes that the U.S. Induced Pluripotent Stem Cells (iPSCs) Market is expected to grow at a CAGR of 10.7% in the forecast period of 2024 to 2031 and is expected to reach USD 488.42 million by 2031 from USD 218.87 million in 2023. The ECM-patterned PAA/ PDMS substrates segment is projected to propel the market growth due to the rising focus on personalized or precision-based research activities.
Key Findings of the Study
Technological Advancement in Regenerative Medicine
Researchers and clinicians are investigating novel therapeutic approaches to address a range of debilitating conditions as regenerative medicine advances because of their extraordinary capacity to differentiate into diverse cell types, which may replace or repair damaged tissues. Regenerative medicine's guiding principles of restoring normal function and promoting healing align with the adaptability of iPSCs, enabling precise and personalized treatments. Thus, the need for induced Pluripotent Stem Cell (iPSCs) in the U.S. is fueled by the increased focus on regenerative medicine applications such as tissue engineering and organ regeneration. The market is positioned as a major player in advancing transformative therapeutic solutions for various medical conditions and the growing utility of induced Pluripotent Stem Cell (iPSCs). The objectives of regenerative medicine align with the capacity of induced Pluripotent Stem Cell (iPSCs) to differentiate into particular cell types needed for tissue repair and regeneration, offering a potent platform to address medical treatment.
Report Scope and Market Segmentation
|
Report Metric
|
Details
|
|
Forecast Period
|
2024 to 2031
|
|
Base Year
|
2023
|
|
Historic Years
|
2022 (Customizable to 2016–2021)
|
|
Quantitative Units
|
Revenue in USD Million
|
|
Segments Covered
|
Model Type (ECM-Patterned PAA/ PDMS Substrates, Microgroove PDMS Substrates, Strained Membranes, 3D Sacffolds, Free-Floating Hydrogel Contructs, and EHTS), Material (Natural Hydrogel-Based Cardiac Models and Synthetic Fibrous Cardiac Models), Cell Source (Skin Cells and Blood Cells), Dimensional Type (2D and 3D), Application (Production of New Cardiac Tissue to Replace Tissue Destroyed by Infraction or Other Diseases, Heart Failure, Isischemic heart disease, chemic Heart Disease, Arrhythmia, Cardiomyopathies, Valvular Heart Disease, Heart Disease Screening, and Others), End Use (Pharmaceutical & Biotechnology Companies, Academic and Government Research Institutes, Contract Development & Manufacturing Organizations, and Others), Distribution Channel (Direct Tender and Retail Sales)
|
|
Countries Covered
|
U.S.
|
|
Market Players Covered
|
Corning Incorporated (U.S.), Lonza (Switzerland), MIMETAS (Netherlands), CN Bio Innovations Ltd (U.K.), 3D BIOTEK LLC (U.S.), AXOL (U.K.), and REPROCELL Inc. (Japan) among others
|
|
Data Points Covered in the Report
|
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework
|
Segment Analysis
U.S. induced Pluripotent Stem Cells (iPSCs) market is segmented into seven notable segments based on model type, material, cell source, dimensional type, application, end use, and distribution channel.
- On the basis of model type, the U.S. induced Pluripotent Stem Cells (iPSCs) market is segmented into ECM-patterned PAA/ PDMS substrates, microgroove PDMS substrates, strained membranes, 3D scaffolds, free-floating hydrogel constructs, and EHTS
In 2024, the ECM- patterned PAA/PDMS substartes segment is expected to dominate the U.S. induced Pluripotent Stem Cells (iPSCs) market
In 2024, the ECM-patterned PAA/ PDMS substrates segment is expected to dominate the market with a market share of 22.66% due to the rising focus on personalized or precision-based research activities to formulate the treatment of diseases.
- On the basis of material, the market is segmented into natural hydrogel based cardiac models and synthetic fibrous cardiac models
In 2024, the natural hydrogel-based cardiac models segment is expected to dominate the U.S. induced Pluripotent Stem Cells (iPSCs) market
In 2024, the natural hydrogel-based cardiac models segment is expected to dominate the market with a market share of 57.37% due to the rising research and development activities.
- On the basis of cell source, the U.S. induced Pluripotent Stem Cells (iPSCs) market is segmented into skin cells and blood cells. In 2024, the skin cells segment is expected to dominate the market with a market share of 63.45%
- On the basis of dimensional type, the U.S. induced Pluripotent Stem Cells (iPSCs) market is segmented into 3D and 2D. In 2024, the 3D segment is expected to dominate the market with a market share of 60.33%
- On the basis of application, the U.S. induced Pluripotent Stem Cells (iPSCs) market is segmented into production of new cardiac tissue to replace tissue destroyed by infraction or other diseases, heart failure, ischemic heart disease, arrhythmia, cardiomyopathies, valvular heart disease, heart disease screening, and others. In 2024, the production of new cardiac tissue to replace tissue destroyed by infraction or other diseases segment is expected to dominate the market with a market share of 22.96%
- On the basis of end use, the U.S. induced Pluripotent Stem Cells (iPSCs) market is segmented into pharmaceutical & biotechnology companies, academic and government research institutes, contract development & manufacturing organizations, and others. In 2024, the pharmaceutical & biotechnology companies segment is expected to dominate the market with a market share of 35.01%
- On the basis of distribution channel, the U.S. induced Pluripotent Stem Cells (iPSCs) market is segmented into direct tender and retail sales. In 2024, the direct tender segment is expected to dominate the market with a market share of 65.90%
Major Players
Data Bridge Market Research analyses Corning Incorporated (U.S.), Lonza (Switzerland), MIMETAS (Netherlands), CN Bio Innovations (U.K.), and REPROCELL Inc. (Japan) as the major players in the U.S. induced Pluripotent Stem Cells (iPSCs) market.
Market Development
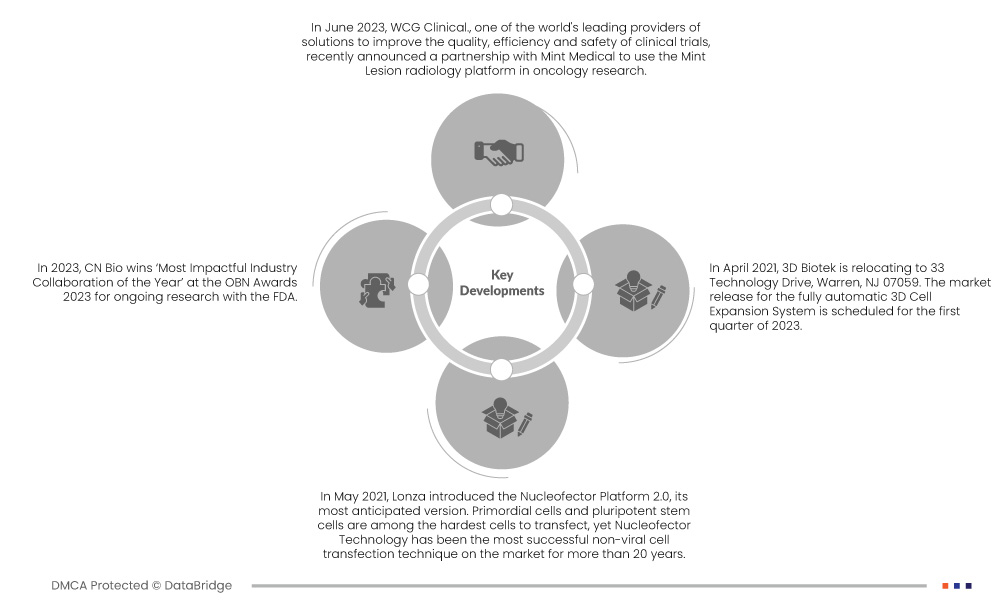
- In June 2023, WCG Clinical., one of the world's leading providers of solutions to improve the quality, efficiency and safety of clinical trials recently announced a partnership with Mint Medical to use the Mint Lesion radiology platform in oncology research. Mint Lesion software is used for standardized and computerized review of medical imaging according to defined protocols, guidelines and workflows. Through this partnership, WCG combines clinical trial management solutions: workflows, quality systems, imaging solutions, technology and a broad network of reviewers with Mint Lesion's validated read-ready platform, structured reporting and site-specific workflows. a best-in-class solution for oncology education. The combination of WCG and Mint Medical offers an effective solution to the market
- In 2023, CN Bio wins ‘Most Impactful Industry Collaboration of the Year’ at the OBN Awards 2023 for ongoing research with the FDA. Such an award helped the company to cater to more customers globally
- In April 2023, 3D Biotek is relocating to 33 Technology Drive, Warren, NJ 07059. The market release for the fully automatic 3D Cell Expansion System is scheduled for the first quarter of 2023
- In May 2021, Lonza introduced the Nucleofector Platform 2.0, its most anticipated version. Primordial cells and pluripotent stem cells are among the hardest cells to transfect, yet Nucleofector Technology has been the most successful non-viral cell transfection technique for more than 20 years. The next generation 4D-Nucleofector Platform now offers flexibility and the same reliable performance with even greater simplicity of use thanks to an improved core unit and even more user-friendly software. This has aided the business in diversifying its line of products
For more detailed information about the U.S. induced Pluripotent Stem Cells (iPSCs) market report, click here – https://www.databridgemarketresearch.com/reports/us-induced-pluripotent-stem-cells-market