Peru Acute Myeloid Leukemia Drugs Market, By Sub-Type (M0 (Undifferentiated Acute Myelobastic Leukemia), M1 (Acute Myeloblastic Leukemia with Minimal Maturation), M2 (Acute Myeloblastic Leukemia with Maturation), M3 (Acute Promyelocytic Leukemia (APL)), M4 (Acute Myelomonocytic Leukemia), M5 (Acute Monocytic Leukemia), M6 (Acute Erythroid Leukemia), M7 (Acute Megakaryoblastic Leukemia), Drugs (Chemotherapy, Targeted Therapy, Immunotherapy), Drug Type (Branded and Generics), Route of Administration (Parenteral, Oral, and Others), Population Type (Geriatric, Adults, and Pediatric), End User (Hospitals, Specialty Clinics, Ambulatory Centers and Others), Distribution Channel (Direct Tender, Hospital Pharmacy, Retail Pharmacy, Online Pharmacy and Others), Industry Trends and Forecast to 2029.
Peru Acute Myeloid Leukemia Drugs Market Analysis and Insights
Acute myeloid leukemia (AML) is a type of blood cancer that affects the bone marrow. In adults, it is the most frequent kind of acute leukemia. If left untreated, this type of cancer usually worsens swiftly. AML is a kind of leukemia that affects the blood cells.
Blood stem cells (immature cells) are produced in the bone marrow and over time they become mature. A blood stem cell can differentiate into a myeloid or lymphoid stem cell. A white blood cell develops from a lymphoid stem cell. A mature blood cell from a myeloid stem cell is one of three types that are red blood cells, granulocytes, and platelets.
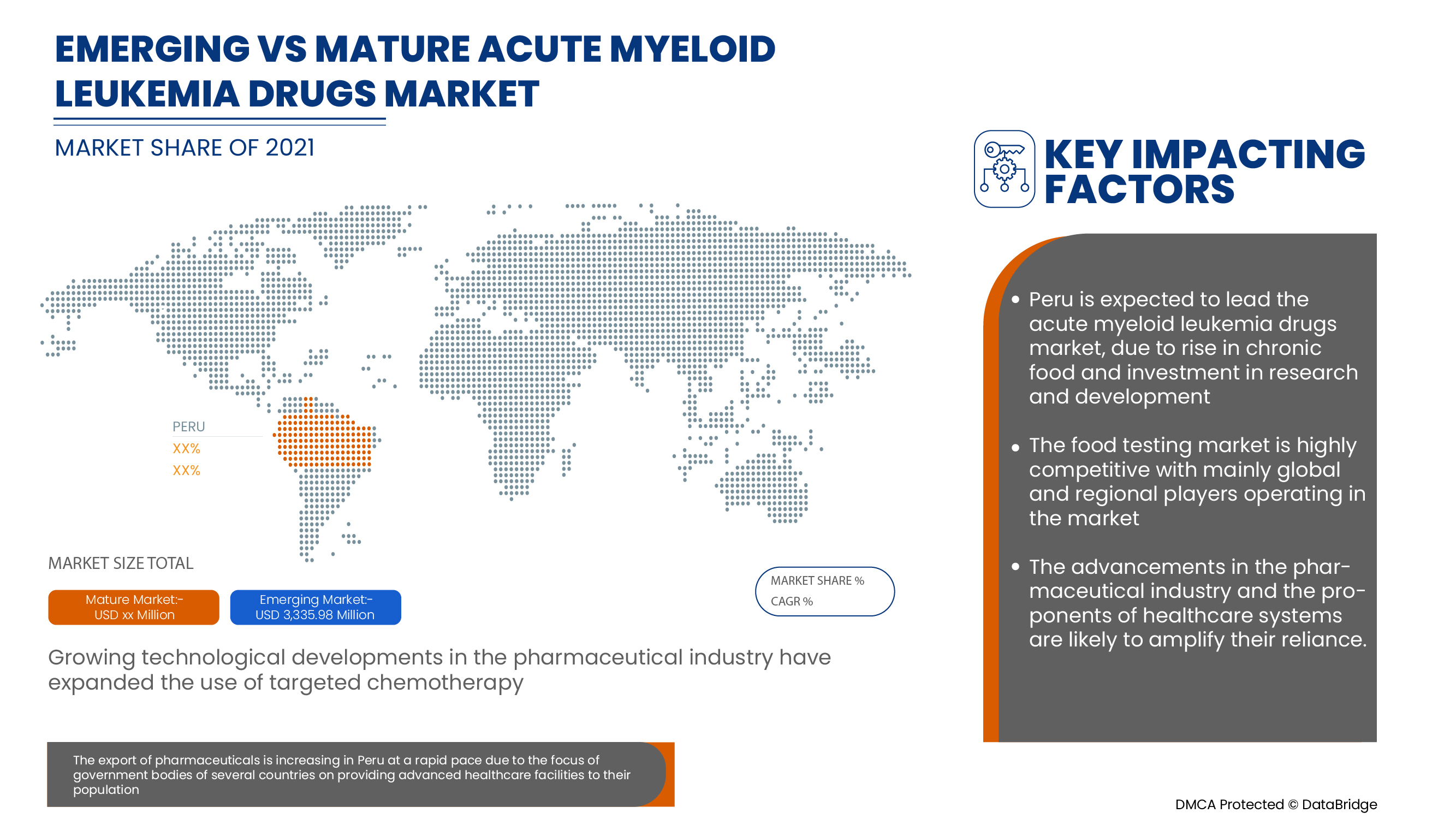
Acute myeloid leukemia drugs are supportive and aim to reduce the severity of the symptoms. Data Bridge Market Research analyses that the Peru acute myeloid leukemia drugs market will grow at a CAGR of 6.5% during the forecast period of 2022 to 2029.
|
Report Metric |
Details |
|
Forecast Period |
2022 to 2029 |
|
Base Year |
2021 |
|
Historic Years |
2020 (Customizable to 2019 - 2014) |
|
Quantitative Units |
Revenue in USD Thousand, Pricing in USD |
|
Segments Covered |
By Sub-Type (M0 (Undifferentiated Acute Myelobastic Leukemia), M1 (Acute Myeloblastic Leukemia with Minimal Maturation), M2 (Acute Myeloblastic Leukemia with Maturation), M3 (Acute Promyelocytic Leukemia (APL)), M4 (Acute Myelomonocytic Leukemia), M5 (Acute Monocytic Leukemia), M6 (Acute Erythroid Leukemia), M7 (Acute Megakaryoblastic Leukemia), Drugs (Chemotherapy, Targeted Therapy, Immunotherapy), Drug Type (Branded and Generics), Route of Administration (Parenteral, Oral, and Others), Population Type (Geriatric, Adults, and Pediatric), End User (Hospitals, Specialty Clinics, Ambulatory Centers and Others), Distribution Channel (Direct Tender, Hospital Pharmacy, Retail Pharmacy, Online Pharmacy and Others) |
|
Country Covered |
Peru |
|
Market Players Covered |
Pfizer Inc., MERCK SHARP & DOHME CORP.(MERCK & CO., INC.의 자회사), Janssen Pharmaceuticals, Inc.(Johnson & Johnson Services, Inc.의 자회사), Novartis AG, Fresenius Kabi AG, F. Hoffmann-La Roche Ltd, Abbvie Inc, Novartis Ag, F. Hoffmann-La Roche Ltd, AstraZeneca, Bohringer Ingelheim International GmbH, Sun Pharmaceutical Industries Ltd., Teva Pharmaceutical Industries Ltd. 등이 있습니다. |
페루 급성 골수성 백혈병 약물 시장 동향
운전자
-
급성 골수성 백혈병의 부담 증가
암 환자의 부담 증가는 급성 골수성 백혈병 약물의 주요 추진 요인 중 하나입니다. 암 환자의 증가로 인해 치료에 대한 수요가 증가합니다.
예를 들어,
- 페루에서는 암으로 인한 사망자 수가 2003년 전체 사망자의 15.4%에서 2016년 18.1%로 증가했습니다(p<0.001).
- 2003년부터 2016년까지 페루에서는 사망자의 거의 5분의 1이 암으로 인한 것으로 나타났습니다.
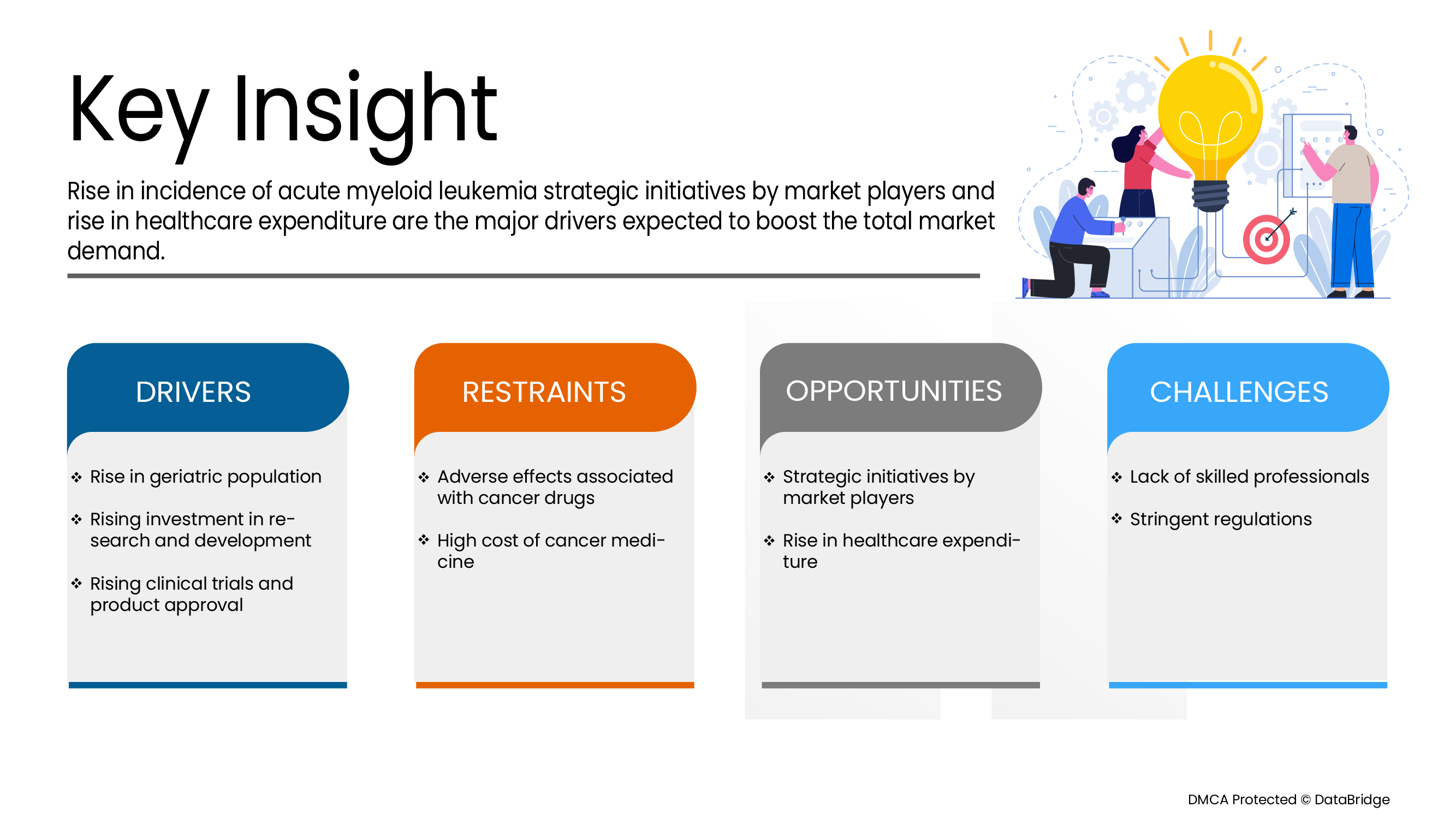
위의 관점에서 우리는 페루와 에콰도르에서 급성 골수성 백혈병의 발병률이 빠르게 증가하고 있으며, 그 결과 급성 골수성 백혈병 약물 시장도 증가하고 있다는 결론을 내릴 수 있습니다. 암 발병률이 증가하는 이유에는 생활 방식의 변화와 환경 변화와 같은 여러 요인이 포함될 수 있으며, 이는 시장 성장을 더욱 촉진할 것으로 예상됩니다.
- 증가하는 임상 시험 및 제품 승인
임상 시험의 증가는 시장의 주요 원동력 중 하나입니다. 더 많은 임상 시험이 수행됨에 따라 새로운 약물 승인 가능성이 높아질 것입니다.
예를 들어,
- 페루에서는 1995년부터 2019년까지 1996건의 임상시험이 이루어졌으며, 그 중 23.5%가 종양학 임상시험이었다.
- 다양한 특성을 지닌 종양학 시험의 수는 수년에 걸쳐 증가하고 있습니다.
위에서 언급한 사항을 바탕으로 페루와 에콰도르에서 종양학 시험이 수년에 걸쳐 증가하고 있으며, 이는 급성 골수성 백혈병 약물 시장의 성장을 촉진할 것으로 예상됩니다.
기회
시장 참여자의 전략적 이니셔티브
페루와 에콰도르에서 급성 골수성 백혈병 치료 약물에 대한 필요성은 급성 골수성 백혈병의 유병률 증가, 백혈병 치료를 위한 진보된 치료법, 그리고 충족되지 않은 의료적 요구가 있는 가장 큰 치료 시장인 종양학으로 인해 증가했습니다. 이러한 요인들은 화학 요법과 면역 요법의 필요성을 높이고 시장 수요를 달성하기 위해 소규모 및 대규모 시장 참여자들이 다양한 전략을 활용하고 있습니다.
주요 기업들은 또한 사업을 원활하게 운영하고, 위험을 피하고, 시장 판매의 장기적 성장을 높이기 위해 제품 출시, 인수, 승인, 확장, 파트너십 등의 구체적인 전략을 고안하려고 노력하고 있습니다.
예를 들어,
- 2020년 6월, AbbVie Inc.는 치료를 받지 않은 급성 골수성 백혈병 환자의 전반적인 생존 혜택과 개선된 완화율에 대해 Venclexta(venetoclax)에 대한 식품의약품(FDA) 승인을 받았습니다. Venclexta는 75세 이상의 성인 또는 집중 유도 화학 요법을 사용할 수 없는 합병증이 있는 새로 진단된 AML의 치료에 대해 azacitidine과 함께 사용하도록 승인을 받았습니다. 멕시코, 이스라엘, 푸에르토리코 및 페루에서도 승인을 받았습니다. 승인을 통해 페루에서 venetoclax의 시판 후 권리와 페루와 에콰도르에 있는 암 연구 병원에서의 임상 사용이 보장되었습니다.
혁신적 치료법의 부상
재발은 급성 골수성 백혈병(AML) 환자를 치료하는 데 있어 여전히 주요 문제입니다. 재발 후의 결과는 모든 AML 환자에게 균일하지 않으며 나이, 유전적 특성, 초기 진단 시 세포 유전학, 첫 번째 완전 관해 기간과 같은 여러 요인에 따라 달라집니다. 따라서 장애물을 극복하기 위해 혁신적인 치료법이 필요하며, 특정 표적을 식별하고 이러한 비정상적인 과정을 극복하는 방법을 기반으로 하는 치료 전략이 필요합니다.
예를 들어,
- 2021년 6월, 브리스톨 마이어스 스퀴브는 오누레그(아자시티딘 정제)에 대한 승인을 받았습니다. 오누레그는 광범위한 급성 골수성 백혈병(AML) 아형을 가진 환자를 위한 최초의 경구 유지 혁신적 요법입니다.
혁신적인 치료법을 사용하면 급성 골수성 백혈병의 진단, 위험 계층화 및 질병 모니터링이 개선될 것으로 예상됩니다. 혁신적인 복합 약물 치료법은 향후 몇 년 안에 환자 결과를 가져올 것으로 예상됩니다. 따라서 혁신적인 치료법을 사용하면 페루와 에콰도르 급성 골수성 백혈병 약물 시장에 수익성 있는 기회가 생길 것으로 예상됩니다.
제약/도전
그러나 저소득 국가의 이용 가능한 인프라 부족과 관련된 높은 비용은 페루 급성 골수성 백혈병 약물 시장의 성장률을 방해할 것입니다. 또한 보조 장치를 사용하는 동안 발생하는 위험은 페루 급성 골수성 백혈병 약물 시장 성장을 방해할 것입니다. 항암제와 관련된 부작용과 전문성 부족은 위에서 언급한 예측 기간 동안 시장에 더욱 큰 도전이 될 것입니다.
페루 급성 골수성 백혈병 약물 시장 보고서는 최근의 새로운 개발, 무역 규정, 수출입 분석, 생산 분석, 가치 사슬 최적화, 시장 점유율, 국내 및 지역 시장 참여자의 영향, 새로운 수익 주머니, 시장 규정의 변화, 전략적 시장 성장 분석, 시장 규모, 범주 시장 성장, 응용 분야 틈새 시장 및 지배력, 제품 승인, 제품 출시, 지리적 확장, 시장의 기술 혁신에 대한 분석 기회를 제공합니다. 페루 급성 골수성 백혈병 약물 시장에 대한 자세한 정보를 얻으려면 Data Bridge Market Research에 연락하여 분석가 브리핑을 받으세요. 저희 팀은 시장 성장을 달성하기 위한 정보에 입각한 시장 결정을 내리는 데 도움을 드립니다.
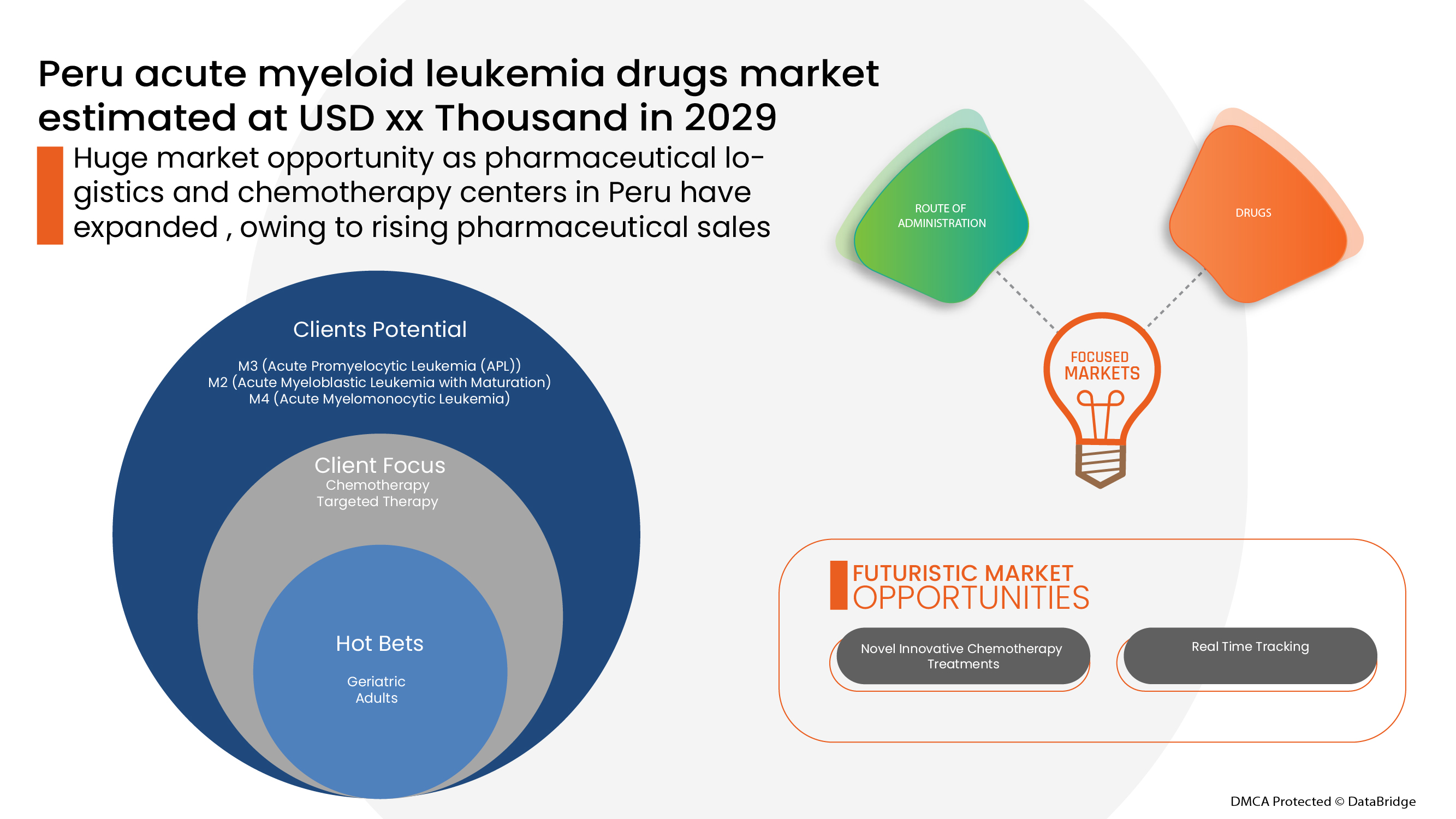
환자 역학 분석
페루 급성 골수성 백혈병 약물은 비교적 흔하지 않고 발병률이 알려지지 않은 만성 발 질환입니다. Science Direct의 연구에 따르면 페루의 급성 골수성 백혈병 발병률은 10만 명의 주민당 4.79건이며, 가장 높은 발병률은 페루 북부의 해안 도시인 피우라에서 10만 명의 주민당 38건의 AML로 관찰되었습니다.
페루 급성 골수성 백혈병 약물 시장은 또한 환자 분석, 예후 및 치료에 대한 자세한 시장 분석을 제공합니다. 유병률, 발생률, 사망률, 준수율은 보고서에서 사용할 수 있는 일부 데이터 변수입니다. 시장 성장에 대한 역학의 직접 또는 간접 영향 분석을 분석하여 성장 기간 동안 시장을 예측하기 위한 보다 견고하고 코호트 다변량 통계 모델을 만듭니다.
페루 급성 골수성 백혈병 약물 시장에 대한 Covid-19 영향
COVID-19는 시장에 부정적인 영향을 미쳤습니다. 팬데믹 동안의 봉쇄와 격리는 진단 관리와 치료를 복잡하게 만듭니다. 일상적인 약물 투여를 위한 의료 시설에 대한 접근성 부족은 시장에 더 큰 영향을 미칠 것입니다. 사회적 고립은 스트레스, 절망, 사회적 지원을 증가시키며, 이 모든 것이 팬데믹 동안 화학 요법 약물 복용을 감소시킬 수 있습니다.
최근 개발
- 2021년 6월, 브리스톨 마이어스 스퀴브는 유럽 위원회로부터 오누레그에 대한 승인을 받았습니다. 오누레그는 급성 골수성 백혈병 성인을 위한 최전선 경구 유지 요법으로 사용됩니다. 승인은 미국, 유럽 및 남미 지역에서 시판 후 승인을 보장할 것입니다. 오누레그는 에콰도르와 페루에서 판매 및 제품 수익을 늘려 시장 성장을 증가시킬 것으로 예상됩니다.
페루 급성 골수성 백혈병 약물 시장 범위
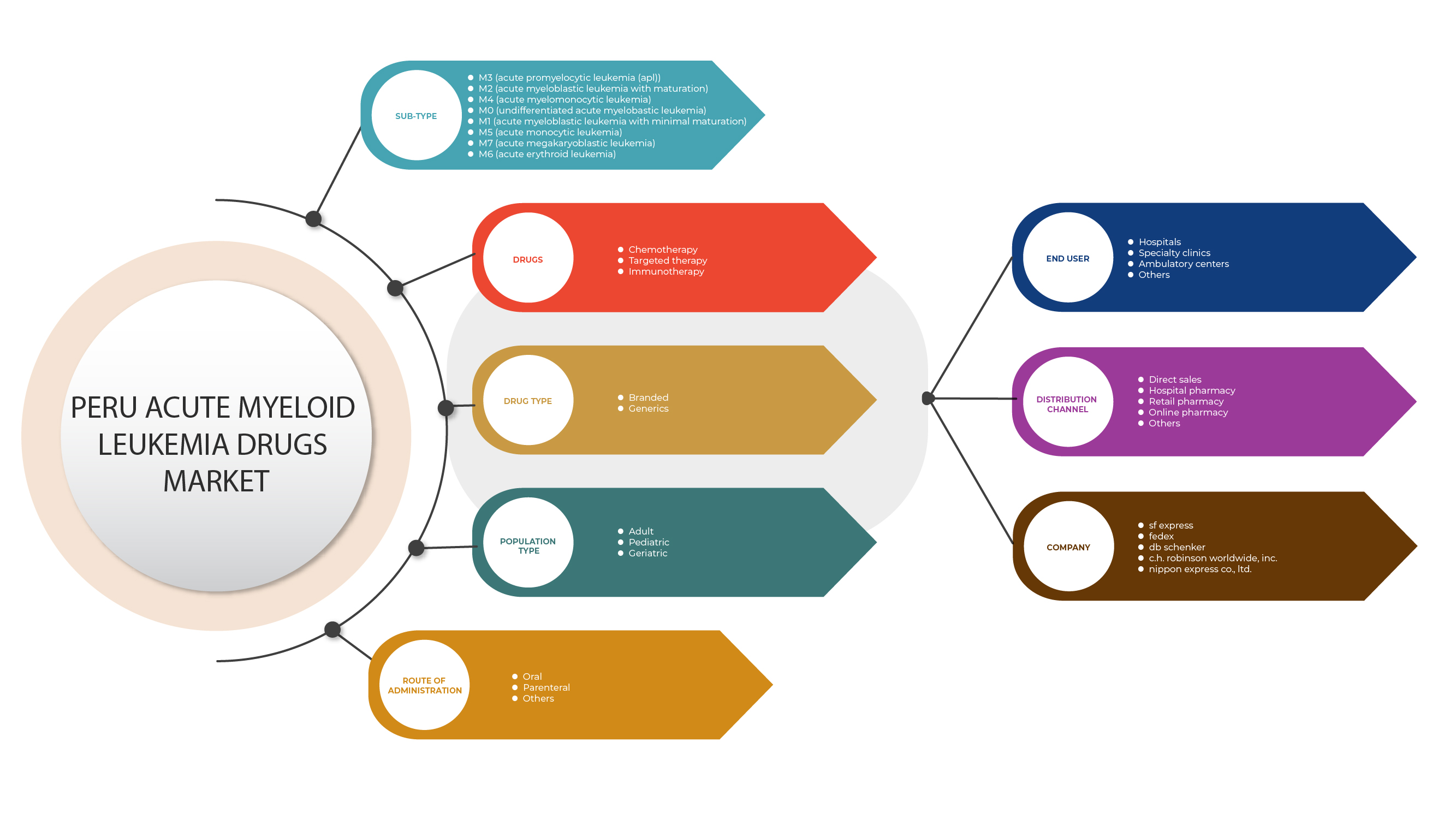
페루 급성 골수성 백혈병 약물 시장은 하위 유형, 약물, 약물 유형, 투여 경로, 인구 유형, 최종 사용자 및 유통 채널을 기준으로 세분화됩니다. 이러한 세그먼트 간의 성장은 산업의 성장 세그먼트를 분석하고 사용자에게 귀중한 시장 개요와 시장 통찰력을 제공하여 핵심 시장 응용 프로그램을 식별하기 위한 전략적 결정을 내리는 데 도움이 됩니다.
하위 유형
- M3(급성 전골수성 백혈병(Apl))
- M2(성숙을 동반한 급성 골수성 백혈병)
- M4(급성 골수구형 백혈병)
- M0(미분화성 급성 골수성 백혈병)
- M1(최소 성숙을 동반한 급성 골수성 백혈병)
- M5(급성 단핵구 백혈병)
- M7(급성 거대모세포성 백혈병)
- M6(급성 적혈구 백혈병)
시장은 하위 유형을 기준으로 M0(미분화 급성 골수성 백혈병), M1(최소 성숙을 동반한 급성 골수성 백혈병), M2(성숙을 동반한 급성 골수성 백혈병), M3(급성 전골수성 백혈병(APL)), M4(급성 골수구 단핵구 백혈병), M5(급성 단핵구 백혈병), M6(급성 적혈구 백혈병), M7(급성 거핵구 백혈병)으로 구분됩니다.
약제
- 화학요법
- 표적 치료
- 면역요법
약물을 기준으로 시장은 화학 요법, 표적 요법, 면역 요법 및 기타로 세분화됩니다. 화학 요법은 cytosar, daunorubicin doxorubicin, methotrexate, decitabin, cladribine 및 기타로 세분화됩니다. 표적 요법은 venetoclax(venclexta), midostaurin(rydapt), enasidenib(idhifa) 및 기타로 세분화됩니다. 면역 요법은 obinutuzumab(gazyva/gazyvaro), rituximab, interferons, alemtuzumab(campath) 및 기타로 세분화됩니다.
약물 유형
- 브랜드화
- 제네릭
약물 유형에 따라 시장은 브랜드와 제네릭으로 세분화됩니다. 브랜드 세그먼트는 cytosar, venclexta, rydapt, idhifa, gazyva/gazyvaro, campath 등으로 세분화됩니다.
투여 경로
- 경구
- 비경구적
- 기타
투여 경로를 기준으로 페루 급성 골수성 백혈병 약물 시장은 경구, 비경구 및 기타로 세분화됩니다. 경구 세그먼트는 정제, 캡슐 및 기타로 세분화됩니다. 비경구 세그먼트는 피하, 정맥 및 기타로 더 세분화됩니다.
인구 유형
- 성인
- 노인
- 소아과
인구 유형을 기준으로 페루 급성 골수성 백혈병 약물 시장은 소아, 성인 및 노인으로 세분화됩니다. 성인은 남성과 여성으로 세분화됩니다.
최종 사용자
- 병원
- 전문 클리닉
- 외래 진료 센터
- 기타
페루 급성 골수성 백혈병 약물 시장은 최종 사용자를 기준으로 병원, 전문 클리닉, 외래 센터 및 기타로 구분됩니다.
유통 채널
- 직접 판매
- 병원 약국
- 소매 약국
- 온라인 약국
- 기타
페루 급성 골수성 백혈병 약물 시장은 유통 채널을 기준으로 직접 판매, 병원 약국, 소매 약국, 온라인 약국 및 기타로 구분됩니다.
파이프라인 분석(제공되지 않음)
- 시타라빈(3상)
- BI 836858(2단계)
- AZD5991, Venetoclax(2상)와 함께
- AZD5991(1상)
- CC-95251(1단계)
- 데시타빈(1상)
- MK-0482(1상)
페루 급성 골수성 백혈병 약물 시장 지역 분석/통찰력
페루 급성 골수성 백혈병 약물 시장을 분석하고, 위에 언급된 대로 국가 하위 유형, 약물, 약물 유형, 투여 경로, 인구 유형, 최종 사용자 및 유통 채널별로 시장 규모에 대한 통찰력과 추세를 제공합니다.
보고서의 국가 섹션은 또한 현재 및 미래 시장 추세에 영향을 미치는 개별 시장 영향 요인과 시장 규정의 변화를 제공합니다. 신규 및 교체 판매, 국가 인구 통계, 질병 역학 및 수출입 관세와 같은 데이터 포인트는 개별 국가의 시장 시나리오를 예측하는 데 사용되는 주요 포인터 중 일부입니다. 또한 글로벌 브랜드의 존재 및 가용성과 국내 및 국내 브랜드와의 치열한 경쟁으로 인해 직면한 과제, 판매 채널의 영향이 국가 데이터에 대한 예측 분석을 제공하는 동안 고려됩니다.
경쟁 환경 및 페루 급성 골수성 백혈병 약물 시장 점유율 분석
페루 급성 골수성 백혈병 약물 시장 경쟁 구도는 경쟁자별 세부 정보를 제공합니다. 포함된 세부 정보에는 회사 개요, 회사 재무, 창출된 수익, 시장 잠재력, 연구 개발 투자, 새로운 시장 이니셔티브, 글로벌 입지, 생산 현장 및 시설, 생산 용량, 회사의 강점과 약점, 제품 출시, 제품 폭과 범위, 응용 분야 우위가 있습니다. 제공된 위의 데이터 포인트는 페루 급성 골수성 백혈병 약물 시장과 관련된 회사의 초점에만 관련이 있습니다.
페루 급성 골수성 백혈병 약물 시장에서 활동하는 주요 기업으로는 Pfizer Inc., MERCK SHARP & DOHME CORP.(MERCK & CO., INC.의 자회사), Janssen Pharmaceuticals, Inc.(Johnson & Johnson Services, Inc.의 자회사), Novartis AG, Fresenius Kabi AG, F. Hoffmann-La Roche Ltd, Abbvie Inc, Novartis Ag, F. Hoffmann-La Roche Ltd, AstraZeneca, Bohringer Ingelheim International GmbH, Sun Pharmaceutical Industries Ltd., Teva Pharmaceutical Industries Ltd. 등이 있습니다.
SKU-
세계 최초의 시장 정보 클라우드 보고서에 온라인으로 접속하세요
- 대화형 데이터 분석 대시보드
- 높은 성장 잠재력 기회를 위한 회사 분석 대시보드
- 사용자 정의 및 질의를 위한 리서치 분석가 액세스
- 대화형 대시보드를 통한 경쟁자 분석
- 최신 뉴스, 업데이트 및 추세 분석
- 포괄적인 경쟁자 추적을 위한 벤치마크 분석의 힘 활용
목차
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF PERU ACUTE MYELOID LEUKEMIA DRUGS MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATIONS
1.6 MARKETS COVERED
2 PERU ACUTE MYELOID LEUKEMIA DRUGS MARKET: SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 DBMR TRIPOD DATA VALIDATION MODEL
2.5 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.6 MULTIVARIATE MODELLING
2.7 PRODUCT SEGMENT LIFELINE CURVE
2.8 DBMR MARKET POSITION GRID
2.9 MARKET END USER COVERAGE GRID
2.1 SECONDARY SOURCES
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL ANALYSIS
4.2 PORTERS FIVE FORCES
5 EPIDERMIOLOGY
6 PIPELINE ANALYSIS FOR PERU ACUTE MYELOID LEUKEMIA DRUGS MARKET
7 SUMMARY WRITE UP (PERU)
7.1 OVERVIEW
8 PERU ACUTE MYELOID LEUKEMIA DRUGS MARKET: REGULATIONS
9 MARKET OVERVIEW
9.1 DRIVERS
9.1.1 RISE IN GERIATRIC POPULATION
9.1.2 RISING INVESTMENT IN RESEARCH AND DEVELOPMENT
9.1.3 RISING CLINIAL TRIALS AND PRODUCT APPROVAL
9.1.4 RISING BURDEN OF ACUTE MYELOID LEUKEMIA
9.2 RESTRAINTS
9.2.1 ADVERSE EFFECTS ASSOCIATED WITH CANCER DRUGS
9.2.2 HIGH COST OF CANCER MEDICINE
9.3 OPPORTUNITIES
9.3.1 STRATEGIC INITIATIVES BY MARKET PLAYERS
9.3.2 RISE IN HEALTHCARE EXPENDITURE
9.3.3 RISE OF INNOVATIVE THERAPIES
9.4 CHALLENGES
9.4.1 LACK OF SKILLED PROFESSIONALS
9.4.2 STRINGENT REGULATIONS
10 PERU ACUTE MYELOID LEUKEMIA DRUGS MARKET, BY COUNTRY
10.1.1 PERU
10.1.2 ECUADOR
11 PERU ACUTE MYELOID LEUKEMIA DRUGS MARKET, BY SUB-TYPE
11.1 OVERVIEW
11.2 M3 (ACUTE PROMYELOCYTIC LEUKEMIA (APL))
11.3 M2 (ACUTE MYELOBLASTIC LEUKEMIA WITH MATURATION)
11.4 M4 (ACUTE MYELOMONOCYTIC LEUKEMIA)
11.5 M0 (UNDIFFERENTIATED ACUTE MYELOBASTIC LEUKEMIA)
11.6 M1 (ACUTE MYELOBLASTIC LEUKEMIA WITH MINIMAL MATURATION)
11.7 M5 (ACUTE MONOCYTIC LEUKEMIA)
11.8 M7 (ACUTE MEGAKARYOBLASTIC LEUKEMIA)
11.9 M6 (ACUTE ERYTHROID LEUKEMIA)
12 PERU ACUTE MYELOID LEUKEMIA DRUGS MARKET, BY DRUGS
12.1 OVERVIEW
12.2 CHEMOTHERAPY
12.2.1 CYTOSAR
12.2.2 DAUNORUBICIN
12.2.3 DOXORUBICIN
12.2.4 METHOTREXATE
12.2.5 DECITABINE
12.2.6 CLADRIBINE
12.2.7 OTHERS
12.3 TARGETED THERAPY
12.3.1 VENETOCLAX (VENCLEXTA)
12.3.2 MIDOSTAURIN (RYDAPT)
12.3.3 ENASIDENIB (IDHIFA)
12.3.4 OTHERS
12.4 IMMUNOTHERAPY
12.4.1 OBINUTUZUMAB (GAZYVA/GAZYVARO)
12.4.2 RITUXIMAB
12.4.3 INTERFERONS
12.4.4 ALEMTUZUMAB (CAMPATH)
12.4.5 OTHERS
13 PERU ACUTE MYELOID LEUKEMIA DRUGS MARKET, BY DRUG TYPE
13.1 OVERVIEW
13.2 BRANDED
13.2.1 CYTOSAR
13.2.2 VENCLEXTA
13.2.3 RYDAPT
13.2.4 IDHIFA
13.2.5 GAZYVA/GAZYVARO
13.2.6 CAMPATH
13.2.7 OTHERS
13.3 GENERICS
14 PERU ACUTE MYELOID LEUKEMIA DRUGS MARKET, BY ROUTE OF ADMINISTRATION
14.1 OVERVIEW
14.2 PARENTERAL
14.2.1 INTRAVENOUS
14.2.2 SUBCUTANEOUS
14.2.3 OTHERS
14.3 ORAL
14.3.1 TABLET
14.3.2 CAPSULE
14.3.3 OTHERS
14.4 OTHERS
15 PERU ACUTE MYELOID LEUKEMIA DRUGS MARKET, BY POPULATION TYPE
15.1 OVERVIEW
15.2 GERIATRIC
15.3 ADULTS
15.3.1 MALE
15.3.2 FEMALE
15.4 PEDIATRIC
16 PERU ACUTE MYELOID LEUKEMIA DRUGS MARKET, BY END USER
16.1 OVERVIEW
16.2 HOSPITALS
16.3 SPECIALTY CLINICS
16.4 AMBULATORY CENTERS
16.5 OTHERS
17 PERU ACUTE MYELOID LEUKEMIA DRUGS MARKET, BY DISTRIBUTION CHANNEL
17.1 OVERVIEW
17.2 DIRECT TENDER
17.3 HOSPITAL PHARMACY
17.4 RETAIL PHARMACY
17.5 ONLINE PHARMACY
17.6 OTHERS
18 PERU ACUTE MYELOID LEUKEMIA DRUGS MARKET: COMPANY LANDSCAPE
18.1 COMPANY SHARE ANALYSIS: PERU
19 SWOT ANALYSIS
20 COMPANY PROFILE
20.1 PFIZER INC.( (2021)
20.1.1 COMPANY SNAPSHOT
20.1.2 REVENUE ANALYSIS
20.1.3 PRODUCT PORTFOLIO
20.1.4 RECENT DEVELOPMENT
20.2 BRISTOL-MYERS SQUIBB COMPANY (2021)
20.2.1 COMPANY SNAPSHOT
20.2.2 REVENUE ANALYSIS
20.2.3 PRODUCT PORTFOLIO
20.2.4 RECENT DEVELOPMENT
20.3 ABBVIE INC. (2021)
20.3.1 COMPANY SNAPSHOT
20.3.2 REVENUE ANALYSIS
20.3.3 PRODUCT PORTFOLIO
20.3.4 RECENT DEVELOPMENTS
20.4 NOVARTIS AG (2021)
20.4.1 COMPANY SNAPSHOT
20.4.2 REVENUE ANALYSIS
20.4.3 PRODUCT PORTFOLIO
20.4.4 RECENT DEVELOPMENT
20.5 F.HOFFMAN-LA ROCHE
20.5.1 COMPANY SNAPSHOT
20.5.2 REVENUE ANALYSIS
20.5.3 PRODUCT PORTFOLIO
20.5.4 RECENT DEVELOPMENT
20.6 MERCK SHARP & DOHME CORP. (A SUBSIDIARY OF MERCK & CO., INC.) (2021)
20.6.1 COMPANY SNAPSHOT
20.6.2 REVENUE ANALYSIS
20.6.3 PRODUCT PORTFOLIO
20.6.4 RECENT DEVELOPMENT
20.7 FRESENIUS KABI AG 2021)
20.7.1 COMPANY SNAPSHOT
20.7.2 REVENUE ANALYSIS
20.7.3 PRODUCT PORTFOLIO
20.7.4 RECENT DEVELOPMENT
20.8 ASTRA ZENECA (2021)
20.8.1 COMPANY SNAPSHOT
20.8.2 REVENUE ANALYSIS
20.8.3 PRODUCT PORTFOLIO
20.8.4 RECENT DEVELOPMENT
20.9 BOHRINGER INGELHEIM INTERNATIONAL GMBH (2021)
20.9.1 COMPANY SNAPSHOT
20.9.2 1.9.2 REVENUE ANALYSIS
20.9.3 PRODUCT PORTFOLIO
20.9.4 RECENT DEVELOPMENT
20.1 JANSSEN PHARMACEUTICALS, INC. (A SUBSIDIARY OF JOHNSON & JOHNSON SERVICES, INC.) (2021)
20.10.1 COMPANY SNAPSHOT
20.10.2 REVENUE ANALYSIS
20.10.3 PRODUCT PORTFOLIO
20.10.4 RECENT DEVELOPMENTS
20.11 SUN PHARMACEUTICAL INDUSTRIES LTD.
20.11.1 COMPANY SNAPSHOT
20.11.2 REVENUE ANALYSIS
20.11.3 PRODUCT PORTFOLIO
20.11.4 RECENT DEVELOPMENT
20.12 TEVA PHARMACEUTICAL INDUSTRIES LTD
20.12.1 COMPANY SNAPSHOT
20.12.2 REVENUE ANALYSIS
20.12.3 PRODUCT PORTFOLIO
20.12.4 PRODUCT PORTFOLIO
20.13 VIATRIS INC. (2021)
20.13.1 COMPANY SNAPSHOT
20.13.2 REVENUE ANALYSIS
20.13.3 PRODUCT PORTFOLIO
20.13.4 RECENT DEVELOPMENTS
21 QUESTIONNAIRE
22 RELATED REPORTS
표 목록
TABLE 1 PERU ACUTE MYELOID LEUKEMIA DRUGS MARKET, BY COUNTRY, 2020-2029 (USD THOUSAND)
TABLE 2 PERU ACUTE MYELOID LEUKEMIA DRUGS MARKET, BY SUB-TYPE, 2020-2029 (USD THOUSAND)
TABLE 3 PERU ACUTE MYELOID LEUKEMIA DRUGS MARKET, BY DRUGS, 2020-2029 (USD THOUSAND)
TABLE 4 PERU CHEMOTHERAPY IN ACUTE MYELOID LEUKEMIA DRUGS MARKET, BY DRUGS, 2020-2029 (USD THOUSAND)
TABLE 5 PERU TARGETED THERAPY IN ACUTE MYELOID LEUKEMIA DRUGS MARKET, BY DRUGS, 2020-2029 (USD THOUSAND)
TABLE 6 PERU IMMUNOTHERAPY IN ACUTE MYELOID LEUKEMIA DRUGS MARKET, BY DRUGS, 2020-2029 (USD THOUSAND)
TABLE 7 PERU ACUTE MYELOID LEUKEMIA DRUGS MARKET, BY DRUG TYPE, 2020-2029 (USD THOUSAND)
TABLE 8 PERU BRANDED IN ACUTE MYELOID LEUKEMIA DRUGS MARKET, BY DRUG TYPE, 2020-2029 (USD THOUSAND)
TABLE 9 PERU ACUTE MYELOID LEUKEMIA DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD THOUSAND)
TABLE 10 PERU PARENTERAL IN ACUTE MYELOID LEUKEMIA DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD THOUSAND)
TABLE 11 PERU ORAL IN ACUTE MYELOID LEUKEMIA DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD THOUSAND)
TABLE 12 PERU ACUTE MYELOID LEUKEMIA DRUGS MARKET, BY POPULATION TYPE, 2020-2029 (USD THOUSAND)
TABLE 13 PERU ADULTS IN ACUTE MYELOID LEUKEMIA DRUGS MARKET, BY POPULATION TYPE, 2020-2029 (USD THOUSAND)
TABLE 14 PERU ACUTE MYELOID LEUKEMIA DRUGS MARKET, BY END USER, 2020-2029 (USD THOUSAND)
TABLE 15 PERU ACUTE MYELOID LEUKEMIA DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD THOUSAND)
TABLE 16 ECUADOR ACUTE MYELOID LEUKEMIA DRUGS MARKET, BY SUB-TYPE, 2020-2029 (USD THOUSAND)
TABLE 17 ECUADOR ACUTE MYELOID LEUKEMIA DRUGS MARKET, BY DRUGS, 2020-2029 (USD THOUSAND)
TABLE 18 ECUADOR CHEMOTHERAPY IN ACUTE MYELOID LEUKEMIA DRUGS MARKET, BY DRUGS, 2020-2029 (USD THOUSAND)
TABLE 19 ECUADOR TARGETED THERAPY IN ACUTE MYELOID LEUKEMIA DRUGS MARKET, BY DRUGS, 2020-2029 (USD THOUSAND)
TABLE 20 ECUADOR IMMUNOTHERAPY IN ACUTE MYELOID LEUKEMIA DRUGS MARKET, BY DRUGS, 2020-2029 (USD THOUSAND)
TABLE 21 ECUADOR ACUTE MYELOID LEUKEMIA DRUGS MARKET, BY DRUG TYPE, 2020-2029 (USD THOUSAND)
TABLE 22 ECUADOR BRANDED IN ACUTE MYELOID LEUKEMIA DRUGS MARKET, BY DRUG TYPE, 2020-2029 (USD THOUSAND)
TABLE 23 ECUADOR ACUTE MYELOID LEUKEMIA DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD THOUSAND)
TABLE 24 ECUADOR PARENTERAL IN ACUTE MYELOID LEUKEMIA DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD THOUSAND)
TABLE 25 ECUADOR ORAL IN ACUTE MYELOID LEUKEMIA DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD THOUSAND)
TABLE 26 ECUADOR ACUTE MYELOID LEUKEMIA DRUGS MARKET, BY POPULATION TYPE, 2020-2029 (USD THOUSAND)
TABLE 27 ECUADOR ADULTS IN ACUTE MYELOID LEUKEMIA DRUGS MARKET, BY POPULATION TYPE, 2020-2029 (USD THOUSAND)
TABLE 28 ECUADOR ACUTE MYELOID LEUKEMIA DRUGS MARKET, BY END USER, 2020-2029 (USD THOUSAND)
TABLE 29 ECUADOR ACUTE MYELOID LEUKEMIA DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD THOUSAND)
TABLE 30 PERU ACUTE MYELOID LEUKEMIA DRUGS MARKET, BY SUB-TYPE, 2020-2029 (USD THOUSAND)
TABLE 31 PERU ACUTE MYELOID LEUKEMIA DRUGS MARKET, BY DRUGS, 2020-2029 (USD THOUSAND)
TABLE 32 PERU CHEMOTHERAPY IN ACUTE MYELOID LEUKEMIA DRUGS MARKET, BY DRUGS, 2020-2029 (USD THOUSAND)
TABLE 33 PERU TARGETED THERAPY IN ACUTE MYELOID LEUKEMIA DRUGS MARKET, BY DRUGS, 2020-2029 (USD THOUSAND)
TABLE 34 PERU IMMUNOTHERAPY IN ACUTE MYELOID LEUKEMIA DRUGS MARKET, BY DRUGS, 2020-2029 (USD THOUSAND)
TABLE 35 PERU ACUTE MYELOID LEUKEMIA DRUGS MARKET, BY DRUG TYPE, 2020-2029 (USD THOUSAND)
TABLE 36 PERU BRANDED IN ACUTE MYELOID LEUKEMIA DRUGS MARKET, BY DRUGS, 2020-2029 (USD THOUSAND)
TABLE 37 PERU ACUTE MYELOID LEUKEMIA DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD THOUSAND)
TABLE 38 PERU PARENTERAL IN ACUTE MYELOID LEUKEMIA DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD THOUSAND)
TABLE 39 PERU ORAL IN ACUTE MYELOID LEUKEMIA DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD THOUSAND)
TABLE 40 PERU ACUTE MYELOID LEUKEMIA DRUGS MARKET, BY POPULATION TYPE, 2020-2029 (USD THOUSAND)
TABLE 41 PERU ADULTS IN ACUTE MYELOID LEUKEMIA DRUGS MARKET, BY POPULATION TYPE, 2020-2029 (USD THOUSAND)
TABLE 42 PERU ACUTE MYELOID LEUKEMIA DRUGS MARKET, BY END USER, 2020-2029 (USD THOUSAND)
TABLE 43 PERU ACUTE MYELOID LEUKEMIA DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD THOUSAND)
그림 목록
FIGURE 1 PERU ACUTE MYELOID LEUKEMIA DRUGS MARKET: SEGMENTATION
FIGURE 2 PERU ACUTE MYELOID LEUKEMIA DRUGS MARKET: DATA TRIANGULATION
FIGURE 3 PERU ACUTE MYELOID LEUKEMIA DRUGS MARKET: DROC ANALYSIS
FIGURE 4 PERU ACUTE MYELOID LEUKEMIA DRUGS MARKET: COUNTRY VS REGIONAL MARKET ANALYSIS
FIGURE 5 PERU ACUTE MYELOID LEUKEMIA DRUGS MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 PERU ACUTE MYELOID LEUKEMIA DRUGS MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 PERU ACUTE MYELOID LEUKEMIA DRUGS MARKET: DBMR POSITION GRID
FIGURE 8 PERU ACUTE MYELOID LEUKEMIA DRUGS MARKET: END USER COVERAGE GRID
FIGURE 9 PERU ACUTE MYELOID LEUKEMIA DRUGS MARKET: SEGMENTATION
FIGURE 10 INCREASED PREVALENCE OF CANCER AND RISE IN ONGOING CLINICAL TRIALS ARE EXPECTED TO DRIVE PERU ACUTE MYELOID LEUKEMIA DRUGS MARKET FROM 2022 TO 2029
FIGURE 11 SUB-TYPE SEGMENT IS EXPECTED TO HAVE THE LARGEST SHARE OF THE PERU ACUTE MYELOID LEUKEMIA DRUGS MARKET FROM 2022 & 2029
FIGURE 12 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF PERU ACUTE MYELOID LEUKEMIA DRUGS MARKET
FIGURE 13 PERU ACUTE MYELOID LEUKEMIA DRUGS MARKET: BY SUB-TYPE, 2021
FIGURE 14 PERU ACUTE MYELOID LEUKEMIA DRUGS MARKET: BY SUB-TYPE, 2022-2029 (USD THOUSAND)
FIGURE 15 PERU ACUTE MYELOID LEUKEMIA DRUGS MARKET: BY SUB-TYPE, CAGR (2022-2029)
FIGURE 16 PERU ACUTE MYELOID LEUKEMIA DRUGS MARKET: BY SUB-TYPE, LIFELINE CURVE
FIGURE 17 PERU ACUTE MYELOID LEUKEMIA DRUGS MARKET: BY DRUGS, 2021
FIGURE 18 PERU ACUTE MYELOID LEUKEMIA DRUGS MARKET: BY DRUGS, 2022-2029 (USD THOUSAND)
FIGURE 19 PERU ACUTE MYELOID LEUKEMIA DRUGS MARKET: BY DRUGS, CAGR (2022-2029)
FIGURE 20 PERU ACUTE MYELOID LEUKEMIA DRUGS MARKET: BY DRUGS, LIFELINE CURVE
FIGURE 21 PERU ACUTE MYELOID LEUKEMIA DRUGS MARKET: BY DRUG TYPE, 2021
FIGURE 22 PERU ACUTE MYELOID LEUKEMIA DRUGS MARKET: BY DRUG TYPE, 2022-2029 (USD THOUSAND)
FIGURE 23 PERU ACUTE MYELOID LEUKEMIA DRUGS MARKET: BY DRUG TYPE, CAGR (2022-2029)
FIGURE 24 PERU ACUTE MYELOID LEUKEMIA DRUGS MARKET: BY DRUG TYPE, LIFELINE CURVE
FIGURE 25 PERU ACUTE MYELOID LEUKEMIA DRUGS MARKET: BY ROUTE OF ADMINISTRATION, 2021
FIGURE 26 PERU ACUTE MYELOID LEUKEMIA DRUGS MARKET: BY ROUTE OF ADMINISTRATION, 2022-2029 (USD THOUSAND)
FIGURE 27 PERU ACUTE MYELOID LEUKEMIA DRUGS MARKET: BY ROUTE OF ADMINISTRATION, CAGR (2022-2029)
FIGURE 28 PERU ACUTE MYELOID LEUKEMIA DRUGS MARKET: BY ROUTE OF ADMINISTRATION, LIFELINE CURVE
FIGURE 29 PERU ACUTE MYELOID LEUKEMIA DRUGS MARKET: BY POPULATION TYPE, 2021
FIGURE 30 PERU ACUTE MYELOID LEUKEMIA DRUGS MARKET: BY POPULATION TYPE, 2022-2029 (USD THOUSAND)
FIGURE 31 PERU ACUTE MYELOID LEUKEMIA DRUGS MARKET: BY POPULATION TYPE, CAGR (2022-2029)
FIGURE 32 PERU ACUTE MYELOID LEUKEMIA DRUGS MARKET: BY POPULATION TYPE, LIFELINE CURVE
FIGURE 33 PERU ACUTE MYELOID LEUKEMIA DRUGS MARKET: BY END USER, 2021
FIGURE 34 PERU ACUTE MYELOID LEUKEMIA DRUGS MARKET: BY END USER, 2022-2029 (USD THOUSAND)
FIGURE 35 PERU ACUTE MYELOID LEUKEMIA DRUGS MARKET: BY END USER, CAGR (2022-2029)
FIGURE 36 PERU ACUTE MYELOID LEUKEMIA DRUGS MARKET: BY END USER, LIFELINE CURVE
FIGURE 37 PERU ACUTE MYELOID LEUKEMIA DRUGS MARKET: BY DISTRIBUTION CHANNEL, 2021
FIGURE 38 PERU ACUTE MYELOID LEUKEMIA DRUGS MARKET: BY DISTRIBUTION CHANNEL, 2022-2029 (USD THOUSAND)
FIGURE 39 PERU ACUTE MYELOID LEUKEMIA DRUGS MARKET: BY DISTRIBUTION CHANNEL, CAGR (2022-2029)
FIGURE 40 PERU ACUTE MYELOID LEUKEMIA DRUGS MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 41 PERU ACUTE MYELOID LEUKEMIA MARKET: COMPANY SHARE 2021 (%)

연구 방법론
데이터 수집 및 기준 연도 분석은 대규모 샘플 크기의 데이터 수집 모듈을 사용하여 수행됩니다. 이 단계에는 다양한 소스와 전략을 통해 시장 정보 또는 관련 데이터를 얻는 것이 포함됩니다. 여기에는 과거에 수집한 모든 데이터를 미리 검토하고 계획하는 것이 포함됩니다. 또한 다양한 정보 소스에서 발견되는 정보 불일치를 검토하는 것도 포함됩니다. 시장 데이터는 시장 통계 및 일관된 모델을 사용하여 분석하고 추정합니다. 또한 시장 점유율 분석 및 주요 추세 분석은 시장 보고서의 주요 성공 요인입니다. 자세한 내용은 분석가에게 전화를 요청하거나 문의 사항을 드롭하세요.
DBMR 연구팀에서 사용하는 주요 연구 방법론은 데이터 마이닝, 시장에 대한 데이터 변수의 영향 분석 및 주요(산업 전문가) 검증을 포함하는 데이터 삼각 측량입니다. 데이터 모델에는 공급업체 포지셔닝 그리드, 시장 타임라인 분석, 시장 개요 및 가이드, 회사 포지셔닝 그리드, 특허 분석, 가격 분석, 회사 시장 점유율 분석, 측정 기준, 글로벌 대 지역 및 공급업체 점유율 분석이 포함됩니다. 연구 방법론에 대해 자세히 알아보려면 문의를 통해 업계 전문가에게 문의하세요.
사용자 정의 가능
Data Bridge Market Research는 고급 형성 연구 분야의 선두 주자입니다. 저희는 기존 및 신규 고객에게 목표에 맞는 데이터와 분석을 제공하는 데 자부심을 느낍니다. 보고서는 추가 국가에 대한 시장 이해(국가 목록 요청), 임상 시험 결과 데이터, 문헌 검토, 재생 시장 및 제품 기반 분석을 포함하도록 사용자 정의할 수 있습니다. 기술 기반 분석에서 시장 포트폴리오 전략에 이르기까지 타겟 경쟁업체의 시장 분석을 분석할 수 있습니다. 귀하가 원하는 형식과 데이터 스타일로 필요한 만큼 많은 경쟁자를 추가할 수 있습니다. 저희 분석가 팀은 또한 원시 엑셀 파일 피벗 테이블(팩트북)로 데이터를 제공하거나 보고서에서 사용 가능한 데이터 세트에서 프레젠테이션을 만드는 데 도움을 줄 수 있습니다.













