Europe Pharmacogenetics Testing in Psychiatry/Depression Market, By Type (Anxiety, Mood Disorders, Depression, Bipolar Disorders, Psychotic Disorders, and Eating Disorders), Test Type (Whole Genome Sequencing and Chromosomal Array-Based Tests), Patient Type (Child, Adult, and Geriatric), Gene Type (CYP2C19, CYP2C9, VKORC1, CYP2D6, HLA-B, HTR2A/C, HLA-A, CYP3A4, SLC6A4, MTHFR, COMT, and Others), Products (Instruments, Consumables, Software & Services), End User (Hospitals and Clinics, Diagnostics Laboratories, cs, and Others), Distribution Channel (Direct Tender, Third Party Distribution Hospital Pharmacy, and Others) – Industry Trends and Forecast to 2029
Europe Pharmacogenetics Testing in Psychiatry/Depression Market Analysis and Insights
Pharmacogenetic testing helps medical professionals by providing information on how a person metabolizes a medication. This information can help doctors and others avoid prescribing antidepressants that could produce undesirable outcomes. Pharmacogenomics has shown promise for predicting antidepressant response and tolerability in treating major depressive disorder (MDD). Pharmacogenomics can improve clinical outcomes by guiding antidepressant selection and dosing. The growing biotechnology sector and increasing health expenditure have accelerated the demand for pharmacogenetic testing in psychiatry/depression.


The growing prevalence of cancer disease, novel technology in the treatment of depression and/or other psychiatric conditions are increasing the adoption of pharmacogenetics testing in psychiatry/depression devices and procedures, and the rising preference for non-surgical procedures are the major drivers, which propelled the demand for the market in the forecast period. However, the high cost associated with the tests, stringent regulation, and lack of awareness may expect to hamper the pharmacogenetics testing in psychiatry/depression market growth in the forecast period.
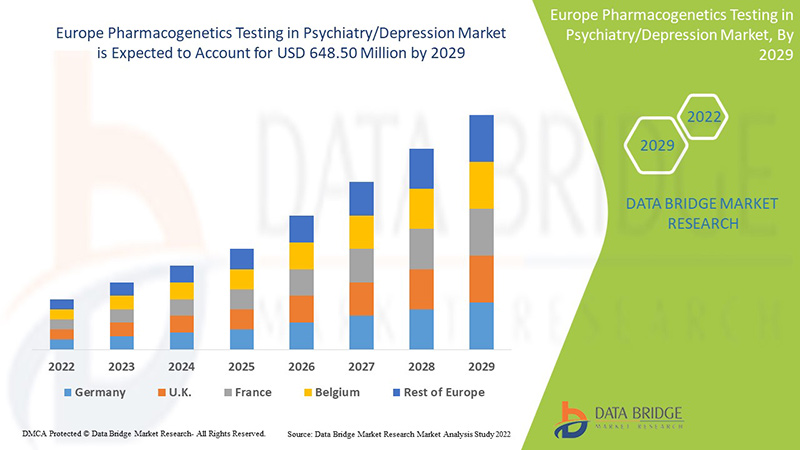
Data Bridge Market Research analyzes that the Europe pharmacogenetics testing in psychiatry/depression market is expected to reach the value of USD 648.50 million by 2029, at a CAGR of 8.9% during the forecast period. Anxiety accounts for the largest type segment in the market due to the increasing depression rate among the Europe population. This market report also covers pricing analysis, patent analysis, and technological advancements in depth.
|
Report Metric |
Details |
|
Forecast Period |
2022 to 2029 |
|
Base Year |
2021 |
|
Historic Years |
2020 |
|
Quantitative Units |
Revenue in USD Million, Volumes in Units, Pricing in USD |
|
Segments Covered |
유럽 정신과/우울증 시장에서의 약물유전학 테스트, 응용 분야별(신약 후보, 약물 최적화 및 재활용 전임상 시험 및 승인, 약물 모니터링, 새로운 질병 관련 표적 및 경로 찾기, 질병 메커니즘 이해, 정보 수집 및 종합, 가설 형성 및 자격, De Novo 약물 설계, 기존 약물의 약물 표적 찾기 등), 기술(머신 러닝, 딥 러닝, 자연어 처리 등), 약물 유형(소분자 및 대분자), 제공(소프트웨어 및 서비스), 적응증(면역 종양학, 신경 퇴행성 질환, 심혈관 질환, 대사 질환 등), 최종 사용(계약 연구 기관(CRO), 제약 및 생명 공학 회사, 연구 센터 및 학술 기관 등) |
|
적용 국가 |
독일, 프랑스, 영국, 이탈리아, 스페인, 러시아, 터키, 네덜란드, 스위스, 헝가리, 오스트리아, 리투아니아, 아일랜드, 노르웨이, 폴란드 및 기타 유럽 국가 |
|
시장 참여자 포함 |
Genelex(Invitae Corporation의 일부), Genewiz(Azenta Life Sciences의 일부), MD Labs, BiogeneiQ, Inc., ONEOME, LLC, Myriad Genetics, Inc., GenXys, Castle Biosciences, Inc., PacBio, QIAGEN, Thermo Fisher Scientific Inc., AB-Biotics.SA, Coriell Life Sciences, Eurofins Scientific, Illumina, Inc., Dynamic DNA Laboratories, STADAPHARM GmbH, Color, cnsdose, Genomind, Inc., Healthspek, myDNA Life Australia Pty Ltd., HudsonAlpha, Sonic Healthcare Limited 등이 있습니다. |
유럽 정신과/우울증 약물유전학 테스트 시장 정의
약물유전체학 검사는 최근 확장 가능하고 주요 우울 장애(MDD)를 안내하는 데 사용할 수 있게 되었습니다. 임상의는 약물유전체학(PGx) 검사를 정신 질환에 대한 약물 결정을 안내하는 필수 도구로 점점 더 인식하고 있습니다. PGx 검사의 광범위한 구현이 예측 기간 동안 시장을 주도하고 있습니다.
개인화된 의학, 계층화된 의학, 정밀 의학이라는 용어는 약리유전학의 가까운 친척이지만, 이는 추가적인 비유전적 요인도 포함하는 더 광범위한 용어입니다. 그럼에도 불구하고, 약리유전학은 이러한 분야의 중요한 구성 요소입니다. 약리유전학은 주로 인간 생식 세포 DNA 변이와 관련이 있지만, 기분 장애와 정신 질환을 이해하는 데 있어서도 최근 중요한 진전이 있었습니다.
약물유전학 검사는 약물과 사람의 유전자 반응 간의 상호작용을 연구하고 약물 효과에 영향을 미치는 유전자 변이를 찾습니다. 많은 연구자와 과학자가 약물과 개별 유전자 간의 고유한 상호작용을 확인하고 이후 맞춤형 또는 개인화된 약물을 개발하는 데 사용할 수 있는 귀중한 통찰력을 제공함에 따라 이 검사에 대한 수요가 높아지고 있습니다.
유럽 정신과/우울증 약물유전학 테스트 시장 동향
이 섹션에서는 시장 동인, 이점, 기회, 제약 및 과제를 이해하는 것을 다룹니다. 이 모든 내용은 아래에서 자세히 설명합니다.
운전자
- 정신과 및 우울증 장애로 고통받는 환자 수 증가
우울증은 전 세계적으로 흔한 질병으로, 인구의 약 3.8%가 영향을 받는 것으로 추산되며, 이 중 5.0%는 성인이고 5.7%는 60세 이상 성인입니다. 우울증은 경미한 것에서 극심한 것까지 심각한 건강 상태가 될 수 있으며, 개인에게 큰 고통을 주고 최악의 경우 자살로 이어질 수 있습니다. 45가지 이상의 항우울제가 있지만, 최적이 아닌 반응은 도전이 되며 유전적 변이, 정신과/우울증의 결과로 간주됩니다. 시간 경과에 따른 우울증 에피소드의 심각성과 패턴에 따라 의료 서비스 제공자는 행동 활성화, 인지 행동 치료, 대인 관계 심리 치료 및/또는 선택적 세로토닌 재흡수 억제제(SSRI) 및 삼환계 항우울제(TCA)와 같은 항우울제 약물과 같은 심리적 진단을 제공할 수 있습니다. 이러한 종류의 정신 장애에는 다양한 약물이 사용됩니다.
우울증 유병률의 증가와 함께, 맞춤형 진단을 제공하기 위해 유전적 변이의 효과를 연구함에 따라 약물유전학 검사에 대한 수요도 증가하고 있습니다. 시장은 산림 기간에 성장할 것으로 예상됩니다.
- 개인화 및 정밀 의학에 대한 수요 증가
약물유전학 검사는 약물의 효과에 영향을 줄 수 있는 유전자 변이를 찾아내기 때문에 의료 전문가가 환자에게 가장 적합한 약을 선택하는 데 도움이 됩니다.
의학은 개인화되고 있으며, 환자들은 점차 개인화된 약물로 개선된 결과와 덜한 부작용에 관심을 표현하고 있습니다. 개인화된 의학은 높은 안전 마진과 최상의 반응으로 치료를 맞춤화할 수 있는 잠재력을 가지고 있습니다. 이러한 추세는 주로 게놈 시퀀싱 개선에 의해 주도됩니다.
개인 건강 관리로의 전환은 의약품 제조의 변화를 의미합니다. 제조업체는 소분자 생성에서 소분자와 유전자 치료의 조합으로 전환하고 있습니다. 스폰서는 비효율적인 대량 배치 생산을 신기술에 대한 투자로 대체하고 개인화된 약물을 생산하는 데 중점을 둡니다.
제지
- 숙련된 의료 및 유전체 전문가 부족
대부분의 임상의는 여전히 약리유전학(PGx) 검사와 그에 따른 데이터 해석에 대한 자신감이 부족하여 이 분야에 대한 지식이 부족하다는 것을 보여줍니다. 이는 약리유전학(PGx) 검사에 대한 전문성과 이해에 관한 의료 전문가의 문해력을 개선해야 할 필요성을 강조합니다.
약리유전학의 가능성에 대한 실무자의 인식 부족과 검사 결과에 대한 설명이 부족하거나 불충분하여 환자를 위한 개인화 기술도 감소합니다. 의대에서 주제별 교육 과정을 개발하는 것 외에도 지속적인 전문 교육 시스템의 교육 주기와 실무 의사를 위한 무료 정보 제공이 필요합니다. 학술 인터넷 포털, 웨비나 등이 필요합니다. 임상 약리학자는 약리유전학 검사 시행에서 중요한 역할을 합니다.
약리유전학 분야에서 임상약리학자의 역량은 매우 중요합니다. 그는 임상 실무에서 유전형 검사의 적용을 조직하고, 검사 결과를 해석하고, 특정 질병 분류를 가진 환자에게 약리유전학의 가능성을 의사에게 알려주는 사람입니다. 즉, 약리유전학을 도입하는 과정에서 과학계, 의료 시스템, 실무 의사 사이의 주요 연결 고리 역할을 합니다.
기회
-
기술의 발전이 증가하다
약물유전체학의 발전으로 정신 질환에 대한 임상 실무에 개인화된 의학을 도입할 수 있는 기회가 점점 더 많아지고 있습니다. 개인화된 의학은 최적의 개인 건강 관리 결정을 내리는 방식으로 질병을 예방, 진단, 치료 및 모니터링하는 포괄적이고 전향적인 접근 방식으로 정의할 수 있습니다. 현재 100개가 넘는 약물에 의료 기술의 발전과 함께 잠재적으로 적용 가능한 약물유전체학 바이오마커와 관련된 미국 식품의약국(FDA) 라벨이 붙어 있습니다. 또한 우울증과 같은 장애에서 약물유전학 검사를 촉진하기 위한 새롭고 진보된 방법이 개발되고 있습니다. 이러한 검사는 진보된 유전자 검사 방법을 사용하여 치료 요법을 형성하기 위한 정확한 결과를 제공합니다. 검사를 지원하는 기술의 발전으로 검사 옵션의 접근성이 개선되었고, 임상의가 이 정보를 사용할 수 있을 때 사용하는 방법을 이해하는 데 도움이 되는 리소스가 늘어나면서 개인화된 또는 정밀 의학의 이러한 측면이 현실이 되고 있습니다. 따라서 제공자는 약물유전체학 검사의 과학적 및 임상적 관련성을 더 잘 알아야 합니다.
이 검사는 또한 약물과 개인의 유전적 구성 사이의 의미 있는 관계를 확립하는 데 도움이 됩니다. 이는 주요 우울증 장애와 기타 정신 질환을 치료하기 위해 환자에게 투여할 약물을 결정하는 데 도움이 됩니다.
도전
- 새로운 제품 및 기기 승인에 대한 엄격한 정부 규제
제품의 효능과 안전성에 대한 우려로 인해 대부분 정부는 새로운 의료 제품이나 테스트 개발을 감독하는 규제 기관과 정책을 개발하게 되었습니다. 이러한 의료 제품의 사용은 제품이 안전하고, 잘 연구되었으며, 부작용이 없음을 보장하는 엄격한 규제 기준을 통과한 후에 이루어질 수 있습니다.
최근 가이드라인과 개정안은 제조업체에 대한 적절한 지침을 제공합니다. 식품, 의약품 및 행정과 같은 국제 규정은 의료 제품의 새로운 출시 또는 시장 진출에 중요한 역할을 합니다. 따라서 시장에 큰 제약이 될 수 있습니다. 따라서 신제품 및 기기 승인에 대한 엄격한 정부 규제는 시장에 영향을 미칠 가능성이 높습니다.
코로나19 이후 유럽 정신과/우울증 시장에서의 약물유전학 테스트에 미치는 영향
COVID-19 발병은 약리유전학 검사 산업의 확장에 유익한 영향을 미쳤습니다. 이 팬데믹은 이 분야의 연구 활동이 일시적으로 중단되고 병원과 진단 센터에 환자가 적게 유입되면서 약리유전체학 시장 성장에 부정적인 영향을 미쳤습니다. 2020년 하반기부터 COVID-19에 대한 특정 약물 및 검사 키트에 대한 연구 수요가 증가하면서 약리유전체학 관행이 유행했습니다.
제조업체들은 COVID-19 이후 회복하기 위해 다양한 전략적 결정을 내리고 있습니다. 업체들은 약물유전학 검사 시장에 관련된 기술과 검사 결과를 개선하기 위해 여러 R&D 활동, 제품 출시 및 전략적 파트너십을 진행하고 있습니다.
최근 개발 사항
- 2022년 4월, Blue Care Network(BCN)는 정밀 의학 프로그램을 시작했습니다. Blue Cross Personalized Medicine은 약물유전체학 또는 유전자 검사를 활용하여 행동 건강, 심장학, 심혈관 및 종양학을 포함한 다양한 진단을 위해 처방된 약물을 검토하여 선택된 회원에게 보다 효과적으로 약물 치료를 개인화하고 맞춤화합니다. OneOme LLC는 BCN이 정밀 의학 프로그램 목표를 달성하고 총 치료 비용을 줄이고 부작용을 줄임으로써 환자 건강 결과를 개선하도록 도왔습니다. 이를 통해 회사는 제품 포트폴리오를 개선할 수 있었습니다.
- 2022년 2월, 고품질, 고정확도 시퀀싱 플랫폼의 선도적 공급업체인 PacBio는 캐나다 토론토에 있는 The Hospital for Sick Children(SickKids)에서 HiFi 전체 게놈 시퀀싱(HiFi WGS)을 사용하여 의학적 및 발달적 상태와 관련이 있을 수 있는 유전적 변이를 잠재적으로 식별하도록 지원한다고 발표했습니다. HiFi WGS를 사용하여 검사한 샘플은 이전에 단편 판독 DNA 시퀀싱 기술을 사용하여 시퀀싱되었지만 여전히 질병을 유발하는 변이를 식별하지 못했습니다. 이를 통해 회사는 제품 사용을 개선하는 데 도움이 되었습니다.
- 2022년 7월, 미국 재향군인부(VA)가 실시한 전국적인 약 2,000명의 재향군인을 대상으로 한 새로운 연구에 따르면, 임상의가 Myriad Genetics, Inc.의 GeneSight Psychotropic 검사 결과를 이용할 수 있었을 때 주요 우울 장애(MDD) 완화율이 크게 개선되었습니다. 이는 이 회사가 약리유전학 검사에서 진전을 보여주는 데 도움이 되었습니다.
- 2021년 1월, myDNA Life Australia Pty Ltd는 미국 휴스턴에 본사를 둔 소비자 DNA 검사 회사인 FamilyTreeDNA와 모회사인 Gene by Gene과 합병하여 약리유전체학 분야에 혁명을 일으켜 진정으로 개인화된 의학을 실현한 다음 영양유전체학으로 확장하여 실행 가능하고 개인화된 영양, 피트니스 및 스킨케어 권장 사항을 제공한다고 발표했습니다. 이를 통해 회사는 사업을 확장할 수 있었습니다.
유럽 정신과/우울증 약물유전학 테스트 시장 범위
정신과/우울증 시장에서 유럽 약물유전학 검사는 유형, 검사 유형, 유전자 유형, 환자 유형, 제품, 최종 사용자 및 유통 채널로 세분화됩니다. 세그먼트 간의 성장은 틈새 성장 포켓과 시장에 접근하고 핵심 응용 분야와 타겟 시장의 차이점을 파악하기 위한 전략을 분석하는 데 도움이 됩니다.
- 유럽 정신과/우울증 약물유전학 테스트 시장, 유형별
- 불안
- 기분 장애
- 우울증
- 양극성 장애
- 정신병 장애
- 섭식 장애
유럽 정신의학/우울증 약물유전학 검사 시장은 유형을 기준으로 불안, 기분 장애, 우울증, 조울증, 정신병 및 섭식 장애로 구분됩니다.
- 유럽 정신과/우울증 시장에서의 약물유전학 테스트, 테스트 유형별
- 전체 게놈 시퀀싱
- 염색체 배열 기반 테스트
유럽 정신의학/우울증 시장에서의 약물유전학 검사는 검사 유형을 기준으로 전체 게놈 시퀀싱 및 염색체 배열 기반 검사로 구분됩니다.
- 유럽 정신과/우울증 시장에서의 약물유전학 테스트, 유전자 유형별
- 사이프러스2C19
- CYP2C9 및 VKORC1
- 사이프러스2D6
- HLA-B
- HTR2A/C
- HLA-A
- 사이프러스3A4
- SLC6A4
- 영어: MTHFR (미국)
- 컴트
- 기타
유전자 유형을 기준으로, 정신과/우울증 시장에서의 유럽 약물유전학 검사는 CYP2C19, CYP2C9, VKORC1, CYP2D6, HLA-B, HTR2A/C, HLA-A, CYP3A4, SLC6A4, MTHFR, COMT 및 기타로 구분됩니다.
- 유럽 정신과/우울증 시장에서의 약물유전학 테스트, 환자 유형별
- 어린이
- 성인
- 노인
유럽 정신과/우울증 약물유전학 검사 시장은 환자 유형을 기준으로 소아, 성인, 노인으로 구분됩니다.
- 유럽 정신과/우울증 시장에서의 약물유전학 테스트, 제품별
- 악기
- 소모품
- 소프트웨어 및 서비스
제품 유형을 기준으로 볼 때, 유럽 정신의학/우울증 약물유전학 검사 시장은 장비, 소모품, 소프트웨어 및 서비스로 구분됩니다.
- 유럽 정신과/우울증 시장에서의 약물유전학 테스트, 최종 사용자별
- 병원 및 진료소
- 진단 연구소
- 학술 및 연구 기관
- 기타
유럽 정신과/우울증 약물유전학 검사 시장은 최종 사용자를 기준으로 병원 및 진료소, 진단 실험실, 학술 및 연구 기관 등으로 세분화됩니다.
- 유럽 약물유전학 테스트 정신과/우울증 시장, 유통 채널별
- 직접 입찰
- 제3자 배포
- 병원 약국
- 기타

On the basis of distribution channel, the Europe pharmacogenetics testing in psychiatry/depression market is segmented into direct tender, third-party distribution hospital pharmacies, and others.
Europe Pharmacogenetics Testing in Psychiatry/Depression Market Regional Analysis/Insights
The Europe pharmacogenetics testing in psychiatry/depression market is analyzed, and market size information is provided by the type, test type, gene type, patient type, product, end user, and distribution channel.
The countries covered in this market report are Germany, France, U.K., Italy, Spain, Russia, Turkey, Netherlands, Switzerland, Hungary, Austria, Lithuania, Ireland, Norway, Poland, and the rest of Europe.
- In 2022, Europe is the second most dominating due to the rise in the mental disorders patient population. Germany is expected to grow due to increased awareness about pharmacogenetics testing.
The country section of the report also provides individual market-impacting factors and changes in regulation in the market domestically that impact the current and future trends of the market. Data points such as new sales, replacement sales, country demographics, regulatory acts, and import-export tariffs are some of the major pointers used to forecast the market scenario for individual countries. Also, the presence and availability of Europe brands and their challenges faced due to large or scarce competition from local and domestic brands, and the impact of sales channels are considered while providing forecast analysis of the country data.
Competitive Landscape and Europe Pharmacogenetics Testing in Psychiatry/Depression Market Share Analysis
Europe pharmacogenetics testing in psychiatry/depression market competitive landscape provides details by the competitor. Details included are company overview, company financials, revenue generated, market potential, investment in R&D, new market initiatives, production sites and facilities, company strengths and weaknesses, product launch, product trials pipelines, product approvals, patents, product width and breath, application dominance, technology lifeline curve. The above data points provided are only related to the company’s focus on the Europe pharmacogenetics testing in psychiatry/depression market.
Some of the major players operating in the Europe pharmacogenetics testing in psychiatry/depression market are
- Genelex (Part of Invitae corporation)
- Genewiz (Part of Azenta Life Sciences)
- MD Labs
- BiogeneiQ, Inc.
- ONEOME, LLC
- Myriad Genetics, Inc.
- GenXys
- Castle Biosciences, Inc.
- PacBio
- QIAGEN
- Thermo Fisher Scientific Inc.
- AB-Biotics, S.A.
- Coriell Life Sciences
- Eurofins Scientific
- Illumina, Inc.
- Dynamic DNA Laboratories
- STADAPHARM GmbH
- Color
- Cnsdose
- Genomind, Inc.
- Healthspek
- myDNA Life Australia Pty Ltd.
- HudsonAlpha
- Sonic Healthcare Limited
Research Methodology: Europe Pharmacogenetics Testing in Psychiatry/Depression Market
Data collection and base year analysis are done using data collection modules with large sample sizes. The market data is analyzed and estimated using market statistical and coherent models. In addition, market share analysis and key trend analysis are the major success factors in the market report. The key research methodology used by DBMR research team is data triangulation, which involves data mining, analysis of the impact of data variables on the market, and primary (industry expert) validation. Apart from this, data models include the Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Company Market Share Analysis, Standards of Measurement, Europe vs Regional, and Vendor Share Analysis. Please request an analyst call in case of further inquiry.
SKU-
세계 최초의 시장 정보 클라우드 보고서에 온라인으로 접속하세요
- 대화형 데이터 분석 대시보드
- 높은 성장 잠재력 기회를 위한 회사 분석 대시보드
- 사용자 정의 및 질의를 위한 리서치 분석가 액세스
- 대화형 대시보드를 통한 경쟁자 분석
- 최신 뉴스, 업데이트 및 추세 분석
- 포괄적인 경쟁자 추적을 위한 벤치마크 분석의 힘 활용
목차
- introduction
- OBJECTIVES OF THE STUDY
- MARKET DEFINITION
- OVERVIEW of Europe pharmacogenetic testing in psychiatry/depression market
- LIMITATIONs
- MARKETS COVERED
- MARKET SEGMENTATION
- MARKETS COVERED
- geographical scope
- years considered for the study
- currency and pricing
- DBMR TRIPOD DATA VALIDATION MODEL
- MULTIVARIATE MODELLING
- type LIFELINE CURVE
- primary interviews with key opinion leaders
- DBMR MARKET POSITION GRID
- market application coverage grid
- vendor share analysis
- secondary sourcEs
- assumptions
- EXECUTIVE SUMMARY
- premium insights
- REGULATIONS: EUROPE PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET
- EUROPEAN UNION
- FRANCE
- market overview
- drivers
- RISING NUMBER OF POPULATION SUFFERING FROM DEPRESSIVE DISORDER
- INITIATIVES TAKEN BY MANUFACTURERS
- GROWING BIOTECHNOLOGY SECTOR ALONG WITH RISING HEALTHCARE EXPENDITURE
- INCREASING INTEREST FOR PERSONALIZED AND PRECISION MEDICATION
- GROWING MEDICAL TOURISM
- RESTRAINTS
- LACK OF STRONG CLINICAL EVIDENCE
- HIGH COST
- LACK OF REIMBURSEMENT
- OPPORTUNITIES
- TECHNOLOGICAL ADVANCEMENTS
- EMERGENCE OF NEW PLAYERS
- Untapped market
- CHALLENGES
- STRINGENT GOVERNMENT REGULATION
- SHORTAGE OF SKILLED PERSONNEL
- COVID-19 IMPACT ON EUROPE PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET
- IMPACT ON PRICE
- IMPACT ON DEMAND
- IMPACT ON SUPPLY
- KEY INITIATIVES BY MARKET PLAYER DURING COVID 19
- CONCLUSION:
- EUROPE PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TYPE
- overview
- WHOLE GENOME SEQUENCING
- array-based TESTS
- EUROPE PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY GENES
- overview
- cyp2c19
- CYP2C9 and VKORC1
- cyp2d6
- HLA-B
- htr2a/c
- HLA-A
- cyp3A4
- slc6a4
- MTHFR
- COMT
- others
- EUROPE PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY DRUG TYPE
- overview
- PRESCRIPTION DRUGS
- Over-the-counter medications
- RECREATIONAL DRUGS
- VITAMINS/NUTRACEUTICals
- EUROPE PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY SAMPLE TYPE
- overview
- SALIVA
- BLOOD
- EUROPE PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY application
- overview
- DRUG DEVELOPMENT
- CLINICAL PRACTICE
- EUROPE PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER
- overview
- pharmaceutical and biotechnology companies
- HEALTHCARE PROVIDERS
- research centers and academic institutes
- others
- EUROPE PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY DISTRIBUTION CHANNEL
- overview
- RETAIL PHARMACIES
- HOSPITAL PHARMACIES
- MAIL-ORDER PHARMACIES
- DIRECt-to-customer services
- EUROPE PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, by Country
- u.k.
- germany
- france
- italy
- spain
- netherlands
- belgium
- russia
- switzerland
- turkey
- rest of Europe
- Europe pharmacogenetic testing in psychiatry/depression market: COMPANY landscape
- company share analysis: Europe
- swot analysis
- company profile
- MYRIAD GENETICS, INC.
- COMPANY SNAPSHOT
- REVENUE ANALYSIS
- PRODUCT PORTFOLIO
- RECENT DEVELOPMENTS
- THERMO FISHER SCIENTIFIC INC.
- COMPANY SNAPSHOT
- REVENUE ANALYSIS
- PRODUCT PORTFOLIO
- RECENT DEVELOPMENT
- STADA ARZNEIMITTEL AG
- COMPANY SNAPSHOT
- REVENUE ANALYSIS
- PRODUCT PORTFOLIO
- RECENT DEVELOPMENTS
- sonic healthcare
- COMPANY SNAPSHOT
- REVENUE ANALYSIS
- PRODUCT PORTFOLIO
- RECENT DEVELOPMENT
- Illumina, Inc.
- COMPANY SNAPSHOT
- REVENUE ANALYSIS
- PRODUCT PORTFOLIO
- RECENT DEVELOPMENTS
- AB-Biotics, S.A.
- COMPANY SNAPSHOT
- REVENUE ANALYSIS
- PRODUCT PORTFOLIO
- RECENT DEVELOPMENTS
- ALTHEADX
- COMPANY SNAPSHOT
- PRODUCT PORTFOLIO
- RECENT DEVELOPMENT
- CORIELL LIFE SCIENCES
- COMPANY SNAPSHOT
- PRODUCT PORTFOLIO
- RECENT DEVELOPMENTS
- luminex corporation
- COMPANY SNAPSHOT
- REVENUE ANALYSIS
- PRODUCT PORTFOLIO
- RECENT DEVELOPMENTS
- mydna life australia pty ltd.
- COMPANY SNAPSHOT
- PRODUCT PORTFOLIO
- RECENT DEVELOPMENT
- PerkinElmer Inc.
- COMPANY SNAPSHOT
- REVENUE ANALYSIS
- PRODUCT PORTFOLIO
- RECENT DEVELOPMENTS
- R-Biopharm AG
- COMPANY SNAPSHOT
- PRODUCT PORTFOLIO
- RECENT DEVELOPMENTS
- questionnaire
- related reports
표 목록
TABLE 1 EUROPE PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TYPE, 2019-2028 (USD million)
TABLE 2 EUROPE PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY GENES, 2019-2028 (USD million)
TABLE 3 EUROPE PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 4 EUROPE PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY SAMPLE TYPE, 2019-2028 (USD MILLION)
TABLE 5 EUROPE PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 6 EUROPE PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2019-2028 (USD MILLION)
TABLE 7 EUROPE PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY DISTRIBUTION CHANNEL, 2019-2028 (USD MILLION)
TABLE 8 Europe pharmacogenetic testing in psychiatry/depression Market, By COUNTRY, 2019-2028 (USD MILLION)
TABLE 9 Europe pharmacogenetic testing in psychiatry/depression Market, By type, 2019-2028 (USD MILLION)
TABLE 10 Europe pharmacogenetic testing in psychiatry/depression Market, By genes, 2019-2028 (USD MILLION)
TABLE 11 Europe pharmacogenetic testing in psychiatry/depression Market, By drug type, 2019-2028 (USD MILLION)
TABLE 12 Europe pharmacogenetic testing in psychiatry/depression Market, By sample, 2019-2028 (USD MILLION)
TABLE 13 Europe pharmacogenetic testing in psychiatry/depression Market, By application, 2019-2028 (USD MILLION)
TABLE 14 Europe pharmacogenetic testing in psychiatry/depression Market, By end user, 2019-2028 (USD MILLION)
TABLE 15 Europe pharmacogenetic testing in psychiatry/depression Market, By distribution channel, 2019-2028 (USD MILLION)
TABLE 16 u.k. pharmacogenetic testing in psychiatry/depression Market, By type, 2019-2028 (USD MILLION)
TABLE 17 u.k. pharmacogenetic testing in psychiatry/depression Market, By genes, 2019-2028 (USD MILLION)
TABLE 18 u.k. pharmacogenetic testing in psychiatry/depression Market, By drug type, 2019-2028 (USD MILLION)
TABLE 19 u.k. pharmacogenetic testing in psychiatry/depression Market, By sample, 2019-2028 (USD MILLION)
TABLE 20 u.k. pharmacogenetic testing in psychiatry/depression Market, By application, 2019-2028 (USD MILLION)
TABLE 21 u.k. pharmacogenetic testing in psychiatry/depression Market, By end user, 2019-2028 (USD MILLION)
TABLE 22 u.k. pharmacogenetic testing in psychiatry/depression Market, By distribution channel, 2019-2028 (USD MILLION)
TABLE 23 germany pharmacogenetic testing in psychiatry/depression Market, By type, 2019-2028 (USD MILLION)
TABLE 24 germany pharmacogenetic testing in psychiatry/depression Market, By genes, 2019-2028 (USD MILLION)
TABLE 25 germany pharmacogenetic testing in psychiatry/depression Market, By drug type, 2019-2028 (USD MILLION)
TABLE 26 germany pharmacogenetic testing in psychiatry/depression Market, By sample, 2019-2028 (USD MILLION)
TABLE 27 germany pharmacogenetic testing in psychiatry/depression Market, By application, 2019-2028 (USD MILLION)
TABLE 28 germany pharmacogenetic testing in psychiatry/depression Market, By end user, 2019-2028 (USD MILLION)
TABLE 29 germany pharmacogenetic testing in psychiatry/depression Market, By distribution channel, 2019-2028 (USD MILLION)
TABLE 30 france pharmacogenetic testing in psychiatry/depression Market, By type, 2019-2028 (USD MILLION)
TABLE 31 france pharmacogenetic testing in psychiatry/depression Market, By genes, 2019-2028 (USD MILLION)
TABLE 32 france pharmacogenetic testing in psychiatry/depression Market, By drug type, 2019-2028 (USD MILLION)
TABLE 33 france pharmacogenetic testing in psychiatry/depression Market, By sample, 2019-2028 (USD MILLION)
TABLE 34 france pharmacogenetic testing in psychiatry/depression Market, By application, 2019-2028 (USD MILLION)
TABLE 35 france pharmacogenetic testing in psychiatry/depression Market, By end user, 2019-2028 (USD MILLION)
TABLE 36 france pharmacogenetic testing in psychiatry/depression Market, By distribution channel, 2019-2028 (USD MILLION)
TABLE 37 italy pharmacogenetic testing in psychiatry/depression Market, By type, 2019-2028 (USD MILLION)
TABLE 38 italy pharmacogenetic testing in psychiatry/depression Market, By genes, 2019-2028 (USD MILLION)
TABLE 39 italy pharmacogenetic testing in psychiatry/depression Market, By drug type, 2019-2028 (USD MILLION)
TABLE 40 italy pharmacogenetic testing in psychiatry/depression Market, By sample, 2019-2028 (USD MILLION)
TABLE 41 italy pharmacogenetic testing in psychiatry/depression Market, By application, 2019-2028 (USD MILLION)
TABLE 42 italy pharmacogenetic testing in psychiatry/depression Market, By end user, 2019-2028 (USD MILLION)
TABLE 43 italy pharmacogenetic testing in psychiatry/depression Market, By distribution channel, 2019-2028 (USD MILLION)
TABLE 44 spain pharmacogenetic testing in psychiatry/depression Market, By type, 2019-2028 (USD MILLION)
TABLE 45 spain pharmacogenetic testing in psychiatry/depression Market, By genes, 2019-2028 (USD MILLION)
TABLE 46 spain pharmacogenetic testing in psychiatry/depression Market, By drug type, 2019-2028 (USD MILLION)
TABLE 47 spain pharmacogenetic testing in psychiatry/depression Market, By sample, 2019-2028 (USD MILLION)
TABLE 48 spain pharmacogenetic testing in psychiatry/depression Market, By application, 2019-2028 (USD MILLION)
TABLE 49 spain pharmacogenetic testing in psychiatry/depression Market, By end user, 2019-2028 (USD MILLION)
TABLE 50 spain pharmacogenetic testing in psychiatry/depression Market, By distribution channel, 2019-2028 (USD MILLION)
TABLE 51 netherlands pharmacogenetic testing in psychiatry/depression Market, By type, 2019-2028 (USD MILLION)
TABLE 52 netherlands pharmacogenetic testing in psychiatry/depression Market, By genes, 2019-2028 (USD MILLION)
TABLE 53 netherlands pharmacogenetic testing in psychiatry/depression Market, By drug type, 2019-2028 (USD MILLION)
TABLE 54 netherlands pharmacogenetic testing in psychiatry/depression Market, By sample, 2019-2028 (USD MILLION)
TABLE 55 netherlands pharmacogenetic testing in psychiatry/depression Market, By application, 2019-2028 (USD MILLION)
TABLE 56 netherlands pharmacogenetic testing in psychiatry/depression Market, By end user, 2019-2028 (USD MILLION)
TABLE 57 netherlands pharmacogenetic testing in psychiatry/depression Market, By distribution channel, 2019-2028 (USD MILLION)
TABLE 58 belgium pharmacogenetic testing in psychiatry/depression Market, By type, 2019-2028 (USD MILLION)
TABLE 59 belgium pharmacogenetic testing in psychiatry/depression Market, By genes, 2019-2028 (USD MILLION)
TABLE 60 belgium pharmacogenetic testing in psychiatry/depression Market, By drug type, 2019-2028 (USD MILLION)
TABLE 61 belgium pharmacogenetic testing in psychiatry/depression Market, By sample, 2019-2028 (USD MILLION)
TABLE 62 belgium pharmacogenetic testing in psychiatry/depression Market, By application, 2019-2028 (USD MILLION)
TABLE 63 belgium pharmacogenetic testing in psychiatry/depression Market, By end user, 2019-2028 (USD MILLION)
TABLE 64 belgium pharmacogenetic testing in psychiatry/depression Market, By distribution channel, 2019-2028 (USD MILLION)
TABLE 65 russia pharmacogenetic testing in psychiatry/depression Market, By type, 2019-2028 (USD MILLION)
TABLE 66 russia pharmacogenetic testing in psychiatry/depression Market, By genes, 2019-2028 (USD MILLION)
TABLE 67 russia pharmacogenetic testing in psychiatry/depression Market, By drug type, 2019-2028 (USD MILLION)
TABLE 68 russia pharmacogenetic testing in psychiatry/depression Market, By sample, 2019-2028 (USD MILLION)
TABLE 69 russia pharmacogenetic testing in psychiatry/depression Market, By application, 2019-2028 (USD MILLION)
TABLE 70 russia pharmacogenetic testing in psychiatry/depression Market, By end user, 2019-2028 (USD MILLION)
TABLE 71 russia pharmacogenetic testing in psychiatry/depression Market, By distribution channel, 2019-2028 (USD MILLION)
TABLE 72 switzerland pharmacogenetic testing in psychiatry/depression Market, By type, 2019-2028 (USD MILLION)
TABLE 73 switzerland pharmacogenetic testing in psychiatry/depression Market, By genes, 2019-2028 (USD MILLION)
TABLE 74 switzerland pharmacogenetic testing in psychiatry/depression Market, By drug type, 2019-2028 (USD MILLION)
TABLE 75 switzerland pharmacogenetic testing in psychiatry/depression Market, By sample, 2019-2028 (USD MILLION)
TABLE 76 switzerland pharmacogenetic testing in psychiatry/depression Market, By application, 2019-2028 (USD MILLION)
TABLE 77 switzerland pharmacogenetic testing in psychiatry/depression Market, By end user, 2019-2028 (USD MILLION)
TABLE 78 switzerland pharmacogenetic testing in psychiatry/depression Market, By distribution channel, 2019-2028 (USD MILLION)
TABLE 79 turkey pharmacogenetic testing in psychiatry/depression Market, By type, 2019-2028 (USD MILLION)
TABLE 80 turkey pharmacogenetic testing in psychiatry/depression Market, By genes, 2019-2028 (USD MILLION)
TABLE 81 turkey pharmacogenetic testing in psychiatry/depression Market, By drug type, 2019-2028 (USD MILLION)
TABLE 82 turkey pharmacogenetic testing in psychiatry/depression Market, By sample, 2019-2028 (USD MILLION)
TABLE 83 turkey pharmacogenetic testing in psychiatry/depression Market, By application, 2019-2028 (USD MILLION)
TABLE 84 turkey pharmacogenetic testing in psychiatry/depression Market, By end user, 2019-2028 (USD MILLION)
TABLE 85 turkey pharmacogenetic testing in psychiatry/depression Market, By distribution channel, 2019-2028 (USD MILLION)
TABLE 86 rest of Europe pharmacogenetic testing in psychiatry/depression Market, By type, 2019-2028 (USD MILLION)
그림 목록
FIGURE 1 Europe PHARMACOGENETIC TESTInG IN PSYCHIATRY/DEPRESSION market: segmentation
FIGURE 2 Europe pharmacogenetic testing in psychiatric/depression market: data triangulation
FIGURE 3 Europe PHARMACOGENETIC TESTING IN PSYCHIATRIC/DEPRESSION market: DROC ANALYSIS
FIGURE 4 Europe PHARMACOGENETIC TESTING IN PSYCHIATRIC/DEPRESSION market: Region vs country MARKET ANALYSIS
FIGURE 5 Europe PHARMACOGENETIC TESTING IN PSYCHIATRIC/DEPRESSION market: COMPANY RESEARCH ANALYSIS
FIGURE 6 Europe pharmacogenetic testing in psychiatric/depression market: DBMR MARKET POSITION GRID
FIGURE 7 Europe pharmacogenetic testing in psychiatry/depression market: MARKET APPLICATION COVERAGE GRID
FIGURE 8 Europe pharmacogenetic testing in psychiatry/depression market: vendor share analysis
FIGURE 9 Europe pharmacogenetic testing in psychiatry/depression market: SEGMENTATION
FIGURE 10 initiatives taken by manufacturers is expected to drive THE Europe pharmacogenetic testing in psychiatry/depression market IN THE FORECAST PERIOD of 2021 to 2028
FIGURE 11 whole genome sequencing segment is expected to account for the largest share of the Europe pharmacogenetic testing in psychiatry/depression market in 2021 & 2028
FIGURE 12 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF EUROPE PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET
FIGURE 13 EUROPE PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY TYPE, 2020
FIGURE 14 EUROPE PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY TYPE, 2019-2028 (USD MILLION)
FIGURE 15 EUROPE PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY TYPE, CAGR (2021-2028)
FIGURE 16 EUROPE PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY TYPE, LIFELINE CURVE
FIGURE 17 EUROPE PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY GENES, 2020
FIGURE 18 EUROPE PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY GENES, 2019-2028 (USD MILLION)
FIGURE 19 EUROPE PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY GENES, CAGR (2021-2028)
FIGURE 20 EUROPE PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY GENES, LIFELINE CURVE
FIGURE 21 EUROPE PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY DRUG TYPE, 2020
FIGURE 22 EUROPE PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY DRUG TYPE, 2019-2028 (USD MILLION)
FIGURE 23 EUROPE PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY DRUG TYPE, CAGR (2021-2028)
FIGURE 24 EUROPE PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY DRUG TYPE, LIFELINE CURVE
FIGURE 25 EUROPE PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY SAMPLE TYPE, 2020
FIGURE 26 EUROPE PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY SAMPLE TYPE, 2019-2028 (USD MILLION)
FIGURE 27 EUROPE PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY DRUG TYPE, CAGR (2021-2028)
FIGURE 28 EUROPE PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY SAMPLE TYPE, LIFELINE CURVE
FIGURE 29 EUROPE PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY APPLICATION, 2020
FIGURE 30 EUROPE PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY APPLICATION, 2019-2028 (USD MILLION)
FIGURE 31 EUROPE PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY APPLICATION, CAGR (2021-2028)
FIGURE 32 EUROPE PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY APPLICATION, LIFELINE CURVE
FIGURE 33 EUROPE PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY END USER, 2020
FIGURE 34 EUROPE PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY END USER, 2019-2028 (USD MILLION)
FIGURE 35 EUROPE PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY END USER, CAGR (2021-2028)
FIGURE 36 EUROPE PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY END USER, LIFELINE CURVE
FIGURE 37 EUROPE PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY DISTRIBUTION CHANNEL, 2020
FIGURE 38 EUROPE PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY DISTRIBUTION CHANNEL, 2019-2028 (USD MILLION)
FIGURE 39 EUROPE PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY DISTRIBUTION CHANNEL, CAGR (2021-2028)
FIGURE 40 EUROPE PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 41 Europe PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: SNAPSHOT (2020)
FIGURE 42 Europe PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY COUNTRY (2020)
FIGURE 43 Europe PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY COUNTRY (2021 & 2028)
FIGURE 44 Europe PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY COUNTRY (2020 & 2028)
FIGURE 45 Europe PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY type (2021-2028)
FIGURE 46 Europe pharmacogenetic testing in psychiatry/depression Market: company share 2020 (%)

연구 방법론
데이터 수집 및 기준 연도 분석은 대규모 샘플 크기의 데이터 수집 모듈을 사용하여 수행됩니다. 이 단계에는 다양한 소스와 전략을 통해 시장 정보 또는 관련 데이터를 얻는 것이 포함됩니다. 여기에는 과거에 수집한 모든 데이터를 미리 검토하고 계획하는 것이 포함됩니다. 또한 다양한 정보 소스에서 발견되는 정보 불일치를 검토하는 것도 포함됩니다. 시장 데이터는 시장 통계 및 일관된 모델을 사용하여 분석하고 추정합니다. 또한 시장 점유율 분석 및 주요 추세 분석은 시장 보고서의 주요 성공 요인입니다. 자세한 내용은 분석가에게 전화를 요청하거나 문의 사항을 드롭하세요.
DBMR 연구팀에서 사용하는 주요 연구 방법론은 데이터 마이닝, 시장에 대한 데이터 변수의 영향 분석 및 주요(산업 전문가) 검증을 포함하는 데이터 삼각 측량입니다. 데이터 모델에는 공급업체 포지셔닝 그리드, 시장 타임라인 분석, 시장 개요 및 가이드, 회사 포지셔닝 그리드, 특허 분석, 가격 분석, 회사 시장 점유율 분석, 측정 기준, 글로벌 대 지역 및 공급업체 점유율 분석이 포함됩니다. 연구 방법론에 대해 자세히 알아보려면 문의를 통해 업계 전문가에게 문의하세요.
사용자 정의 가능
Data Bridge Market Research는 고급 형성 연구 분야의 선두 주자입니다. 저희는 기존 및 신규 고객에게 목표에 맞는 데이터와 분석을 제공하는 데 자부심을 느낍니다. 보고서는 추가 국가에 대한 시장 이해(국가 목록 요청), 임상 시험 결과 데이터, 문헌 검토, 재생 시장 및 제품 기반 분석을 포함하도록 사용자 정의할 수 있습니다. 기술 기반 분석에서 시장 포트폴리오 전략에 이르기까지 타겟 경쟁업체의 시장 분석을 분석할 수 있습니다. 귀하가 원하는 형식과 데이터 스타일로 필요한 만큼 많은 경쟁자를 추가할 수 있습니다. 저희 분석가 팀은 또한 원시 엑셀 파일 피벗 테이블(팩트북)로 데이터를 제공하거나 보고서에서 사용 가능한 데이터 세트에서 프레젠테이션을 만드는 데 도움을 줄 수 있습니다.













