The rising burden of duchenne muscular dystrophy (DMD) is expected to drive the market growth in recent years. DMD is a rare and debilitating genetic disorder characterized by progressive muscle weakness and loss of function. It primarily affects males, with symptoms typically appearing in early childhood. The number of individuals diagnosed with DMD is also increasing, and this rising prevalence has created a pressing need for effective treatments and therapies as the global population continues to grow.
Thus, the increasing prevalence and impact of DMD is expected to drive the market growth as it has raised awareness, accelerated research and development efforts, and streamlined regulatory processes.
Access Full Report @ https://www.databridgemarketresearch.com/reports/global-duchenne-muscular-dystrophy-treatment-market
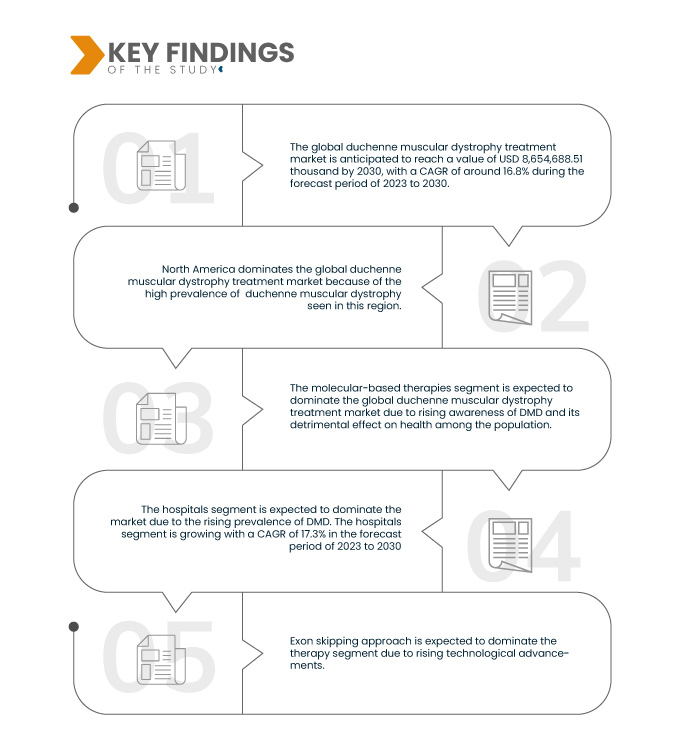
Data Bridge Market Research analyzes the Global Duchenne Muscular Dystrophy Treatment Market is expected to reach USD 8,654,688.51 thousand by 2030 from USD 2,595,090.00 thousand in 2022, growing with a substantial CAGR of 16.8% in the forecast period of 2023 to 2030.
Key Findings of the Study
Introduction of Novel Therapies for DMD Disorder is Expected to Drive the Market Growth
The introduction of novel therapies for DMD stands as a transformative force that is expected to drive the market growth. The emergence of innovative treatment approaches is reshaping the landscape of DMD care and contributing significantly to market expansion. Traditional treatment options primarily focused on symptom management and supportive care. However, the introduction of novel therapies represents a paradigm shift as they aim to address the root genetic mutations responsible for DMD.
Moreover, exon-skipping drugs have emerged as another promising class of therapies for DMD. These drugs are designed to "skip" specific exons in the dystrophin gene, allowing for the production of a truncated but partially functional dystrophin protein. Exon-skipping drugs can significantly slow the progression of the disease and improve the quality of life for individuals with DMD. The development and approval of these therapies have provided renewed expectation to DMD patients and further drive market growth.
The introduction of novel DMD therapies has inspired a sense of hope and optimism among the DMD community. This heightened optimism and awareness among people, which is further expected to drive the market growth.
Report Scope and Market Segmentation
|
Report Metric
|
Details
|
|
Forecast Period
|
2023 to 2030
|
|
Base Year
|
2022
|
|
Historic Years
|
2021 (Customizable to 2015-2020)
|
|
Quantitative Units
|
Revenue in USD Thousand
|
|
Segments Covered
|
Treatment Type (Molecular-Based Therapies, Steroid Therapy, and Others), Therapy (Exon Skipping Approach, Mutation Suppression, and Dystrophin-Targeted Therapies), Route of Administration (Oral, Parenteral, and Others), End User (Hospitals, Specialty Clinics, Homecare, and Others), Distribution Channel (Hospital Pharmacy, Retail Pharmacy, and Online Pharmacy)
|
|
Countries Covered
|
U.S., Canada, Mexico, Germany, France, U.K., Italy, Spain, Russia, Turkey, Belgium, Netherlands, Switzerland, Rest of Europe, Japan, China, South Korea, India, Australia, Singapore, Thailand, Malaysia, Indonesia, Philippines, Rest of Asia-Pacific, Brazil, Argentina, Rest of South America, South Africa, Saudi Arabia, U.A.E., Egypt, Israel, and Rest of Middle East and Africa
|
|
Market Players Covered
|
Sarepta Therapeutics, Inc. (U.S.), Solid Biosciences Inc. (U.S.), Capricor Therapeutics, Inc. (U.S.), Dyne Therapeutics (U.S.), BioMarin (U.S.), Stealth BioTherapeutics Inc (U.S.), Avidity Biosciences (U.S.), ReveraGen BioPharma, Inc. (U.S.), TAIHO PHARMACEUTICAL CO., LTD.(Japan), Akashi RX (U.S.), ITALFARMACO S.p.A (Italy), NS Pharma, Inc. (U.S.), PTC Therapeutics. (U.S.), Pfizer Inc. (U.S.), FibroGen, Inc. (U.S.), SANTHERA PHARMACEUTICALS (Switzerland), and F. Hoffmann-La Roche Ltd (Switzerland) and among others
|
|
Data Points Covered in the Report
|
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
|
Segment Analysis:
The global duchenne muscular dystrophy treatment market is segmented into five notable segments such as treatment type, therapy, route of administration, end user, and distribution channel.
- On the basis of treatment type, the market is segmented into molecular-based therapies, steroid therapy, and others
In 2023, the molecular-based therapies segment is expected to dominate the global duchenne muscular dystrophy treatment market
In 2023, the molecular-based therapies segment is expected to dominate with a market share of 51.70%, due to rising clinical trials and regulatory approvals.
- On the basis of therapy, the market is segmented into exon skipping approach, mutation suppression, and dystrophin-targeted therapies.
In 2023, the exon skipping approach segment is expected to dominate the global duchenne muscular dystrophy treatment market
In 2023, the exon skipping approach segment is expected to dominate the market with a market share of 49.77%, due to its potential to restore Dystrophin production, thereby slowing the progression of muscle degeneration in DMD patients.
- On the basis of route of administration, the market is segmented into parenteral, oral, and others. In 2023, the parenteral segment is expected to dominate the market with a market share of 70.41%.
- On the basis of end user, the market is segmented into hospitals, specialty clinics, homecare, and others. In 2023, the hospitals segment is expected to dominate the market with a market share of 49.22%.
- On the basis of distribution channel, the market is segmented into hospital pharmacy, retail pharmacy, and online pharmacy. In 2023, the hospital pharmacy segment is expected to dominate the market with a market share of 60.66%.
Major Players
Data Bridge Market Research analyzes Sarepta Therapeutics, Inc. (U.S.), F. Hoffmann-La Roche Ltd (Switzerland), PTC Therapeutics. (U.S.), and Pfizer Inc. (U.S.) as the major market players in the global duchenne muscular dystrophy treatment market.
Market Developments
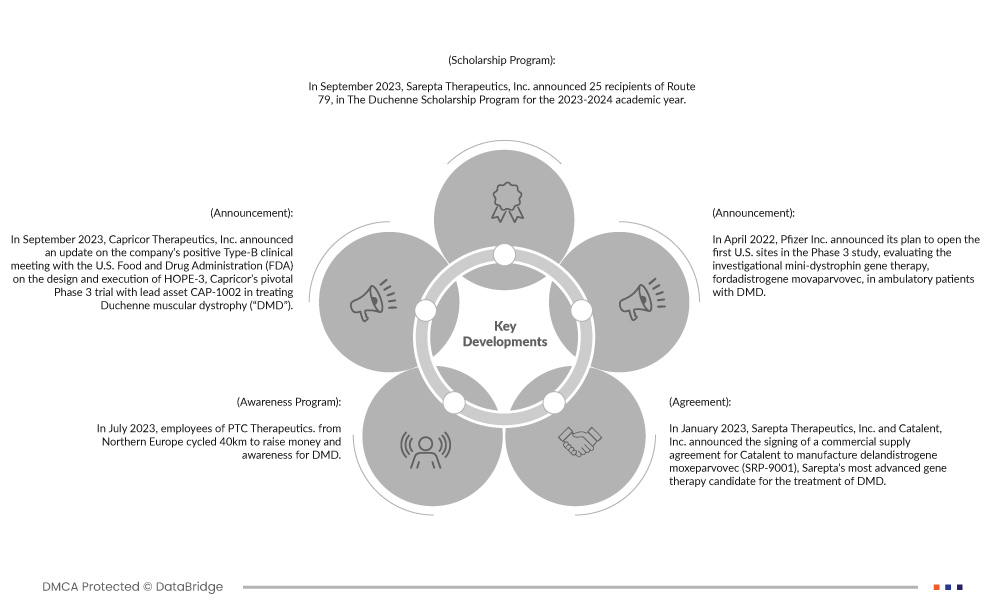
- In September 2023, Sarepta Therapeutics, Inc. announced 25 recipients of Route 79, in The Duchenne Scholarship Program for the 2023-2024 academic year. Academic scholarships will be awarded to 20 individuals out of the 25 recipients, living with Duchenne Muscular Dystrophy (DMD) and to five siblings of individuals living with DMD. Each recipient will receive a scholarship of up to USD 5,000. This will enhance the company’s brand image.
- In September 2023, Capricor Therapeutics, Inc. announced an update on the company’s positive Type-B clinical meeting with the U.S. Food and Drug Administration (FDA) on the design and execution of HOPE-3, Capricor’s pivotal Phase 3 trial with lead asset CAP-1002 in treating DMD. Feedback from the FDA on trial design and timeline confirms CAP-1002’s path towards a future Biologics License Application (BLA) submission. This helps the organization in increasing in its product category and overall revenue.
- In July 2023, employees of PTC Therapeutics. from Northern Europe cycled 40km to raise money and awareness for DMD. The initiative supported ‘De Duchenne 40’, organized by Duchenne Parent Project Netherlands, to further research and better care for children with DMD, so that they can live at least 40 years.
- In January 2023, Sarepta Therapeutics, Inc. and Catalent, Inc. announced the signing of a commercial supply agreement for Catalent to manufacture delandistrogene moxeparvovec (SRP-9001), Sarepta’s most advanced gene therapy candidate for the treatment of DMD. The agreement also structures how Catalent may support multiple gene therapy candidates in Sarepta’s pipeline for limb-girdle muscular dystrophy (LGMD). This helps the organisation in developing the overall product category.
- In April 2022, Pfizer Inc. announced its plan to open the first U.S. sites in the Phase 3 study, evaluating the investigational mini-dystrophin gene therapy, fordadistrogene movaparvovec, in ambulatory patients with DMD.
Regional Analysis
Geographically, the countries covered in the global duchenne muscular dystrophy treatment market report are U.S., Canada, Mexico, Germany, France, U.K., Italy, Spain, Russia, Turkey, Belgium, Netherlands, Switzerland, Rest of Europe, Japan, China, South Korea, India, Australia, Singapore, Thailand, Malaysia, Indonesia, Philippines, Rest of Asia-Pacific, Brazil, Argentina, Rest of South America, South Africa, Saudi Arabia, U.A.E., Egypt, Israel, and Rest of Middle East and Africa.
As per Data Bridge Market Research analysis:
North America is expected to dominate the global Duchenne Muscular Dystrophy treatment market
North America is expected to dominate the market due to the higher level of investments by various manufacturers, increasing demand for pharmaceutical products manufacturing, and the rising awareness about DMD treatment among the population in the region.
Asia-Pacific is the fastest-growing region in the global Duchenne Muscular Dystrophy treatment market
Asia-Pacific is expected to be the fastest growing region in the market due to the rising technological advancements and strategic initiatives by the major market players.
For more detailed information about the global duchenne muscular dystrophy treatment market report, click here – https://www.databridgemarketresearch.com/reports/global-duchenne-muscular-dystrophy-treatment-market