Treatment for viral respiratory infections includes a wide range of medical procedures intended to fight diseases brought on by viruses that impact the respiratory system. Many ailments, including the common cold, influenza (flu), Respiratory Syncytial Virus (RSV), adenovirus, and more recently, new viruses such as SARS-CoV-2, which is responsible for COVID-19, are caused by these illnesses, which mostly affect the nose, throat, sinuses, and lungs. A variety of strategies are usually used to treat these illnesses, such as antiviral drugs, supportive care to reduce symptoms, immunization to ward off particular diseases, and, in more serious situations, hospitalization with cutting-edge techniques such as oxygen therapy or mechanical ventilation. The development of novel antiviral medications, vaccinations, and therapeutic approaches to improve treatment efficacy, lessen symptom intensity, and stop the transmission of these extremely contagious respiratory infections is constantly fueled by advances in medical science.
Access Full Report @ https://www.databridgemarketresearch.com/reports/global-viral-respiratory-infections-treatment-market
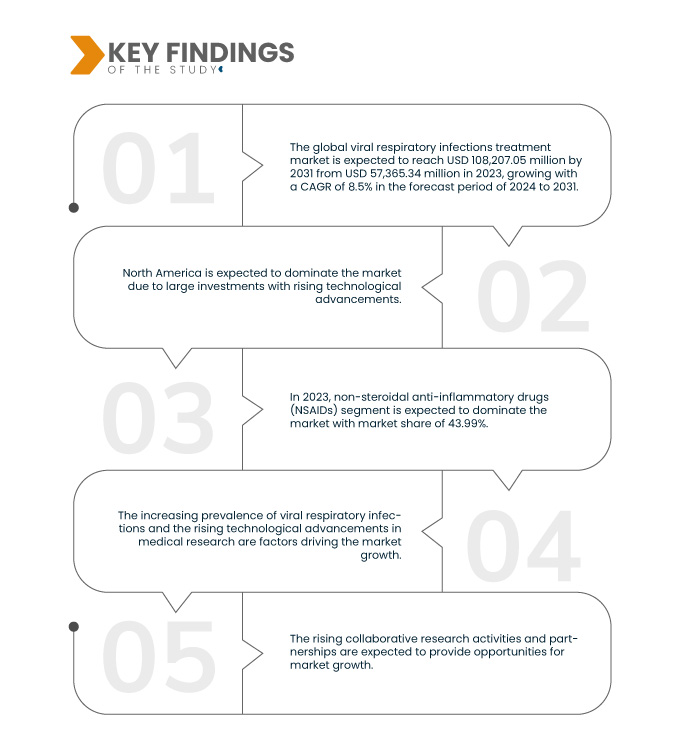
Data Bridge Market Research analyzes that the Global Viral Respiratory Infections Treatment Market is expected to reach USD 108,207.05 million by 2031 from USD 57,365.34 million in 2023, growing at a CAGR of 8.5% in the forecast period of 2024 to 2031.
Key Findings of the Study
Advancements in Medical Research and Technology
The relentless pursuit of innovative solutions has led to the development of novel antiviral treatments, diagnostic tools, and therapeutic approaches. These breakthroughs are reshaping the landscape of viral respiratory infection management, offering more effective and targeted interventions.
In recent years, the integration of cutting-edge technologies such as genomics and proteomics has empowered researchers to gain a deeper understanding of viral respiratory pathogens. This enhanced understanding has paved the way for the discovery of specific molecular targets for therapeutic interventions. Precision medicine approaches, enabled by advancements in genetic research, are fostering the development of personalized treatments tailored to individual patients, optimizing efficacy and minimizing side effects.
Report Scope and Market Segmentation
|
Report Metric
|
Details
|
|
Forecast Period
|
2024 to 2031
|
|
Base Year
|
2023
|
|
Historic Year
|
2022 (Customizable to 2016 – 2021)
|
|
Quantitative Units
|
Revenue in USD Million
|
|
Segments Covered
|
Treatment Type (Antiviral Medications, Vaccine, Monoclonal Antibodies, Non-steroidal Anti-inflammatory Drugs (NSAIDs), and Others), Type (OTC and Prescription Based), Route of Administration (Oral, Nasal, and Injectable), Disease Type (Covid, Influenza (FLU), Respiratory Syncytial Virus (RSV), Parainfluenza, Adenoviruses, Rhinovirus (Common Cold), Enterovirus, Anti-inflammatory, and Others), Infection Type (Upper Respiratory Infections and Lower Respiratory Infections), Population (Pediatric, Adults, and Geriatric), Gender (Male and Female), End User (Hospitals, Home Care Settings, Clinics, Research Institutes and Academic Centers, and Ambulatory Surgical Centers), Distribution Channel (Direct Tender, Retail Sales, and Others)
|
|
Countries Covered
|
U.S., Canada, Mexico, Germany, Italy, U.K., France, Switzerland, Spain, Russia, Turkey, Belgium, Netherlands, Rest of Europe, China, India, South Korea, Japan, Thailand, Australia, Singapore, Indonesia, Malaysia, Philippines, Rest of Asia-Pacific, Brazil, Argentina, Rest of South America, Saudi Arabia, U.A.E., South Africa, Egypt, Israel and Rest of Middle East and Africa
|
|
Market Players Covered
|
AstraZeneca (U.K.), Pfizer Inc. (U.S.), GSK plc (U.K.), F.Hoffmann-La Roche Ltd (Switzerland), SINOVAC (China), Lilly (U.S.), Regenron Pharmaceuticals Inc. (U.S.), Bharat Biotech. (India), Johnson & Johnson, Inc. (U.S.), Biological E Limited (India), Gilead Sciences, Inc. (U.S.), Serum Institute of India Pvt. Ltd (India), Swedish Orphan Biovitrum AB (publ) (Sweden), Celltrion Healthcare Co., Ltd (South Korea), InflaRx N.V. (Germany) and among others
|
|
Data Points Covered in the Report
|
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
|
Segment Analysis
The global viral respiratory infections treatment market is segmented into nine notable segments based on treatment type, type, route of administration, disease type, infection type, population, gender, end user, and distribution channel.
- On the basis of treatment type, the market is segmented into antiviral medications, vaccine, monoclonal antibodies, non-steroidal anti-inflammatory drugs (NSAIDs), and others
In 2024, the nonsteroidal anti-inflammatory drugs (NSAIDs) segment is expected to dominate the global viral respiratory infections treatment market
In 2024, the non-steroidal anti-inflammatory drugs (NSAIDs) segment is expected to dominate the market with a market share of 44.24% due to the rising prevalence of respiratory disorders.
- On the basis of type, the market is segmented into OTC and prescription based
In 2024, the prescription based segment is expected to dominate the global viral respiratory infections treatment market
In 2024, the prescription based segment is expected to dominate the market with a market share of 76.08% due to the easy accessibility and availability of drugs and vaccines.
- On the basis of route of administration, the market is segmented into oral, nasal, and injectable. In 2024, the nasal segment is expected to dominate the market with a market share of 60.43%
- On the basis of disease type, the market is segmented into COVID, influenza (FLU), respiratory syncytial virus (RSV), parainfluenza, adenovirus, rhinovirus (common cold), enterovirus, and others. In 2024, the Covid segment is expected to dominate the market with a market share of 29.13% due to rising incidences of infectious disorders after the pandemic
- On the basis of infection type, the market is segmented into upper respiratory infections and lower respiratory infections. In 2024, the lower respiratory infections segment is expected to dominate the market with a market share of 59.58%
- On the basis of population, the market is segmented into pediatric, adults, and geriatric. In 2024, the geriatric segment is expected to dominate the market with a market share of 44.23%
- On the basis of gender, the market is segmented into male and female. In 2024, the male segment is expected to dominate the market with a market share of 62.11%
- On the basis of end user, the market is segmented into hospitals, home care settings, clinics, research institutes and academic centers, and ambulatory surgical centers. In 2024, the hospitals segment is expected to dominate the market with a market share of 39.00%
- On the basis of distribution channel, the market is segmented into direct tender, retail sales, and others. In 2024, the direct tender segment is expected to dominate the market with a market share of 51.44%
Major Players
Data Bridge Market Research analyzes Pfizer Inc. (U.S.), GSK plc (U.K.), Gilead Sciences, Inc. (U.S.), F.Hoffmann-La Roche Ltd (Switzerland), and Johnson & Johnson, Inc. (U.S.) as the major market players in global viral respiratory infections treatment market.
Market Developments
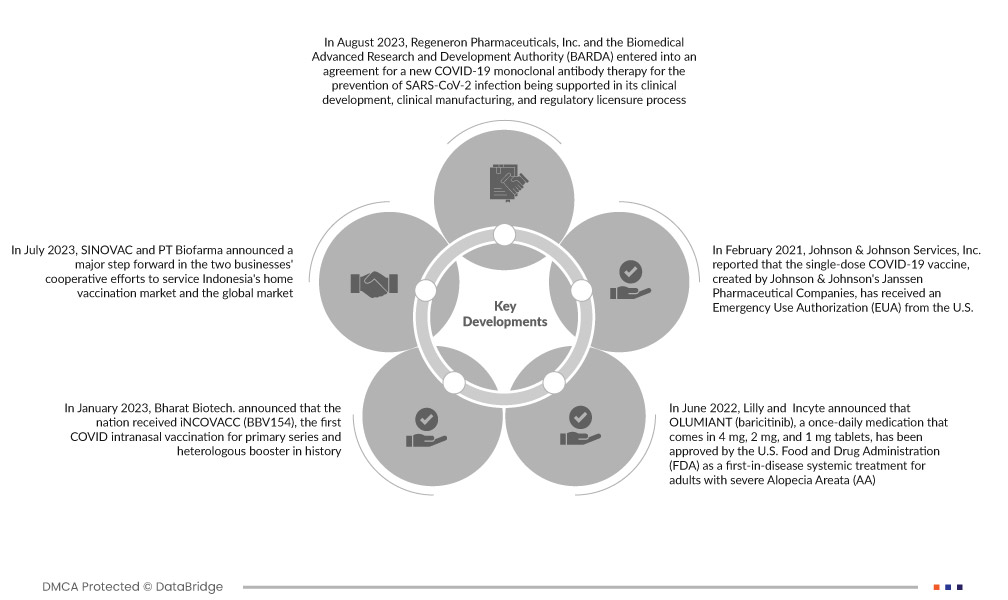
- In August 2023, Regeneron Pharmaceuticals, Inc. and the Biomedical Advanced Research and Development Authority (BARDA) entered into an agreement for a new COVID-19 monoclonal antibody therapy for the prevention of SARS-CoV-2 infection being supported in its clinical development, clinical manufacturing, and regulatory licensure process. The deal is a component of the U.S. Department of Health and Human Services (HHS) Project NextGen, which aims to develop a pipeline of novel COVID-19 vaccines and treatments
- In July 2023, SINOVAC and PT Biofarma announced a major step forward in the two businesses' cooperative efforts to service Indonesia's home vaccination market and the global market. On July 27, at the Indonesia-China Healthcare and Biotech Investment Forum in Chengdu, the capital of Sichuan Province in southwest China, a partnership agreement was signed. This helped the company to expand its business
- In January 2023, Bharat Biotech. announced that the nation received iNCOVACC (BBV154), the first COVID intranasal vaccination for primary series and heterologous booster in history. The Central Drugs Standard Control Organisation (CDSCO) gave Bharat Biotech. permission to provide iNCOVACC as a heterologous booster dose and for the primary series. A booster dose of the vaccination becomes essential due to the rising number of COVID-19 cases and the emergence of new, highly transmissible virus types. This helped the company to showcase its resources and expand its product offerings
- In June 2022, Lilly and Incyte announced that OLUMIANT (baricitinib), a once-daily medication that comes in 4 mg, 2 mg, and 1 mg tablets, has been approved by the U.S. Food and Drug Administration (FDA) as a first-in-disease systemic treatment for adults with severe Alopecia Areata (AA)
- In February 2021, Johnson & Johnson Services, Inc. reported that the single-dose COVID-19 vaccine, created by Johnson & Johnson's Janssen Pharmaceutical Companies, has received an Emergency Use Authorization (EUA) from the U.S. Food and Drug Administration (FDA) to prevent COVID-19 in people 18 years of age and older. The EUA's provisions permit vaccination usage while additional information is being obtained. Later in 2021, the company intends to submit a Biologics Licence Application (BLA) to the FDA
Regional Analysis
Geographically, the countries covered in the global viral respiratory infections treatment market report are U.S., Canada, Mexico, Germany, Italy, U.K., France, Switzerland, Spain, Russia, Turkey, Belgium, Netherlands, Rest of Europe, China, India, South Korea, Japan, Thailand, Australia, Singapore, Indonesia, Malaysia, Philippines, Rest of Asia-Pacific, Brazil, Argentina, Rest of South America, Saudi Arabia, U.A.E., South Africa, Egypt, Israel and Rest of Middle East and Africa.
As per Data Bridge Market Research analysis:
North America is expected to dominate and to be fastest growing region in global viral respiratory infections market
North America is expected to dominate and to be fastest growing region in market owing to the higher level of investments by U.S. manufacturers and the increasing advancement in the treatment options in the U.S. North America will continue to dominate the market in terms of market share and market revenue and will continue to flourish its dominance during the forecast period. This is due to the growing adoption of advanced treatment technology and the rapid development of medical facilities in emerging economies in this region. In addition to this, the rising level of healthcare expenditure and increasing per-capita income are expected to propel the market growth in this region.
For more detailed information about the global viral respiratory infections treatment market report, click here – https://www.databridgemarketresearch.com/reports/global-viral-respiratory-infections-treatment-market