U.S. Acute Respiratory Distress Syndrome (ARDS) Market, By Cause (Coronavirus Disease 2019 (COVID-19), Sepsis, Inhalation Of Harmful Substances, Severe Pneumonia And Others), Type (Diagnosis And Treatment), Route Of Administration (Oral, Parenteral And Others), End User (Hospitals, Specialty Clinics, Home Healthcare And Others), Distribution Channel (Direct Tender, Hospital Pharmacy, Retail Pharmacy, Online Pharmacy And Others) - Industry Trends and Forecast to 2029.
U.S. Acute Respiratory Distress Syndrome (ARDS)Market Analysis and Insights
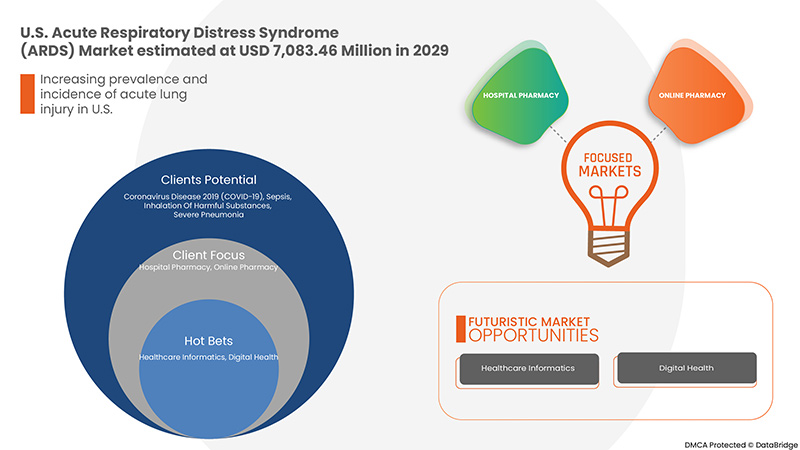
The U.S. acute respiratory distress syndrome (ARDS) market is expected to grow significantly in the forecast period of 2022 to 2029. Data Bridge Market Research analyses that the market is growing with a CAGR of 10.6% in the forecast period of 2022 to 2029 and is expected to reach USD 7,083.46 million by 2029. The major factor driving the growth of the market is the Increasing prevalence and incidence of acute lung injury, a wide range of risk factors for ARDS and acceleration in a patient pool of covid-19 with ARDS, the rising rate of air pollution and lifestyle-related diseases, and increasing accident rates and trauma causing ARDS.
Acute respiratory distress syndrome (ARDS) is a life-threatening lung injury that allows fluid to leak into the lungs. Most people who get ARDS are already hospitalized for trauma or illness like COVID-19. The syndrome usually occurs when fluids build up in the lungs' tiny, elastic air sacs called alveoli. This fluid build-up causes less oxygen reaches the bloodstream. This deprives the organs of getting enough oxygen for their normal function. People with other illness develops ARDS within a few hours to days after the precipitating injury or infection. The risk of death increases with age, and depending on the severity of the illness, patients surviving the syndrome becomes hard. Severe illness or injury causing damage to the membrane sacs of the lungs leads to ARDS. The most common underlying causes for the said diseases include sepsis, inhalation of harmful substances, severe pneumonia, head, chest, or another major injury, coronavirus disease 2019 (COVID-19), and others.
The U.S. acute respiratory distress syndrome (ARDS) market report provides details of market share, new developments, and the impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, products approvals, strategic decisions, product launches, geographic expansions, and technological innovations in the market. To understand the analysis and the market scenario, contact us for an Analyst Brief. Our team will help you create a revenue impact solution to achieve your desired goal.
|
Report Metric |
Details |
|
Forecast Period |
2022 to 2029 |
|
Base Year |
2021 |
|
역사적 연도 |
2020 (2019-2014까지 사용자 정의 가능) |
|
양적 단위 |
수익 (단위: USD 백만) |
|
다루는 세그먼트 |
원인별(코로나바이러스감염증 2019(COVID-19), 패혈증, 유해물질 흡입, 중증 폐렴 등), 유형별(진단 및 치료), 투여 경로별(경구, 비경구 등), 최종 사용자별(병원, 전문 병원, 가정 의료 등), 유통 채널별(직접 입찰, 병원 약국, 소매 약국, 온라인 약국 등) |
|
적용 국가 |
우리를 |
|
시장 참여자 포함 |
Gilead Sciences, Inc., Terumo Medical Corporation, Getinge AB., Medtronic, LivaNova PLC, Fresenius SE & Co. KGaA, Drägerwerk AG & Co. KGaA, F. Hoffmann-La Roche Ltd, Fisher & Paykel Healthcare Limited., Hamilton Medical, NIPRO, Pfizer Inc., ResMed, Smiths Medical, WEINMANN Emergency Medical Technology GmbH + Co. KG |
시장 정의
급성 폐 손상의 유병률과 발생률이 증가하는 것은 미국 급성 호흡 곤란 증후군(ARDS) 시장의 중요한 원동력입니다. ARDS에 대한 광범위한 위험 요소와 ARDS가 있는 covid-19 환자 풀의 가속화는 시장 성장을 가속화합니다. 주요 제약은 미국 급성 호흡 곤란 증후군(ARDS) 시장에 부정적인 영향을 미칠 수 있습니다. 치료와 관련된 합병증 및 장치 및 치료의 높은 비용. 증가하는 노령 인구는 시장 기회를 제공할 것으로 예상됩니다. 그러나 엄격한 규칙과 규정은 미국 급성 호흡 곤란 증후군(ARDS) 시장 성장에 도전할 것으로 예상됩니다.
미국 급성 호흡곤란증후군(ARDS) 시장 동향
운전자
- 급성 폐손상의 유병률 및 발생률 증가
급성 폐 손상 환자는 노령 인구 증가, 패혈증 및 폐렴 환자 증가 등 여러 요인으로 인해 널리 보고되고 있습니다. 그러나 대부분의 사람들은 폐 손상 및 급성 호흡 곤란 증후군 진단을 후기 단계에만 받습니다. 따라서 ARDS의 발생률과 유병률은 계속 증가하고 있으며, 이 질병은 전 세계적으로 높은 이환율과 사망률 부담을 지닌 주요 임상 문제로 널리 인식되고 있습니다. 따라서 급성 폐 손상 및 수반되는 급성 호흡 곤란 증후군의 유병률과 발생률이 증가함에 따라 미국 급성 호흡 곤란 증후군(ARDS) 시장이 주도되고 있습니다.
- ARDS의 광범위한 위험 요소
급성 호흡곤란증후군에 대한 위험 요인의 범위가 매우 넓다고 보고되었습니다. 이 증후군에는 환경적 위험 요인과 개인적 위험 요인이 관련되어 있습니다. 일부 만성 알코올 소비와 적극적 또는 수동적 흡연은 일반적으로 연관되어 있습니다. 따라서 연령에 관계없이 알코올 소비가 증가하면 증후군과 유사한 ARDS가 발생합니다. 호흡곤란, 고혈압 , 당뇨병 등과 같은 다른 위험 요인은 ARDS 발병으로 이어집니다. 따라서 급성 호흡곤란증후군과 관련된 이 광범위한 위험 요인은 임상적 상태를 발병할 가능성을 높이고 동시에 질병 사례를 증가시킵니다. 이러한 긍정적인 위험 요인은 시장 성장을 촉진할 것으로 예상됩니다.
- 급성 호흡곤란증후군(ARDS)을 동반한 코로나19 환자 풀의 가속화
전 세계가 COVID-19 감염으로 엄청난 고통을 겪고 있기 때문에 COVID-19와 급성 호흡곤란 증후군 사이에도 직접적인 상관관계가 있습니다. 중증 COVID-19 질환은 궁극적으로 ARDS와 폐렴으로 이어집니다. 이는 감염된 개인에게 치명적인 것으로 입증되었습니다. COVID-19를 유발하는 바이러스가 신체에 들어가면 상기도 세포에 부착됩니다. 이는 염증을 유발하고 기침, 인후통, 발열과 같은 증상을 유발하는 면역 반응을 일으킵니다. 일부 심각한 경우 바이러스는 상기도를 넘어 폐를 통과하여 폐포에 도달합니다. 따라서 COVID-19 사례가 증가함에 따라 사람들 사이에서 급성 호흡곤란 증후군의 가능성도 높아져 급성 호흡곤란 증후군 시장 성장의 원동력이 될 것으로 예상됩니다.
- 대기 오염과 생활 습관 관련 질병의 증가율
낮은 수준에서 중간 수준의 대기 오염에 장기간 노출되면 ARDS를 포함한 모든 폐 감염이 발생할 위험 요소가 커집니다. 이는 급성 호흡 곤란 증후군에 대한 새롭고 잠재적으로 수정 가능한 환경적 위험 요소입니다. 세계보건기구에 따르면 대기 오염은 심장 질환 및 폐 및 호흡기 감염과 같은 질병의 부담을 급성 및 만성 수준에서 증가시켜 건강에 가장 큰 환경적 위험 요소 중 하나입니다. 따라서 선진국과 개발도상국의 대기 오염률이 증가함에 따라 사람들이 급성 호흡 곤란 증후군과 같은 증후군에 걸릴 가능성이 커집니다. 따라서 이 요소가 시장 성장을 견인할 것으로 예상됩니다.
- 사고율 증가 및 급성 호흡곤란증후군(ARDS)을 유발하는 외상
사고로 인한 중대한 외상은 ARDS 발병의 잘 알려진 위험 요인입니다. 급성 호흡 곤란 증후군은 췌장 염증, 위 내용물이 폐로 흡입, 수혈, 주택 화재 및 연기 흡입, 심각한 감염, 익사 외상 및 약물에 대한 심각한 반응으로 인해 신체에 부상을 입어 발생할 수 있습니다. 심지어 자동차 사고도 ARDS의 원인입니다. 따라서 연기를 흡입하면 기도 내강을 막는 피브린, 호중구, 점액 및 상피 세포 파편과 같은 특정 물질이 방출되어 환기에 변화가 발생합니다. 이러한 상태는 저산소증으로 이어지며 이는 ARDS의 주요한 이유입니다.
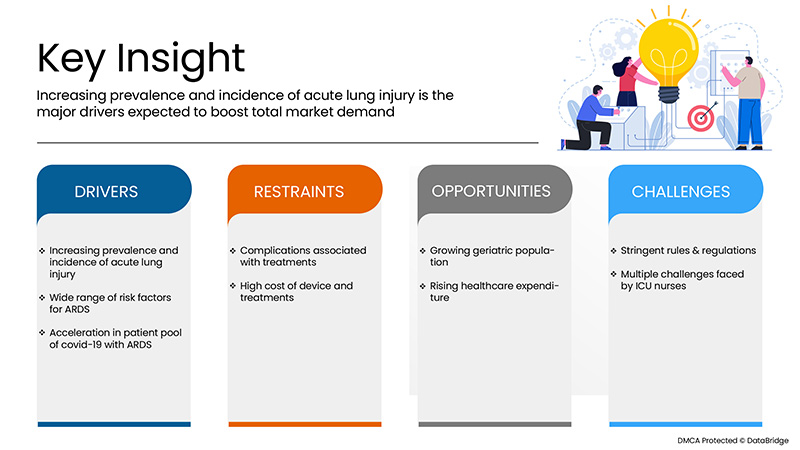
기회
- 노령 인구 증가
'세계의 노령 인구는 빠른 속도로 증가하고 있습니다. 노인은 중환자실 입원 인구의 점점 더 흔한 인구가 되었습니다. National Center for Biotechnology Information(NCBI)의 기사에 따르면, 급성 호흡곤란증후군(ARDS)과 관련된 사망률은 노인 인구에서 69%~80%로 보고되어 노인에게 더 위험합니다. 따라서 노인 인구의 증가 속도는 면역 체계가 약해 중증 호흡기 질환에 더 취약하기 때문에 급성 호흡곤란증후군 치료에 대한 수요가 증가하고 있음을 보여줍니다. 향후 전 세계적으로 증가할 것으로 예상되는 노령 인구의 증가는 예측 기간 동안 시장 성장의 기회를 창출할 것으로 예상됩니다.
- 증가하는 의료비
다양한 국가의 사람들의 가처분 소득이 증가함에 따라 전 세계적으로 의료비가 증가했습니다. 게다가 인구 요구 사항을 충족하기 위해 정부 기관과 의료 기관은 의료비 지출을 가속화함으로써 주도권을 잡고 있습니다. 의료비 지출의 증가는 최근 몇 년 동안 이 질환이 매우 만연했기 때문에 의료 시설에서 급성 호흡곤란증후군에 대한 치료 시설을 개선하는 데 도움이 됩니다. 증가하는 의료비 지출은 또한 경제 및 의료 부문의 추가 성장에 이롭습니다. 주로 인공호흡기 및 기타 ARDS 치료 장치를 사용하여 더 나은 고급 치료 옵션의 개발에 상당한 영향을 미치기 때문에 유익합니다.
- 시장 참여자들의 전략적 이니셔티브
COVID-19와 같은 감염성 질환과 폐암과 같은 만성 질환의 증가율로 인해 ARDS와 같은 증후군의 동시적 부담이 전 세계적으로 널리 나타나고 있습니다. 연구 품질의 극적인 상승과 연구 기회의 증가는 이러한 참여자들이 취하는 다양한 전략적 이니셔티브 때문입니다. 그들은 수년에 걸쳐 제품 출시, 협업, 합병, 인수 등과 같은 이니셔티브를 취하고 있으며 시장에서 더 많은 기회를 주도하고 창출할 것으로 예상됩니다. 급성 호흡곤란 증후군 시장에서 주요 회사가 수행한 이러한 전략적 제품 출시, 인수 및 합병은 다양한 지역의 회사에 기회를 열었습니다. 이 전략을 통해 회사는 시장에서 입지를 강화할 수 있습니다.
- 급성 호흡곤란증후군 (ARDS) 에 대한 인식 개선
급성 호흡곤란증후군은 여러 가지 다른 원인이 있기 때문에 일반적으로 일반적인 사망 원인으로 무시됩니다. 고급 기술 치료와 상태에 대한 적절한 인식이 필요하면 ARDS 발생률을 상당히 줄일 수 있습니다. 시기적절한 진단과 예방이 예방 또는 더 빠른 회복에 중요하기 때문에 대중의 관심이 가장 중요합니다. 현재 정부와 기관은 폐 손상 연구 범위를 확대하여 ARDS의 1차 예방을 포함하고 증후군의 이환율이나 사망률을 줄였습니다. 따라서 다양한 '협회' 지원으로 ARDS에 대한 인식을 높이면 미국 급성 호흡곤란증후군 '시장이 미래에 광범위하게 성장할 수 있는 기회가 커집니다.
제약/도전
- 치료와 관련된 합병증
급성 호흡곤란증후군은 사망률과 이환율을 예방하기 위해 적절하고 시기적절한 치료와 관리가 필요한 임상적 상태입니다. 그러나 ARDS 치료 중에 환자는 병원에서 많은 합병증에 직면할 수 있습니다. ARDS에 대한 치료법이 있어 사람들이 생존하는 데 도움이 되지만, 치료 중과 치료 후 합병증은 여전히 환자에게 부담입니다. 가장 잠재적이고 지속적인 영향에는 호흡 문제, 우울증, 기억 및 사고 문제, 피로 및 근육 약화 등이 있습니다. 급성 호흡곤란증후군과 관련된 이러한 합병증과 치료 후 합병증은 예측 기간 동안 시장 성장을 방해할 것으로 예상됩니다.
- 장비 및 치료 비용이 높음
급성 호흡곤란증후군은 광범위한 고급 치료 옵션을 제공받고 있지만, 평균 소득 계층에서는 장기 치료 비용을 감당하기 어렵습니다. 중환자 치료 및 집중 치료실 서비스 이용은 전 세계적으로 증가하고 있으며, 그 비싼 비용은 현재 의료 시스템에서 주요 관심사입니다. ARDS 환자는 일반적으로 빈번한 화폐화 및 인공호흡기 사용과 함께 장기 입원을 해야 하며, 상당한 양의 의료 자원을 소모합니다. 따라서 치료 및 인공호흡기의 높은 비용은 환자가 완전히 회복될 때까지 장기적으로 신뢰할 수 있는 치료법을 감당하기 매우 어렵습니다. 이는 시장 성장을 방해할 것으로 예상됩니다.
- 숙련된 인력 부족
갑작스러운 COVID-19 위기는 인력 부족을 포함한 의료 기관이 직면해야 할 다양한 과제를 만들어냈습니다. 이 팬데믹은 미국과 같은 선진국을 강타했습니다. 그들은 이러한 과제를 극복하기 위해 여러 가지 방법을 시도했지만, 뉴스 기사에서는 국가들이 인력 부족에 시달리고 있다고 보도했습니다. 미국에서는 팬데믹이 시작되기 전부터도 간호사가 부족했습니다. 이 지역은 호흡기 질환 인구가 많기 때문입니다. 따라서 ICU에서 적절한 기술과 훈련을 받은 간호사가 부족한 것은 급성 호흡 곤란 증후군(ARDS) 시장의 성장을 방해할 것으로 예상되는 더욱 주목할 만한 제약이 될 것입니다.
- 엄격한 규칙 및 규정
전 세계적으로 인공호흡기 사용이 급속히 증가하고 있으며, 노령 인구가 증가하고 조기 진단과 시기적절한 치료로 예방할 수 있는 급성 호흡곤란증후군을 포함한 여러 급성 폐질환이 있습니다. 동시에, 시장에 있는 급성 호흡곤란증후군 제조업체의 플레이어는 기계적 인공호흡기나 가습기와 같은 제품을 출시하기 위해 상위 당국의 승인을 받기 위해 특정 규정을 따라야 합니다. 이러한 엄격한 지침을 따라야 하며, 이는 모든 단계 중에서 가장 어려운 작업 중 하나입니다. 예를 들어, 미국 식품의약국(FDA)은 미국에서 호흡기 질환 제품을 규제합니다. 따라서 제품 승인을 위한 엄격한 규칙과 규정은 시장 성장에 도전합니다.
- ICU 간호사가 직면한 다양한 과제
중환자실은 대규모 환자 풀과 병원 자원 부족으로 인해 팬데믹 동안 많은 어려움을 겪습니다. COVID-19의 첫 번째 물결 이후 병원은 'ICU'의 용량과 잠재력을 늘렸고, 이로 인해 ICU 의료진의 근무 시간이 늘어났습니다. ICU에서 일하는 간호사는 감염된 환자를 다루는 여러 가지 이유로 항상 두려움에 떨고 있습니다. 따라서 가장 큰 어려움은 급성 호흡곤란 증후군과 중증 급성 호흡기 증후군을 다루는 간호사와 다른 의료진이 겪는 어려움입니다. 조직은 새로운 혁신적인 아이디어와 더 많은 인력을 배치하여 이를 극복하려고 노력하고 있습니다.
최근 개발
- 2020년 7월, Gilead Sciences, Inc.는 COVID-19의 잠재적 외래 치료를 위한 흡입형 Remdesivir 용액의 임상 시험을 시작했습니다. 이 시험은 회사가 항바이러스 제품 포트폴리오를 강화하는 데 도움이 되었으며, 이를 통해 시장에서의 입지를 강화했습니다.
미국 급성 호흡곤란증후군(ARDS) 시장 범위
미국 급성 호흡곤란증후군(ARDS) 시장은 원인, 유형, 투여 경로, 최종 사용자 및 유통 채널을 기준으로 분류됩니다. 이러한 세그먼트 간의 성장은 산업의 주요 성장 세그먼트를 분석하고 사용자에게 핵심 시장 응용 프로그램을 식별하기 위한 전략적 결정을 내릴 수 있는 귀중한 시장 개요와 시장 통찰력을 제공하는 데 도움이 됩니다.
원인
- 코로나바이러스감염증 2019(COVID-19)
- 부패
- 유해물질 흡입
- 중증 폐렴
- 기타
미국 급성 호흡곤란증후군(ARDS) 시장은 원인에 따라 코로나바이러스감염증-19(COVID-19), 패혈증, 유해물질 흡입, 중증 폐렴 등으로 분류됩니다.
유형
- 진단
- 치료
미국 급성 호흡곤란증후군(ARDS) 시장은 유형에 따라 진단과 치료로 분류됩니다.
투여 경로
- 경구
- 비경구적
- 기타
미국 급성 호흡곤란증후군(ARDS) 시장은 투여 경로를 기준으로 경구, 비경구 및 기타로 분류됩니다.
최종 사용자
- 병원
- 전문 클리닉
- 홈 헬스케어
- 기타
미국 급성 호흡곤란증후군(ARDS) 시장은 최종 사용자를 기준으로 병원, 전문 클리닉, 재택 건강관리 및 기타로 분류됩니다.
유통 채널
- 직접 입찰
- 병원 약국
- 소매 약국
- 온라인 약국
- 기타
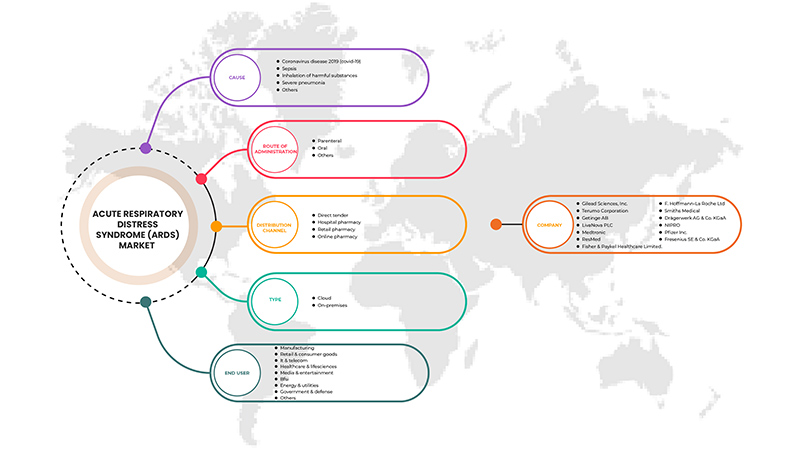
미국 급성 호흡곤란증후군(ARDS) 시장은 유통 채널을 기준으로 직접 입찰, 병원 약국, 소매 약국, 온라인 약국 및 기타로 분류됩니다.
경쟁 환경 및 미국 급성 호흡곤란증후군(ARDS) 시장 점유율 분석
미국 급성 호흡곤란증후군(ARDS) 시장 경쟁 구도는 경쟁자에 대한 세부 정보를 제공합니다. 포함된 세부 정보에는 회사 개요, 회사 재무, 창출된 수익, 시장 잠재력, 연구 개발 투자, 새로운 시장 이니셔티브, 생산 현장 및 시설, 회사의 강점과 약점, 제품 출시, 제품 시험 파이프라인, 제품 승인, 특허, 제품 폭과 폭, 응용 분야 우세, 기술 수명선 곡선이 있습니다. 위에 제공된 데이터 포인트는 미국 급성 호흡곤란증후군(ARDS) 시장과 관련된 회사의 초점에만 관련이 있습니다.
미국 급성 호흡곤란증후군(ARDS) 시장에서 활동하는 대표적인 기업으로는 Gilead Sciences, Inc., Terumo Medical Corporation, Getinge AB., Medtronic, LivaNova PLC, Fresenius SE & Co. KGaA, Drägerwerk AG & Co. KGaA, F. Hoffmann-La Roche Ltd, Fisher & Paykel Healthcare Limited., Hamilton Medical, NIPRO, Pfizer Inc., ResMed, Smiths Medical, WEINMANN Emergency Medical Technology GmbH + Co. KG 등이 있습니다.
연구 방법론
데이터 수집 및 기준 연도 분석은 대규모 샘플 크기의 데이터 수집 모듈을 사용하여 수행됩니다. 시장 데이터는 시장 통계 및 일관된 모델을 사용하여 분석 및 추정됩니다. 또한 시장 점유율 분석 및 주요 추세 분석은 시장 보고서의 주요 성공 요인입니다. DBMR 연구팀에서 사용하는 주요 연구 방법론은 데이터 마이닝, 시장에 대한 데이터 변수의 영향 분석 및 기본(산업 전문가) 검증을 포함하는 데이터 삼각 측량입니다. 이 외에도 데이터 모델에는 공급업체 포지셔닝 그리드, 시장 타임라인 분석, 시장 개요 및 가이드, 회사 포지셔닝 그리드, 회사 시장 점유율 분석, 측정 표준, 미국 대 지역 및 공급업체 점유율 분석이 포함됩니다. 추가 문의 사항이 있는 경우 분석가에게 전화를 요청하십시오.
SKU-
세계 최초의 시장 정보 클라우드 보고서에 온라인으로 접속하세요
- 대화형 데이터 분석 대시보드
- 높은 성장 잠재력 기회를 위한 회사 분석 대시보드
- 사용자 정의 및 질의를 위한 리서치 분석가 액세스
- 대화형 대시보드를 통한 경쟁자 분석
- 최신 뉴스, 업데이트 및 추세 분석
- 포괄적인 경쟁자 추적을 위한 벤치마크 분석의 힘 활용
목차
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 CAUSE LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 MARKET END USER COVERAGE GRID
2.11 VENDOR SHARE ANALYSIS
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL ANALYSIS
4.2 PORTER'S FIVE FORCES MODEL
4.3 PIPELINE ANALYSIS
5 INSURANCE REIMBURSEMENT
5.1 CENTER FOR MEDICARE SERVICES (CMS)–ELSO (EXTRACORPOREAL LIFE SUPPORT ORGANIZATION)
5.2 HEALTH RESOURCES AND SERVICES ADMINISTRATION
5.3 ABBOTT CODING GUIDE FOR ECMO
5.4 CERN HEALTH INSURANCE SCHEME
5.5 AMERICAN SOCIETY OF CLINICAL ONCOLOGY (ASCO) – (MEDICARE & MEDICAID)
5.6 AMERICAN HOSPITAL ASSOCIATION
5.7 CONCLUSION
6 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: REGULATIONS
6.1 REGULATION IN U.S.
6.2 REGULATION FOR VENTILATORS AND RESPIRATORY DEVICES AS PER FDA
6.3 LABELING OF MODIFIED DEVICES
7 COUNTRY SUMMERY
8 MARKET OVERVIEW
8.1 DRIVERS
8.1.1 INCREASING PREVALENCE AND INCIDENCE OF ACUTE LUNG INJURY
8.1.2 WIDE RANGE OF RISK FACTORS FOR ARDS
8.1.3 ACCELERATION IN PATIENT POOL OF COVID-19 WITH ARDS
8.1.4 RISING RATE OF AIR POLLUTION AND LIFESTYLE-RELATED DISEASES
8.1.5 INCREASING ACCIDENT RATES AND TRAUMA CAUSING ARDS
8.2 RESTRAINTS
8.2.1 COMPLICATIONS ASSOCIATED WITH TREATMENTS
8.2.2 HIGH COST OF DEVICE AND TREATMENTS
8.2.3 LACK OF SKILLED WORKFORCE
8.3 OPPORTUNITIES
8.3.1 GROWING GERIATRIC POPULATION
8.3.2 RISING HEALTHCARE EXPENDITURE
8.3.3 STRATEGIC INITIATIVES BY MARKET PLAYERS
8.3.4 IMPROVING AWARENESS REGARDING ARD SYNDROME
8.4 CHALLENGES
8.4.1 STRINGENT RULES & REGULATIONS
8.4.2 MULTIPLE CHALLENGES FACED BY ICU NURSES
9 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE
9.1 OVERVIEW
9.2 CORONAVIRUS DISEASE 2019 (COVID-19)
9.3 SEPSIS
9.4 INHALATION OF HARMFUL SUBSTANCES
9.5 SEVERE PNEUMONIA
9.6 OTHERS
10 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE
10.1 OVERVIEW
10.2 DIAGNOSIS
10.2.1 IMAGING TESTS
10.2.1.1 CHEST X-RAY
10.2.1.2 CT SCAN
10.2.1.3 ULTRASOUND
10.2.1.4 OTHERS
10.2.2 BLOOD TEST
10.2.3 RESPIRATORY RATE
10.2.4 SPO2 TEST
10.2.5 OTHERS
10.3 TREATMENT
10.3.1 MECHANICAL VENTILATION
10.3.1.1 HIGH-FLOW NASAL O2
10.3.1.2 BI-LEVEL POSITIVE AIRWAY PRESSURE
10.3.1.3 CONTINOUS POSITIVE AIRWAY PRESSURE
10.3.1.4 PRONE POSITIVE VENTILATION
10.3.1.5 OTHERS
10.3.2 CORTICOSTEROIDS
10.3.2.1 METHYLPREDNISOLONE
10.3.2.2 DEXAMETHASONE
10.3.2.3 OTHERS
10.3.3 ANTIVIRAL MEDICATION
10.3.3.1 REMDESIVIR
10.3.3.2 COMBINATION DRUGS
10.3.3.3 OTHERS
10.3.4 EXTRACORPOREAL MEMBRANE OXYGENATION (ECMO)
10.3.5 TOCILIZUMAB
10.3.6 OTHERS
11 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION
11.1 OVERVIEW
11.2 PARENTERAL
11.2.1 INTRAVENOUS
11.2.2 INTRAMUSCULAR
11.3 ORAL
11.4 OTHERS
12 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER
12.1 OVERVIEW
12.2 HOSPITALS
12.3 SPECIALTY CLINICS
12.4 HOME HEALTHCARE
12.5 OTHERS
13 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL
13.1 OVERVIEW
13.2 DIRECT TENDER
13.3 HOSPITAL PHARMACY
13.4 RETAIL PHARMACY
13.5 ONLINE PHARMACY
14 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: COMPANY LANDSCAPE
14.1 COMPANY SHARE ANALYSIS: U.S
15 SWOT ANALYSIS
16 COMPANY PROFILE
16.1 GILEAD SCIENCES INC.
16.1.1 COMPANY SNAPSHOT
16.1.2 REVENUS ANALYSIS
16.1.3 PRODUCT PORTFOLIO
16.1.4 RECENT DEVELOPMENT
16.2 TERUMO CORPORATION
16.2.1 COMPANY SNAPSHOT
16.2.2 REVENUS ANALYSIS
16.2.3 PRODUCT PORTFOLIO
16.2.4 RECENT DEVELOPMENT
16.3 GETINGE AB
16.3.1 COMPANY SNAPSHOT
16.3.2 REVENUS ANALYSIS
16.3.3 PRODUCT PORTFOLIO
16.3.4 RECENT DEVELOPMENT
16.4 LIVANOVA PLC
16.4.1 COMPANY SNAPSHOT
16.4.2 REVENUE ANALYSIS
16.4.3 PRODUCT PORTFOLIO
16.4.4 RECENT DEVELOPMENTS
16.5 MEDTRONIC
16.5.1 COMPANY SNAPSHOT
16.5.2 REVENUE ANALYSIS
16.5.3 PRODUCT PORTFOLIO
16.5.4 RECENT DEVELOPMENTS
16.6 DRÄGERWERK AG & CO. KGAA
16.6.1 COMPANY SNAPSHOT
16.6.2 REVENUE ANALYSIS
16.6.3 PRODUCT PORTFOLIO
16.6.4 RECENT DEVELOPMENTS
16.7 F. HOFFMANN-LA ROCHE LTD
16.7.1 COMPANY SNAPSHOT
16.7.2 RECENT ANALYSIS
16.7.3 PRODUCT PORTFOLIO
16.7.4 RECENT DEVELOPMENTS
16.8 FISHER & PAYKEL HEALTHCARE LIMITED
16.8.1 COMPANY SNAPSHOT
16.8.2 REVENUE ANALYSIS
16.8.3 PRODUCT PORTFOLIO
16.8.4 RECENT DEVELOPMENTS
16.9 FRESENIUS SE & CO. KGAA
16.9.1 COMPANY SNAPSHOT
16.9.2 REVENUS ANALYSIS
16.9.3 PRODUCT PORTFOLIO
16.9.4 RECENT DEVELOPMENTS
16.1 HAMILTON MEDICAL
16.10.1 COMPANY SNAPSHOT
16.10.2 PRODUCT PORTFOLIO
16.10.3 RECENT DEVELOPMENT
16.11 NIPRO
16.11.1 COMPANY SNAPSHOT
16.11.2 REVENUS ANALYSIS
16.11.3 PRODUCT PORTFOLIO
16.11.4 RECENT DEVELOPMENT
16.12 PFIZER INC.
16.12.1 COMPANY SNAPSHOT
16.12.2 REVENUS ANALYSIS
16.12.3 PRODUCT PORTFOLIO
16.12.4 RECENT DEVELOPMENT
16.13 RESMED
16.13.1 COMPANY SNAPSHOT
16.13.2 REVENUE ANALYSIS
16.13.3 PRODUCT PORTFOLIO
16.13.4 RECENT DEVELOPMENT
16.14 SMITHS MEDICAL
16.14.1 COMPANY SNAPSHOT
16.14.2 REVENUS ANALYSIS
16.14.3 PRODUCT PORTFOLIO
16.14.4 RECENT DEVELOPMENTS
16.15 WEINMANN EMERGENCY MEDICAL TECHNOLOGY GMBH + CO. KG
16.15.1 COMPANY SNAPSHOT
16.15.2 PRODUCT PORTFOLIO
16.15.3 RECENT DEVELOPMENT
17 QUESTIONNAIRE
18 RELATED REPORTS
표 목록
TABLE 1 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: PIPELINE ANALYSIS
TABLE 2 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2020-2029 (USD MILLION)
TABLE 3 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 4 U.S. DIAGNOSIS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 5 U.S. IMAGING TESTS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 6 U.S. TREATMENT IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 7 U.S. MECHANICAL VENTILATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 8 U.S. CORTICOSTEROIDS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 9 U.S. ANTIVIRAL MEDICATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 10 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD MILLION)
TABLE 11 U.S. PARENTERAL IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD MILLION)
TABLE 12 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 13 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
그림 목록
FIGURE 1 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: SEGMENTATION
FIGURE 2 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: DATA TRIANGULATION
FIGURE 3 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: DROC ANALYSIS
FIGURE 4 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: COUNTRY MARKET ANALYSIS
FIGURE 5 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: DBMR MARKET POSITION GRID
FIGURE 8 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: MARKET END USER COVERAGE GRID
FIGURE 9 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: SEGMENTATION
FIGURE 11 ACCELERATION IN PATIENT POOL OF COVID-19 WITH ARDS IS EXPECTED TO DRIVE THE U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET IN THE FORECAST PERIOD OF 2022 TO 2029
FIGURE 12 CORONAVIRUS DISEASE 2019 (COVID-19) SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET IN 2022 & 2029
FIGURE 13 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET
FIGURE 14 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY CAUSE, 2021
FIGURE 15 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY CAUSE, 2022-2029 (USD MILLION)
FIGURE 16 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY CAUSE, CAGR (2022-2029)
FIGURE 17 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY CAUSE, LIFELINE CURVE
FIGURE 18 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY TYPE, 2021
FIGURE 19 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY TYPE, 2022-2029 (USD MILLION)
FIGURE 20 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY TYPE, CAGR (2022-2029)
FIGURE 21 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY TYPE, LIFELINE CURVE
FIGURE 22 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY ROUTE OF ADMINISTRATION, 2021
FIGURE 23 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY ROUTE OF ADMINISTRATION, 2022-2029 (USD MILLION)
FIGURE 24 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY ROUTE OF ADMINISTRATION, CAGR (2022-2029)
FIGURE 25 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY ROUTE OF ADMINISTRATION, LIFELINE CURVE
FIGURE 26 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY END USER, 2021
FIGURE 27 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY END USER, 2022-2029 (USD MILLION)
FIGURE 28 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY END USER, CAGR (2022-2029)
FIGURE 29 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY END USER, LIFELINE CURVE
FIGURE 30 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: DISTRIBUTION CHANNEL, 2021
FIGURE 31 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY DISTRIBUTION CHANNEL, 2022-2029 (USD MILLION)
FIGURE 32 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY DISTRIBUTION CHANNEL, CAGR (2022-2029)
FIGURE 33 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 34 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: COMPANY SHARE 2021 (%)

연구 방법론
데이터 수집 및 기준 연도 분석은 대규모 샘플 크기의 데이터 수집 모듈을 사용하여 수행됩니다. 이 단계에는 다양한 소스와 전략을 통해 시장 정보 또는 관련 데이터를 얻는 것이 포함됩니다. 여기에는 과거에 수집한 모든 데이터를 미리 검토하고 계획하는 것이 포함됩니다. 또한 다양한 정보 소스에서 발견되는 정보 불일치를 검토하는 것도 포함됩니다. 시장 데이터는 시장 통계 및 일관된 모델을 사용하여 분석하고 추정합니다. 또한 시장 점유율 분석 및 주요 추세 분석은 시장 보고서의 주요 성공 요인입니다. 자세한 내용은 분석가에게 전화를 요청하거나 문의 사항을 드롭하세요.
DBMR 연구팀에서 사용하는 주요 연구 방법론은 데이터 마이닝, 시장에 대한 데이터 변수의 영향 분석 및 주요(산업 전문가) 검증을 포함하는 데이터 삼각 측량입니다. 데이터 모델에는 공급업체 포지셔닝 그리드, 시장 타임라인 분석, 시장 개요 및 가이드, 회사 포지셔닝 그리드, 특허 분석, 가격 분석, 회사 시장 점유율 분석, 측정 기준, 글로벌 대 지역 및 공급업체 점유율 분석이 포함됩니다. 연구 방법론에 대해 자세히 알아보려면 문의를 통해 업계 전문가에게 문의하세요.
사용자 정의 가능
Data Bridge Market Research는 고급 형성 연구 분야의 선두 주자입니다. 저희는 기존 및 신규 고객에게 목표에 맞는 데이터와 분석을 제공하는 데 자부심을 느낍니다. 보고서는 추가 국가에 대한 시장 이해(국가 목록 요청), 임상 시험 결과 데이터, 문헌 검토, 재생 시장 및 제품 기반 분석을 포함하도록 사용자 정의할 수 있습니다. 기술 기반 분석에서 시장 포트폴리오 전략에 이르기까지 타겟 경쟁업체의 시장 분석을 분석할 수 있습니다. 귀하가 원하는 형식과 데이터 스타일로 필요한 만큼 많은 경쟁자를 추가할 수 있습니다. 저희 분석가 팀은 또한 원시 엑셀 파일 피벗 테이블(팩트북)로 데이터를 제공하거나 보고서에서 사용 가능한 데이터 세트에서 프레젠테이션을 만드는 데 도움을 줄 수 있습니다.













