Switzerland Foot and Ankle Devices Market, By Product (Orthopedic Implants and Devices, Prostheses, Bracing and Support Devices, Osteotomy Wedge, Staple System and Others), Application (Trauma and Hairline Fractures, Osteoarthritis, Rheumatoid Arthritis, Diabetic Foot Diseases, Ligament Injuries, Neurological Disorders, Hammertoe, Osteoporosis and Other Applications), Surgery (Metatarsal Foot Surgery, Bunions Foot Surgery, Hammertoe Foot Surgery, Plantar Fasciitis Foot Surgery, Ankle Arthritis Surgeries, Achilles Tendon Disorders, Morton’s Neuroma Foot Surgery, Tibialis Posterior Dysfunction Foot Surgery and Other Surgeries), Type (Passive Devices and Powered Devices), Age (Pediatric, Adult, Geriatric), End User (Hospitals, Ambulatory Surgery Centers, Orthopedic Clinics, Trauma Centers, Rehabilitation Centers and Others) Distribution Channel (Direct Sales, Third Party Distributor and Online Sales) Industry Trends & Forecast to 2029.
Switzerland Foot and Ankle Devices Market Analysis and Insights
The foot and ankle devices are used to treat and heal various foot and ankle fractures due to conditions such as trauma and hairline fractures, osteoarthritis, rheumatoid arthritis, diabetic foot diseases, ligament injuries, neurological disorders, hammertoe, and osteoporosis. The foot and ankle devices cure. An orthotic (foot and ankle orthoses) is a device that can be placed in the shoes' sole to correct any abnormality in the feet and ankles. Foot orthotics lessen the foot pain caused by medical conditions such as arthritis, bunions, plantar fasciitis, flat feet, and diabetes. These foot devices can offset stress levels as pressure is exerted on them, allowing feet to function properly.
The advantages offered by the foot and ankle devices are superior comfort, improvement in balance and gait, aids in absorbance of shock, redirect pressure away from painful areas in the foot and ankle and improved athletic performance, and lowered the risk of injury.
Switzerland foot and ankle devices is supportive and aims to reduce the severity of the symptoms. Data Bridge Market Research analyses that the Switzerland foot and ankle devices market will grow at a CAGR of 4.8% during the forecast period of 2022 to 2029.
|
Report Metric |
Details |
|
Forecast Period |
2022 to 2029 |
|
Base Year |
2021 |
|
Historic Years |
2020 (Customizable to 2019 - 2014) |
|
Quantitative Units |
Revenue in USD Million, Pricing in USD |
|
Segments Covered |
제품별(정형외과 임플란트 및 장치, 보철물, 보조 및 지지 장치, 골절술 웨지, 스테이플 시스템 및 기타), 응용 분야(외상 및 모발 골절, 골관절염, 류마티스 관절염, 당뇨병성 발 질환, 인대 부상, 신경계 질환, 망치발가락, 골다공증 및 기타 응용 분야), 수술(중족골 발 수술, 외반모 발 수술, 망치발가락 발 수술, 발바닥 근막염 발 수술, 발목 관절염 수술, 아킬레스건 질환, 모튼 신경종 발 수술, 경골 후부 기능 장애 발 수술 및 기타 수술), 유형(수동 장치 및 전동 장치), 연령(소아, 성인, 노인), 최종 사용자(병원, 외래 수술 센터, 정형외과 병원, 외상 센터, 재활 센터 및 기타) 유통 채널(직접 판매, 제3자 유통업체 및 온라인 판매) |
|
국가 커버 |
스위스 |
|
시장 참여자 포함 |
Smith+Nephew(영국), Össur(아이슬란드), OTTOBOCK(독일), Globus Medical(미국), Medartis AG(바젤), DePuy Synthes(Johnson & Johnson Private Limited의 자회사)(미국), GROUP FH ORTHO(프랑스), Stryker(미국), Zimmer Biomet(미국), Arthrex, Inc.(미국), DJO, LLC(미국) 등이 있습니다. |
스위스 발과 발목 장치 시장 동향
운전자
- 만성 발 및 발목 질환의 유병률 및 발생률 증가
정형외과 질환은 전 세계적으로 계속 증가하고 있습니다. 관절염, 뼈 박차, 외반모지, 평발과 같은 질환이 날로 증가하고 있습니다. 염좌와 골절은 가장 흔한 발목 부상입니다. 스위스에서는 발과 발목 장애가 흔하며 그 발생률도 증가했습니다. 인구 증가, 비만, 스포츠 활동에 대한 보다 광범위한 참여가 스위스에서 발과 발목 장애 발생률의 주요 원인입니다. 발목 골절은 남성(53%)에서 여성(47%)보다 약간 더 흔합니다. 따라서 시장 성장률 확대로 이어질 주요 동인으로 작용합니다.
예를 들어,
국립 생명공학 정보 센터(NCBI) 보고서에 따르면 스위스에서 220만 명 이상이 근골격계 질환을 앓고 있으며 관절염이 가장 흔합니다. 16세 미만의 어린이 약 6,000명이 류마티스 관절염을 앓고 있습니다.
- 제품 출시 증가
스위스 발과 발목 장치의 성장률에 영향을 미치는 또 다른 중요한 요소는 새로운 발과 발목 보조기(정형외과 임플란트 및 장치, 내부 힘줄 고정 시스템, 압박 나사, 이종이식) 출시와 같은 제품 출시로, 산업 성장을 개선하고 발과 발목 장치의 판매와 유통을 늘릴 것으로 기대됩니다. 제품 출시는 새로운 파트너십과 사업 협력을 창출할 것입니다.
- 발과 발목 장치의 기술 개발의 성장
발과 발목 장치 분야에서 기술적 발전이 이루어졌습니다. 최근의 기술적 발전은 특히 보조기와 보철물 분야에서 의료적 발전을 촉진했습니다. 다양한 시장 참여자와의 협력과 최근의 기술적 접근 방식은 만성 통증 감소, 보행 개선, 자세 개선과 같은 임상 관리 운영에 새로운 기술이 어떤 영향을 미치는지 강조했습니다. 스위스 발과 발목 장치 시장의 성장을 더욱 강화할 것입니다.
또한, 의료 기술의 발전, 공공 및 민간 기관의 인식 확산을 위한 이니셔티브 증가, 정부 자금 지원 증가는 시장을 확대할 요인입니다. 효과적인 치료법에 대한 수요 증가, 시기적절한 진단에 대한 인식 증가와 같은 다른 요인은 스위스 발과 발목 장치 시장의 성장률에 긍정적인 영향을 미칠 것입니다. 또한, 높은 가처분 소득, 발작 사례 증가, 라이프스타일의 변화로 인해 스위스 발과 발목 장치 시장이 확대될 것입니다.
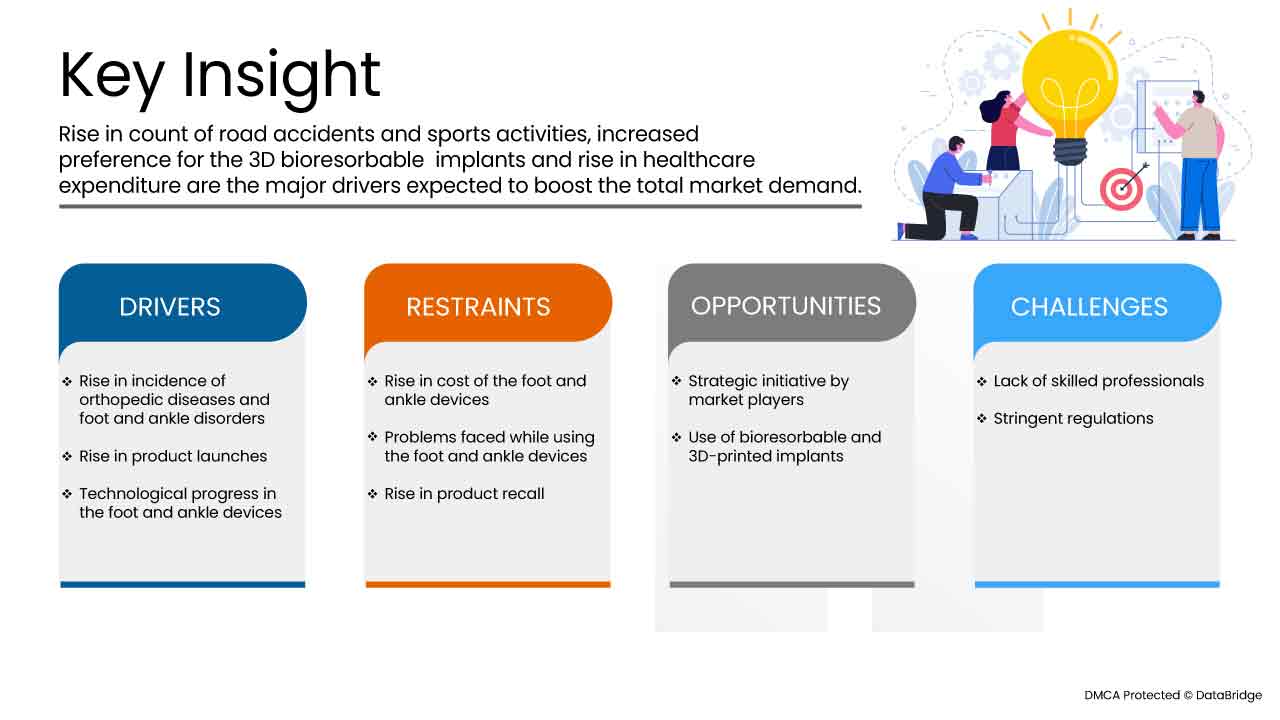
기회
- 의료비 지출은 모든 의료 서비스, 검사 장치, 가족 계획 활동, 건강을 위해 지정된 응급 처치를 포함하지만 식수와 위생 시설은 제외합니다. 모든 국가의 의료비 지출을 결정하는 요인은 소득(1인당 GDP), 기술 진보 및 의료 관행의 변화, 의료 시스템 특성입니다. 스위스의 의료비 지출 증가는 스위스에 있는 시장 참여자들의 발과 발목 장치 개발에 대한 투자를 증가시키고, 인적 자본을 강화하고 생산성을 개선하며, 재활, 정형외과 및 외상 센터를 설립하고 서비스 범위와 재정적 보호를 개선하여 발과 발목 질환 치료에 자금을 지원할 것으로 예상됩니다. 스위스의 개인 가구는 약 64%를 지불하여 스위스 의료 시스템 자금 조달에 가장 큰 기여를 합니다.
게다가 정부와 민간 기관의 연구 개발 활동이 늘어나고 투자도 늘어나면서 시장 성장률에 새로운 기회가 생길 것입니다.
또한 효과적인 치료법과 지속적인 임상 시험의 출시는 2022-2029년 예측 기간 동안 스위스 족부 및 발목 장치 시장에 유익한 기회를 제공할 것입니다. 또한, 현재 충족되지 않은 높은 수요와 의료 기술의 발전은 앞으로 스위스 족부 및 발목 장치 시장의 성장률을 높일 것입니다.
제약/도전
그러나 저소득 국가의 이용 가능한 인프라 부족과 관련된 높은 비용은 스위스 족부 및 발목 장치 시장의 성장률을 방해할 것입니다. 또한 보조 장치를 사용하는 동안 발생하는 위험은 스위스 족부 및 발목 장치 시장 성장을 방해할 것입니다. 이 신경학적 질환에 대한 치료법의 부족과 유전 및 희귀 질환에 대한 인식 부족은 위에서 언급한 예측 기간 동안 시장에 더욱 큰 도전이 될 것입니다.
스위스 족부 및 발목 장치 시장 보고서는 최근의 새로운 개발, 무역 규정, 수출입 분석, 생산 분석, 가치 사슬 최적화, 시장 점유율, 국내 및 지역 시장 참여자의 영향, 새로운 수익 창출처, 시장 규정의 변화, 전략적 시장 성장 분석, 시장 규모, 범주 시장 성장, 응용 분야 틈새 시장 및 지배력, 제품 승인, 제품 출시, 지리적 확장, 시장의 기술 혁신에 대한 분석 기회를 제공합니다. 스위스 족부 및 발목 장치 시장에 대한 자세한 정보를 얻으려면 Data Bridge Market Research에 연락하여 분석가 브리핑을 받으세요. 저희 팀은 시장 성장을 달성하기 위한 정보에 입각한 시장 결정을 내리는 데 도움을 드립니다.
환자 역학 분석
스위스 족부 및 발목 장치는 비교적 흔하지 않고 발생률이 알려지지 않은 만성 발 질환입니다. 미국 아카데미와 정형외과 의사의 연구에 따르면 스포츠 부상의 25%가 족부 및 발목과 관련이 있으며, 특히 축구, 야구, 축구, 달리기, 하키와 같이 점프와 달리기가 필요한 스포츠에서 그렇습니다.
스위스 발과 발목 장치 시장은 또한 환자 분석, 예후 및 치료에 대한 자세한 시장 분석을 제공합니다. 유병률, 발생률, 사망률, 준수율은 보고서에서 사용할 수 있는 일부 데이터 변수입니다. 시장 성장에 대한 역학의 직접 또는 간접 영향 분석을 분석하여 성장 기간 동안 시장을 예측하기 위한 보다 견고하고 코호트 다변량 통계 모델을 만듭니다.
COVID-19가 스위스 발과 발목 장치 시장에 미치는 영향
COVID-19는 시장에 부정적인 영향을 미쳤습니다. 팬데믹 동안의 봉쇄와 격리는 진단 관리와 치료를 복잡하게 만듭니다. 일상적이고 약물 투여를 위한 의료 시설에 대한 접근성 부족은 시장에 더 큰 영향을 미칠 것입니다. 사회적 고립은 스트레스, 절망, 사회적 지원을 증가시키며, 이 모든 것이 팬데믹 동안 항경련제 약물 복용을 감소시킬 수 있습니다. 시장 참여자들의 전략적 이니셔티브는 시장 규모를 늘릴 것으로 예상됩니다.
최근 개발
- 2021년 5월, Stryker는 Wright Medical의 제품을 통합한 새로운 발과 발목 포트폴리오를 선보이기 위해 Wright Medical과의 인수를 발표했습니다. 이 인수를 통해 회사는 제품 포트폴리오를 성장시키고 전 세계적으로 새로운 발과 발목 제품을 출시할 수 있었습니다.
스위스 발과 발목 장치 시장 범위
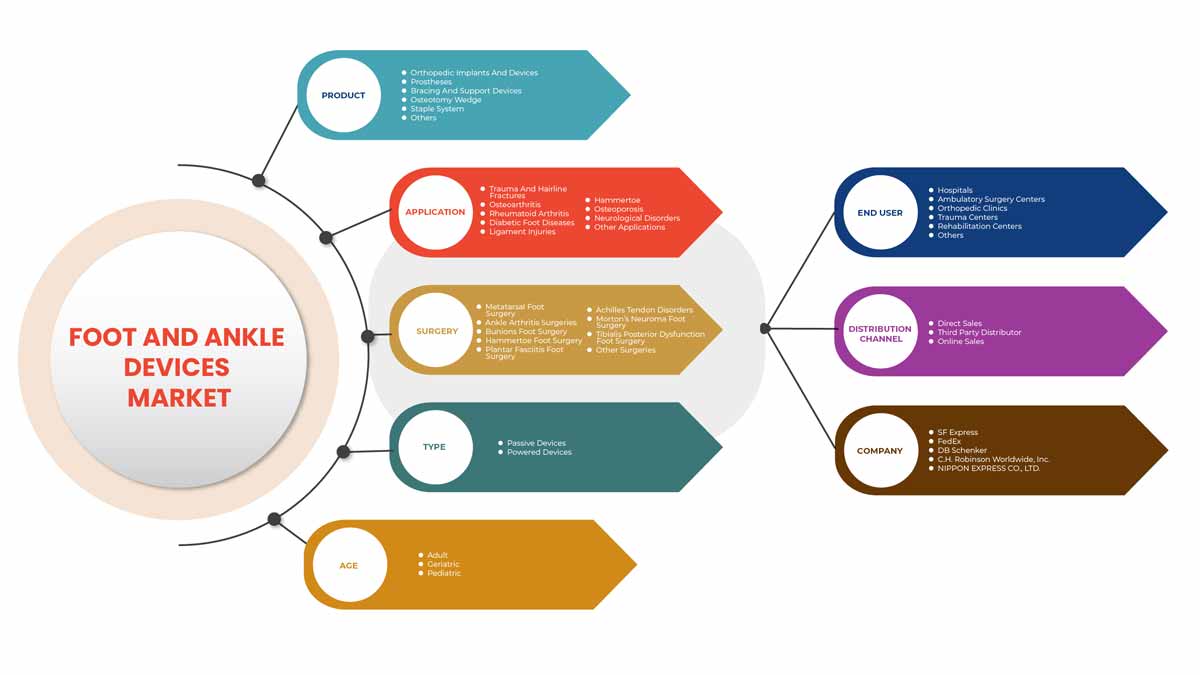
스위스 발과 발목 장치 시장은 제품, 응용 프로그램, 수술, 유형, 연령, 최종 사용자 및 유통 채널을 기준으로 세분화됩니다. 이러한 세그먼트 간의 성장은 산업의 빈약한 성장 세그먼트를 분석하고 사용자에게 귀중한 시장 개요와 시장 통찰력을 제공하여 핵심 시장 응용 프로그램을 식별하기 위한 전략적 결정을 내리는 데 도움이 됩니다.
제품
- 정형외과 임플란트 및 장치
- 보철물
- 브레이싱 및 지지 장치
- 골절술 웨지
- 스테이플 시스템
- 기타
정형외과 임플란트 및 장치는 제품을 기준으로 정형외과 임플란트 및 장치, 보철물, 보조 및 지지 장치, 골절술 웨지, 스테이플 시스템 및 기타로 세분화됩니다.
애플리케이션
- 외상 및 모발 골절
- 골관절염
- 류머티스성 관절염
- 당뇨발질환
- 인대 부상
- 망치족지
- 골다공증
- 신경계 질환
- 다른 응용 프로그램
스위스 발 및 발목 장치 시장은 응용 분야별로 외상 및 미세 골절, 골관절염, 류마티스 관절염, 당뇨성 발 질환, 인대 부상, 신경 장애, 망치발가락 , 골다공증 및 기타 응용 분야로 구분됩니다.
수술
- 중족골 발 수술
- 발목 관절염 수술
- 엄지발가락 외반증 발 수술
- 망치발가락 수술
- 발바닥 근막염 발 수술
- 아킬레스건 장애
- 모튼 신경종 발 수술
- 후경골근 기능 장애 발 수술
- 기타 수술
수술을 기준으로 볼 때, 스위스 발 및 발목 의료기기 시장은 중족골 발 수술, 외반모지 발 수술, 망치발가락 발 수술, 발바닥 근막염 발 수술, 발목 관절염 수술, 아킬레스건 장애, 모튼 신경종 발 수술, 후경골근 기능 장애 발 수술 및 기타 수술로 구분됩니다.
유형
- 수동 장치
- 전원 장치
유형을 기준으로 스위스 발 및 발목 장치 시장은 수동 장치와 전원 장치로 구분됩니다.
나이
- 성인
- 노인
- 소아과
스위스 발 및 발목 장치 시장은 연령을 기준으로 소아용, 성인용, 노인용으로 구분됩니다.
최종 사용자
- 병원
- 외래 수술 센터
- 정형외과 병원
- 트라우마 센터
- 재활 센터
- 기타
스위스 발 및 발목 장치 시장은 최종 사용자를 기준으로 병원, 외래 수술 센터, 정형외과 병원, 외상 센터, 재활 센터 등으로 구분됩니다.
유통 채널
- 직접 판매
- 제3자 유통업체
- 온라인 판매
스위스 발 및 발목 장치 시장은 유통 채널을 기준으로 직접 판매, 제3자 유통업체, 온라인 판매로 구분됩니다.
스위스 발과 발목 장치 시장 국가 분석/통찰력
스위스 발 및 발목 장치 시장을 분석하고, 위에 언급된 대로 국가, 제품, 응용 분야, 수술, 유형, 연령, 최종 사용자 및 유통 채널별로 시장 규모에 대한 통찰력과 추세를 제공합니다.
스위스 발 및 발목 장치 시장 보고서에서 다루는 국가는 스위스입니다.
보고서의 국가 섹션은 또한 개별 시장 영향 요인과 국내 시장의 현재 및 미래 트렌드에 영향을 미치는 규제의 변화를 제공합니다. 신규 판매, 교체 판매, 국가 인구 통계, 질병 역학 및 수출입 관세와 같은 데이터 포인트는 개별 국가의 시장 시나리오를 예측하는 데 사용되는 주요 포인터 중 일부입니다. 또한 글로벌 브랜드의 존재 및 가용성과 지역 및 국내 브랜드와의 대규모 또는 희소한 경쟁으로 인해 직면한 과제, 판매 채널의 영향은 국가 데이터에 대한 예측 분석을 제공하는 동안 고려됩니다.
경쟁 환경 및 스위스 발 및 발목 장치 시장 점유율 분석
스위스 족부 및 발목 장치 시장 경쟁 구도는 경쟁자별 세부 정보를 제공합니다. 포함된 세부 정보에는 회사 개요, 회사 재무, 창출된 수익, 시장 잠재력, 연구 개발 투자, 새로운 시장 이니셔티브, 글로벌 입지, 생산 현장 및 시설, 생산 용량, 회사의 강점과 약점, 제품 출시, 제품 폭과 범위, 응용 분야 우위가 있습니다. 위에 제공된 데이터 포인트는 스위스 족부 및 발목 장치 시장과 관련된 회사의 초점에만 관련이 있습니다.
스위스 발 및 발목 장치 시장의 주요 기업으로는 Smith+Nephew, Össur, OTTOBOCK, Globus Medical, Medartis AG, DePuy Synthes(Johnson & Johnson Services, Inc의 자회사), GROUP FH ORTHO, Stryker, Zimmer Biomet., Arthrex, Inc., DJO, LLC 등이 있습니다.
SKU-
세계 최초의 시장 정보 클라우드 보고서에 온라인으로 접속하세요
- 대화형 데이터 분석 대시보드
- 높은 성장 잠재력 기회를 위한 회사 분석 대시보드
- 사용자 정의 및 질의를 위한 리서치 분석가 액세스
- 대화형 대시보드를 통한 경쟁자 분석
- 최신 뉴스, 업데이트 및 추세 분석
- 포괄적인 경쟁자 추적을 위한 벤치마크 분석의 힘 활용
목차
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF SWITZERLAND FOOT AND ANKLE DEVICES MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATIONS
1.6 MARKETS COVERED
2 SWITZERLAND FOOT AND ANKLE DEVICES MARKET: SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 DBMR TRIPOD DATA VALIDATION MODEL
2.5 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.6 MULTIVARIATE MODELLING
2.7 PRODUCT SEGMENT LIFELINE CURVE
2.8 DBMR MARKET POSITION GRID
2.9 MARKET END USER COVERAGE GRID
2.1 SECONDARY SOURCES
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 SWITZERLAND FOOT AND ANKLE DEVICES MARKET (2020-2029) , NUMBER OF SURGERIES (IN THOUSANDS)
4.2 PESTEL
4.3 PORTER'S FIVE FORCES
5 SUMMARY WRITE UP (SWITZERLAND)
5.1 OVERVIEW
6 SWITZERLAND FOOT AND ANKLE DEVICES MARKET: REGULATIONS
7 MARKET OVERVIEW
7.1 DRIVERS
7.1.1 RISE IN INCIDENCE OF ORTHOPEDIC DISEASES AND FOOT AND ANKLE DISORDERS
7.1.2 RISE IN NUMBER OF ROAD ACCIDENTS AND SPORTS ACTIVITIES
7.1.3 GROWING AWARENESS IN PUBLIC AND PRIVATE INSTITUTES RELATED TO TREATMENT AND POST-OPERATIVE CARE
7.1.4 RISE IN PRODUCT LAUNCHES
7.1.5 TECHNOLOGICAL PROGRESS IN FOOT AND ANKLE DEVICES
7.2 RESTRAINTS
7.2.1 RISE IN COST OF FOOT AND ANKLE DEVICES
7.2.2 PROBLEMS FACED WHILE USING FOOT AND ANKLE DEVICES
7.2.3 RISE IN PRODUCT RECALL
7.2.4 RISE IN CONCERN REGARDING METAL SENSITIVITY IN PATIENTS WITH FOOT AND ANKLE DEVICES
7.3 OPPORTUNITIES
7.3.1 STRATEGIC INITIATIVES BY MARKET PLAYERS
7.3.2 RISE IN HEALTHCARE EXPENDITURE
7.3.3 USE OF BIORESORBABLE AND 3D-PRINTED IMPLANTS
7.4 CHALLENGES
7.4.1 LACK OF SKILLED PROFESSIONALS
7.4.2 STRINGENT REGULATIONS
8 SWITZERLAND FOOT AND ANKLE DEVICES MARKET, BY PRODUCT
8.1 OVERVIEW
8.2 ORTHOPEDIC IMPLANTS AND DEVICES
8.2.1 FIXATION DEVICES
8.2.1.1 INTERNAL FIXATION DEVICES
8.2.1.1.1 PLATES
8.2.1.1.1.1 MEDIAL DISTAL TIBIA PLATES
8.2.1.1.1.2 ANTEROLATERAL DISTAL TIBIA PLATES
8.2.1.1.1.3 DISTAL TIBIA T AND L PLATES
8.2.1.1.1.4 LATERAL DISTAL FIBULA PLATES
8.2.1.1.1.5 OTHERS
8.2.1.1.2 SCREWS
8.2.1.1.2.1 BY TYPE
8.2.1.1.2.1.1 CANNULATED SCREW
8.2.1.1.2.1.2 HEADLESS COMPRESSION SCREW
8.2.1.1.2.1.3 HEADED-FOREFOOT COMPRESSION SCREWS
8.2.1.1.2.1.4 SNAP-OFF SCREWS
8.2.1.1.2.1.5 OTHERS
8.2.1.1.2.2 BY MATERIAL
8.2.1.1.2.2.1 STAINLESS STEEL
8.2.1.1.2.2.2 TITANIUM
8.2.1.1.2.2.3 OTHERS
8.2.1.1.3 FUSION NAILS
8.2.1.1.3.1 WITH INTERNAL COMPRESSION
8.2.1.1.3.2 WITHOUT INTERNAL COMPRESSION
8.2.1.1.3.3 WIRES & PINS
8.2.1.1.3.4 OTHERS
8.2.1.2 EXTERNAL FIXATION DEVICES
8.2.1.2.1 RING ANKLE FIXATORS
8.2.1.2.2 UNILATERAL FIXATORS
8.2.1.2.3 HYBRID FIXATORS
8.2.1.2.4 CIRCULAR FIXATORS
8.2.2 JOINT IMPLANTS
8.2.2.1 ANKLE IMPLANTS (ANKLE REPLACEMENT DEVICES)
8.2.2.2 PHALANGEAL IMPLANTS
8.2.2.3 SUBTALAR JOINT IMPLANTS (SUBTALAR JOINT RECONSTRUCTION DEVICES)
8.2.2.4 OTHERS
8.2.3 SOFT-TISSUE ORTHOPEDIC DEVICES
8.2.3.1 MUSCULOSKELETAL REINFORCEMENT DEVICES
8.2.3.2 ARTIFICIAL TENDONS AND LIGAMENTS
8.2.3.3 HIGH STRENGTH SUTURES
8.2.3.4 OTHERS
8.3 PROSTHESES
8.3.1 SOLID ANKLE CUSHION HEEL PROSTHESES
8.3.2 ELASTIC (FLEXIBLE) KEEL FOOT
8.3.3 SINGLE AXIAL PROSTHESES
8.3.4 MULTIAXIAL PROSTHESES
8.3.5 DYNAMIC RESPONSE/ ENERGY STORING PROSTHESES
8.3.6 MICROPROCESSOR-CONTROLLED PROSTHESES
8.4 BRACING AND SUPPORT DEVICES
8.4.1 SOFT BRACES AND SUPPORT DEVICES
8.4.2 HARD BRACES AND SUPPORT DEVICES
8.4.3 HINGED BRACES AND SUPPORT DEVICES
8.4.4 DYNAMIC ANKLE FOOT ORTHOTIC
8.4.5 OTHERS
8.5 OSTEOTOMY WEDGE
8.5.1 ALLOGRAFT BONE
8.5.2 WEDGE SYSTEM
8.5.3 OTHERS
8.6 STAPLE SYSTEM
8.7 OTHERS
9 SWITZERLAND FOOT AND ANKLE DEVICES MARKET, BY APPLICATION
9.1 OVERVIEW
9.2 TRAUMA AND HAIRLINE FRACTURES
9.3 OSTEOARTHRITIS
9.4 RHEUMATOID ARTHRITIS
9.5 DIABETIC FOOT DISEASES
9.6 LIGAMENT INJURIES
9.7 HAMMERTOE
9.8 OSTEOPOROSIS
9.9 NEUROLOGICAL DISORDERS
9.1 OTHER APPLICATIONS
10 SWITZERLAND FOOT AND ANKLE DEVICES MARKET, BY SURGERY
10.1 OVERVIEW
10.2 METATARSAL FOOT SURGERY
10.3 ANKLE ARTHRITIS SURGERIES
10.4 BUNIONS FOOT SURGERY
10.5 HAMMERTOE FOOT SURGERY
10.6 PLANTAR FASCIITIS FOOT SURGERY
10.7 ACHILLES TENDON DISORDERS
10.8 MORTON’S NEUROMA FOOT SURGERY
10.9 TIBIALIS POSTERIOR DYSFUNCTION FOOT SURGERY
10.1 OTHER SURGERIES
11 SWITZERLAND FOOT AND ANKLE DEVICES MARKET, BY TYPE
11.1 OVERVIEW
11.2 PASSIVE DEVICES
11.3 POWERED DEVICES
12 SWITZERLAND FOOT AND ANKLE DEVICES MARKET, BY AGE
12.1 OVERVIEW
12.2 ADULT
12.3 GERIATRIC
12.4 PEDIATRIC
13 SWITZERLAND FOOT AND ANKLE DEVICES MARKET, BY END USER
13.1 OVERVIEW
13.2 HOSPITALS
13.3 AMBULATORY SURGERY CENTERS
13.4 ORTHOPEDIC CLINICS
13.5 TRAUMA CENTERS
13.6 REHABILITATION CENTERS
13.7 OTHERS
14 SWITZERLAND FOOT AND ANKLE DEVICES MARKET, BY DISTRIBUTION CHANNEL
14.1 OVERVIEW
14.2 DIRECT SALES
14.3 THIRD PARTY DISTRIBUTOR
14.4 ONLINE SALES
15 SWITZERLAND FOOT AND ANKLE DEVICES MARKET: COMPANY LANDSCAPE
15.1 COMPANY SHARE ANALYSIS: SWITZERLAND
16 SWOT ANALYSIS
17 COMPANY PROFILE
17.1 DEPUY SYNTHES (A SUBSIDIARY OF JOHNSON & JOHNSON SERVICES, INC)
17.1.1 COMPANY SNAPSHOT
17.1.2 REVENUE ANALYSIS
17.1.3 PRODUCT PORTFOLIO
17.1.4 RECENT DEVELOPMENT
17.1.4.1 ACQUISITION
17.2 STRYKER
17.2.1 COMPANY SNAPSHOT
17.2.2 REVENUE ANALYSIS
17.2.3 PRODUCT PORTFOLIO
17.2.4 RECENT DEVELOPMENTS
17.2.4.1 ACQUISITION
17.3 ARTHREX, INC.
17.3.1 COMPANY SNAPSHOT
17.3.2 PRODUCT PORTFOLIO
17.3.3 RECENT DEVELOPMENT
17.4 MEDARTIS AG
17.4.1 COMPANY SNAPSHOT
17.4.2 REVENUE ANALYSIS
17.4.3 PRODUCT PORTFOLIO
17.4.4 RECENT DEVELOPMENT
17.4.4.1 ACQUISITION
17.5 ZIMMER BIOMET
17.5.1 COMPANY SNAPSHOT
17.5.2 REVENUE ANALYSIS
17.5.3 PRODUCT PORTFOLIO
17.5.4 RECENT DEVELOPMENT
17.5.4.1 ACQUISITION
17.6 ÖSSUR
17.6.1 COMPANY SNAPSHOT
17.6.2 REVENUE ANALYSIS
17.6.3 PRODUCT PORTFOLIO
17.6.4 RECENT DEVELOPMENTS
17.6.4.1 PRODUCT LAUNCH
17.7 DJO, LLC
17.7.1 COMPANY SNAPSHOT
17.7.2 1.7.2 PRODUCT PORTFOLIO
17.7.3 RECENT DEVELOPMENTS
17.7.3.1 ACQUISITION
17.8 SMITH & NEPHEW
17.8.1 COMPANY SNAPSHOT
17.8.2 REVENUE ANALYSIS
17.8.3 PRODUCT PORTFOLIO
17.8.4 RECENT DEVELOPMENTS
17.9 OTTOBOCK
17.9.1 COMPANY SNAPSHOT
17.9.2 PRODUCT PORTFOLIO
17.9.3 RECENT DEVELOPMENT
17.9.3.1 PARTNERSHIP
17.1 ACUMED
17.10.1 COMPANY SNAPSHOT
17.10.2 PRODUCT PORTFOLIO
17.10.3 RECENT DEVELOPMENT
17.11 GROUP FH ORTHO
17.11.1 COMPANY SNAPSHOT
17.11.2 PRODUCT PORTFOLIO
17.11.3 RECENT DEVELOPMENTS
17.12 GLOBUS MEDICAL, INC.
17.12.1 COMPANY SNAPSHOT
17.12.2 REVENUE ANALYSIS
17.12.3 PRODUCT PORTFOLIO
17.12.4 RECENT DEVELOPMENT
17.12.4.1 ACQUISITION
18 QUESTIONNAIRE
19 RELATED REPORTS
표 목록
TABLE 1 SWITZERLAND FOOT AND ANKLE DEVICES MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 2 SWITZERLAND ORTHOPEDIC IMPLANTS AND DEVICES IN FOOT AND ANKLE DEVICES MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 3 SWITZERLAND FIXATION DEVICES IN FOOT AND ANKLE DEVICES MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 4 SWITZERLAND INTERNAL FIXATION DEVICES IN FOOT AND ANKLE DEVICES MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 5 SWITZERLAND PLATES IN FOOT AND ANKLE DEVICES MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 6 SWITZERLAND SCREWS IN FOOT AND ANKLE DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 7 SWITZERLAND SCREWS IN FOOT AND ANKLE DEVICES MARKET, BY MATERIAL, 2020-2029 (USD MILLION)
TABLE 8 SWITZERLAND FUSION NAILS IN FOOT AND ANKLE DEVICES MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 9 SWITZERLAND EXTERNAL FIXATION DEVICES IN FOOT AND ANKLE DEVICES MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 10 SWITZERLAND JOINT IMPLANTS IN FOOT AND ANKLE DEVICES MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 11 SWITZERLAND SOFT-TISSUE ORTHOPEDIC DEVICES IN FOOT AND ANKLE DEVICES MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 12 SWITZERLAND PROSTHESES IN FOOT AND ANKLE DEVICES MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 13 SWITZERLAND BRACING AND SUPPORT DEVICES IN FOOT AND ANKLE DEVICES MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 14 SWITZERLAND OSTEOTOMY WEDGE IN FOOT AND ANKLE DEVICES MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 15 SWITZERLAND FOOT AND ANKLE DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 16 SWITZERLAND FOOT AND ANKLE DEVICES MARKET, BY SURGERY, 2020-2029 (USD MILLION)
TABLE 17 SWITZERLAND FOOT AND ANKLE DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 18 SWITZERLAND FOOT AND ANKLE DEVICES MARKET, BY AGE, 2020-2029 (USD MILLION)
TABLE 19 SWITZERLAND FOOT AND ANKLE DEVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 20 SWITZERLAND FOOT AND ANKLE DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
그림 목록
FIGURE 1 SWITZERLAND FOOT AND ANKLE DEVICES MARKET: SEGMENTATION
FIGURE 2 SWITZERLAND FOOT AND ANKLE DEVICES MARKET: DATA TRIANGULATION
FIGURE 3 SWITZERLAND FOOT AND ANKLE DEVICES MARKET: DROC ANALYSIS
FIGURE 4 SWITZERLAND FOOT AND ANKLE DEVICES MARKET: COUNTRY VS REGIONAL MARKET ANALYSIS
FIGURE 5 SWITZERLAND FOOT AND ANKLE DEVICES MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 SWITZERLAND FOOT AND ANKLE DEVICES MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 SWITZERLAND FOOT AND ANKLE DEVICES MARKET: DBMR POSITION GRID
FIGURE 8 SWITZERLAND FOOT AND ANKLE DEVICES MARKET: END USER COVERAGE GRID
FIGURE 9 SWITZERLAND FOOT AND ANKLE DEVICES MARKET: SEGMENTATION
FIGURE 10 INCREASED PREVALENCE OF CHRONIC FOOT AND ANKLE DISORDERS, RISE IN TECHNOLOGICAL DEVELOPMENTS, INCREASED PREFERENCE FOR BIORESORBABLE IMPLANTS, AND PRODUCT APPROVAL IS EXPECTED TO DRIVE SWITZERLAND FOOT AND ANKLE DEVICES MARKET FROM 2022 TO 2029
FIGURE 11 PRODUCT SEGMENT IS EXPECTED TO HAVE THE LARGEST SHARE OF THE SWITZERLAND FOOT AND ANKLE DEVICES MARKET FROM 2022 & 2029
FIGURE 12 NUMBER OF SURGERIES (IN THOUSANDS)
FIGURE 13 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF THE SWITZERLAND FOOT AND ANKLE DEVICES MARKET
FIGURE 14 TOTAL ANKLE REPLACEMENT SURGERIES IN SWITZERLAND FROM 2000- 2010
FIGURE 15 TOTAL ANKLE REPLACEMENT SURGERIES IN SWITZERLAND FROM 2018-2020
FIGURE 16 SWITZERLAND FOOT AND ANKLE DEVICES MARKET: BY PRODUCT, 2021
FIGURE 17 SWITZERLAND FOOT AND ANKLE DEVICES MARKET: BY PRODUCT, 2022-2029 (USD MILLION)
FIGURE 18 SWITZERLAND FOOT AND ANKLE DEVICES MARKET: BY PRODUCT, CAGR (2022-2029)
FIGURE 19 SWITZERLAND FOOT AND ANKLE DEVICES MARKET: BY PRODUCT, LIFELINE CURVE
FIGURE 20 SWITZERLAND FOOT AND ANKLE DEVICES MARKET: BY APPLICATION, 2021
FIGURE 21 SWITZERLAND FOOT AND ANKLE DEVICES MARKET: BY APPLICATION, 2022-2029 (USD MILLION)
FIGURE 22 SWITZERLAND FOOT AND ANKLE DEVICES MARKET: BY APPLICATION, CAGR (2022-2029)
FIGURE 23 SWITZERLAND FOOT AND ANKLE DEVICES MARKET: BY APPLICATION, LIFELINE CURVE
FIGURE 24 SWITZERLAND FOOT AND ANKLE DEVICES MARKET: BY SURGERY, 2021
FIGURE 25 SWITZERLAND FOOT AND ANKLE DEVICES MARKET: BY SURGERY, 2022-2029 (USD MILLION)
FIGURE 26 SWITZERLAND FOOT AND ANKLE DEVICES MARKET: BY SURGERY, CAGR (2022-2029)
FIGURE 27 SWITZERLAND FOOT AND ANKLE DEVICES MARKET: BY SURGERY, LIFELINE CURVE
FIGURE 28 SWITZERLAND FOOT AND ANKLE DEVICES MARKET: BY TYPE, 2021
FIGURE 29 SWITZERLAND FOOT AND ANKLE DEVICES MARKET: BY TYPE, 2022-2029 (USD MILLION)
FIGURE 30 SWITZERLAND FOOT AND ANKLE DEVICES MARKET: BY TYPE, CAGR (2022-2029)
FIGURE 31 SWITZERLAND FOOT AND ANKLE DEVICES MARKET: BY TYPE, LIFELINE CURVE
FIGURE 32 SWITZERLAND FOOT AND ANKLE DEVICES MARKET: BY AGE, 2021
FIGURE 33 SWITZERLAND FOOT AND ANKLE DEVICES MARKET: BY AGE, 2022-2029 (USD MILLION)
FIGURE 34 SWITZERLAND FOOT AND ANKLE DEVICES MARKET: BY AGE, CAGR (2022-2029)
FIGURE 35 SWITZERLAND FOOT AND ANKLE DEVICES MARKET: BY AGE, LIFELINE CURVE
FIGURE 36 SWITZERLAND FOOT AND ANKLE DEVICES MARKET: BY END USER, 2021
FIGURE 37 SWITZERLAND FOOT AND ANKLE DEVICES MARKET: BY END USER, 2022-2029 (USD MILLION)
FIGURE 38 SWITZERLAND FOOT AND ANKLE DEVICES MARKET: BY END USER, CAGR (2022-2029)
FIGURE 39 SWITZERLAND FOOT AND ANKLE DEVICES MARKET: BY END USER, LIFELINE CURVE
FIGURE 40 SWITZERLAND FOOT AND ANKLE DEVICES MARKET: BY DISTRIBUTION CHANNEL, 2021
FIGURE 41 SWITZERLAND FOOT AND ANKLE DEVICES MARKET: BY DISTRIBUTION CHANNEL, 2022-2029 (USD MILLION)
FIGURE 42 SWITZERLAND FOOT AND ANKLE DEVICES MARKET: BY DISTRIBUTION CHANNEL, CAGR (2022-2029)
FIGURE 43 SWITZERLAND FOOT AND ANKLE DEVICES MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 44 SWITZERLAND FOOT AND ANKLE DEVICES MARKET: COMPANY SHARE 2021 (%)

연구 방법론
데이터 수집 및 기준 연도 분석은 대규모 샘플 크기의 데이터 수집 모듈을 사용하여 수행됩니다. 이 단계에는 다양한 소스와 전략을 통해 시장 정보 또는 관련 데이터를 얻는 것이 포함됩니다. 여기에는 과거에 수집한 모든 데이터를 미리 검토하고 계획하는 것이 포함됩니다. 또한 다양한 정보 소스에서 발견되는 정보 불일치를 검토하는 것도 포함됩니다. 시장 데이터는 시장 통계 및 일관된 모델을 사용하여 분석하고 추정합니다. 또한 시장 점유율 분석 및 주요 추세 분석은 시장 보고서의 주요 성공 요인입니다. 자세한 내용은 분석가에게 전화를 요청하거나 문의 사항을 드롭하세요.
DBMR 연구팀에서 사용하는 주요 연구 방법론은 데이터 마이닝, 시장에 대한 데이터 변수의 영향 분석 및 주요(산업 전문가) 검증을 포함하는 데이터 삼각 측량입니다. 데이터 모델에는 공급업체 포지셔닝 그리드, 시장 타임라인 분석, 시장 개요 및 가이드, 회사 포지셔닝 그리드, 특허 분석, 가격 분석, 회사 시장 점유율 분석, 측정 기준, 글로벌 대 지역 및 공급업체 점유율 분석이 포함됩니다. 연구 방법론에 대해 자세히 알아보려면 문의를 통해 업계 전문가에게 문의하세요.
사용자 정의 가능
Data Bridge Market Research는 고급 형성 연구 분야의 선두 주자입니다. 저희는 기존 및 신규 고객에게 목표에 맞는 데이터와 분석을 제공하는 데 자부심을 느낍니다. 보고서는 추가 국가에 대한 시장 이해(국가 목록 요청), 임상 시험 결과 데이터, 문헌 검토, 재생 시장 및 제품 기반 분석을 포함하도록 사용자 정의할 수 있습니다. 기술 기반 분석에서 시장 포트폴리오 전략에 이르기까지 타겟 경쟁업체의 시장 분석을 분석할 수 있습니다. 귀하가 원하는 형식과 데이터 스타일로 필요한 만큼 많은 경쟁자를 추가할 수 있습니다. 저희 분석가 팀은 또한 원시 엑셀 파일 피벗 테이블(팩트북)로 데이터를 제공하거나 보고서에서 사용 가능한 데이터 세트에서 프레젠테이션을 만드는 데 도움을 줄 수 있습니다.













