Endotoxin testing is indispensable in the quality control of pharmaceuticals and medical devices. Its primary role is identifying and quantifying endotoxins, bacterial byproducts that can harm medical products. Advancements in this field have introduced more sensitive and rapid testing methods such as recombinant Factor C (RFC) assays, offering improved accuracy and reduced testing time. These innovations ensure the safety and efficacy of healthcare products, crucial for safeguarding patient health and compliance with regulatory standards.
Access Full Report @ https://www.databridgemarketresearch.com/reports/australia-endotoxin-testing-market
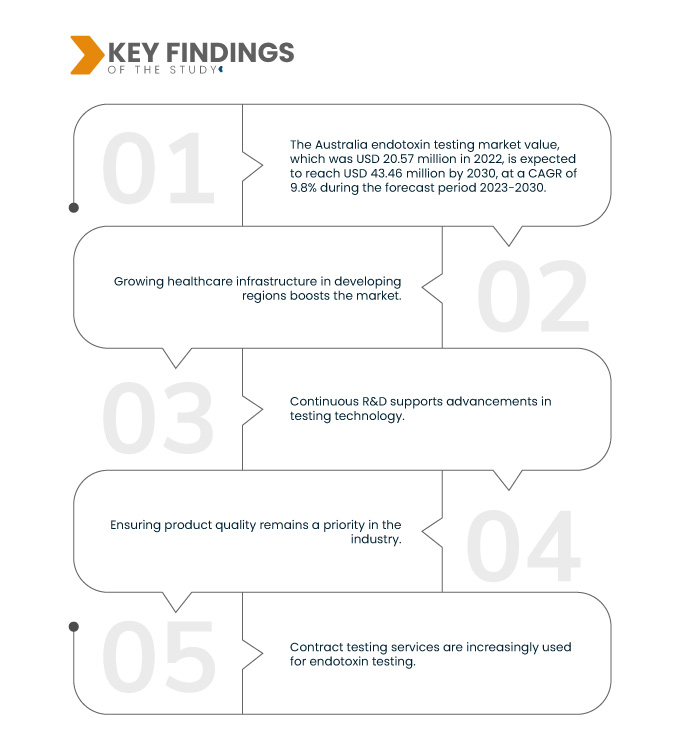
Data Bridge Market Research analyses that the Australia Endotoxin Testing Market value, which was USD 20.57 million in 2022, is expected to reach USD 43.46 million by 2030, at a CAGR of 9.8% during the forecast period 2023-2030. Increasing healthcare expenditure drives the need for stringent quality control in medical product manufacturing. Endotoxin testing is critical to ensure the safety and efficacy of pharmaceuticals and medical devices, aligning with growing healthcare spending and patient safety concerns.
Key Findings of the Study
Regulatory requirements are expected to drive the market's growth rate
Stringent regulations are a cornerstone of pharmaceutical and medical device production. They mandate endotoxin testing to guarantee the safety of these products. These regulations ensure that endotoxin levels are within acceptable limits, preventing patient exposure to harmful bacterial components. Compliance with these standards is essential for product approval and market access, as it assures the quality, efficacy, and safety of medical products, aligning with the industry's commitment to upholding the highest healthcare standards.
Report Scope and Market Segmentation
Report Metric
|
Details
|
Forecast Period
|
2023 to 2030
|
Base Year
|
2022
|
Historic Years
|
2021 (Customizable to 2015-2020)
|
Quantitative Units
|
Revenue in USD Million, Volumes in Units, Pricing in USD
|
Segments Covered
|
Product Type (Endotoxin Detection Kits and Reagents, Instruments, Systems, and Software, Endotoxin Testing Services and Consumables and accessories), Test Type (Limulus Amoebocyte Lysate (LAL) Test, Monocyte Activation Test (MAT), Rabbit Pyrogen Test and Recombinant C (RFC) Assay), Application (Pharmaceutical Manufacturing, Medical Device Manufacturing, Raw Materials Production and Packaging Manufacture), Method (Gel Clot Endotoxin Test, Chromogenic Endotoxin Test, and Turbidimetric Endotoxin Test), Mode of Purchase (Large Group, Mid and Small Group and Individual), End User (Pharmaceutical Companies, Biotechnology Companies, Medical Device Companies, Contract Research Organization (CRO), Contract Manufacturing Organization (CMO), Academic Research Institute and Others)
|
Countries Covered
|
China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC).
|
Market Players Covered
|
Eurofins Scientific (France), Thermo Fisher Scientific (U.S.), Pall Corporation. (U.S.), Lonza (Switzerland), Charles River Laboratories (U.S.), Merck KGaA (Germany), STERIS (U.K.), Société Générale de Surveillance SA (France), Sartorius AG (Germany), and GenScript (U.S.)
|
Data Points Covered in the Report
|
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
|
Segment Analysis:
The Australia endotoxin testing market is segmented based on product type, test type, application, method, mode of purchase, and end user.
- On the basis of product type, the Australia endotoxin testing market is segmented into endotoxin detection kits and reagents, instruments, systems, and software, endotoxin testing services, and consumables and accessories.
- On the basis of test type, the Australia endotoxin testing market is segmented into limulus amoebocyte lysate (LAL) test, monocyte activation test (MAT), rabbit pyrogen test, and recombinant C (RFC) assay.
- On the basis of application, the Australia endotoxin testing market is segmented into pharmaceutical manufacturing, medical device manufacturing, raw materials production, and packaging manufacturing.
- On the basis of method, the Australia endotoxin testing market is segmented into gel clot endotoxin test, chromogenic endotoxin test, and turbidimetric endotoxin test.
- On the basis of mode of purchase, the Australia endotoxin testing market is segmented into large group, mid and small group, and individual.
- On the basis of end user, the Australia endotoxin testing market is segmented into pharmaceutical companies, biotechnology companies, medical device companies, contract research organization (CRO), contract manufacturing organization (CMO), academic research institute, and others.
Major Players
Data Bridge Market Research recognizes the following companies as the Australia endotoxin testing market players in Australia endotoxin testing market are Eurofins Scientific (France), Thermo Fisher Scientific (U.S.), Pall Corporation. (U.S.), Lonza (Switzerland), Charles River Laboratories (U.S.), Merck KGaA (Germany),
Market Developments
- In January 2022, EmpowerDX, a division of Eurofins, introduced PFAS Exposure to the U.S. market. This at-home, direct-to-consumer test allows individuals to assess their blood levels of PFAS (per- and polyfluoroalkyl substances), including "forever chemicals." PFAS Exposure offers a convenient way for consumers to monitor their exposure to these potentially harmful compounds, empowering them with insights into their PFAS levels and promoting awareness of environmental and health concerns.
- In June 2020, Fujifilm Wako unveiled endotoxin detection reagents in the U.S. market. These reagents are crucial tools for assessing and measuring endotoxins, harmful bacterial components that can contaminate medical and pharmaceutical products. Fujifilm Wako's entry into the U.S. market with these reagents contributes to healthcare and pharmaceutical products' safety and quality control by enabling accurate endotoxin testing, aligning with stringent industry standards and regulations.
For more detailed information about the Australia endotoxin testing market report, click here – https://www.databridgemarketresearch.com/reports/australia-endotoxin-testing-market