North America Medical Device Regulatory Affairs Outsourcing Market
시장 규모 (USD 10억)
연평균 성장률 :
% 
 USD
2.93 Billion
USD
7.46 Billion
2025
2033
USD
2.93 Billion
USD
7.46 Billion
2025
2033
| 2026 –2033 | |
| USD 2.93 Billion | |
| USD 7.46 Billion | |
|
|
|
|
북미 의료 기기 규제 업무 아웃소싱 시장, 서비스별(규제 업무 서비스, 품질 컨설팅 및 의료 문서 작성), 제품(완제품, 전자 제품 및 원자재), 기기 유형(클래스 I, 클래스 II 및 클래스 III), 응용 분야(심장학, 진단 영상, 정형외과, IVD, 안과, 일반 및 성형외과, 약물 전달, 치과, 내시경, 당뇨병 관리 및 기타), 최종 사용자(소규모 의료 기기 회사, 중규모 의료 기기 회사 및 대규모 의료 기기 회사), 국가(미국, 캐나다, 멕시코) 산업 동향 및 2028년까지의 예측
 시장 분석 및 통찰력: 북미 의료 기기 규제 업무 아웃소싱 시장
시장 분석 및 통찰력: 북미 의료 기기 규제 업무 아웃소싱 시장
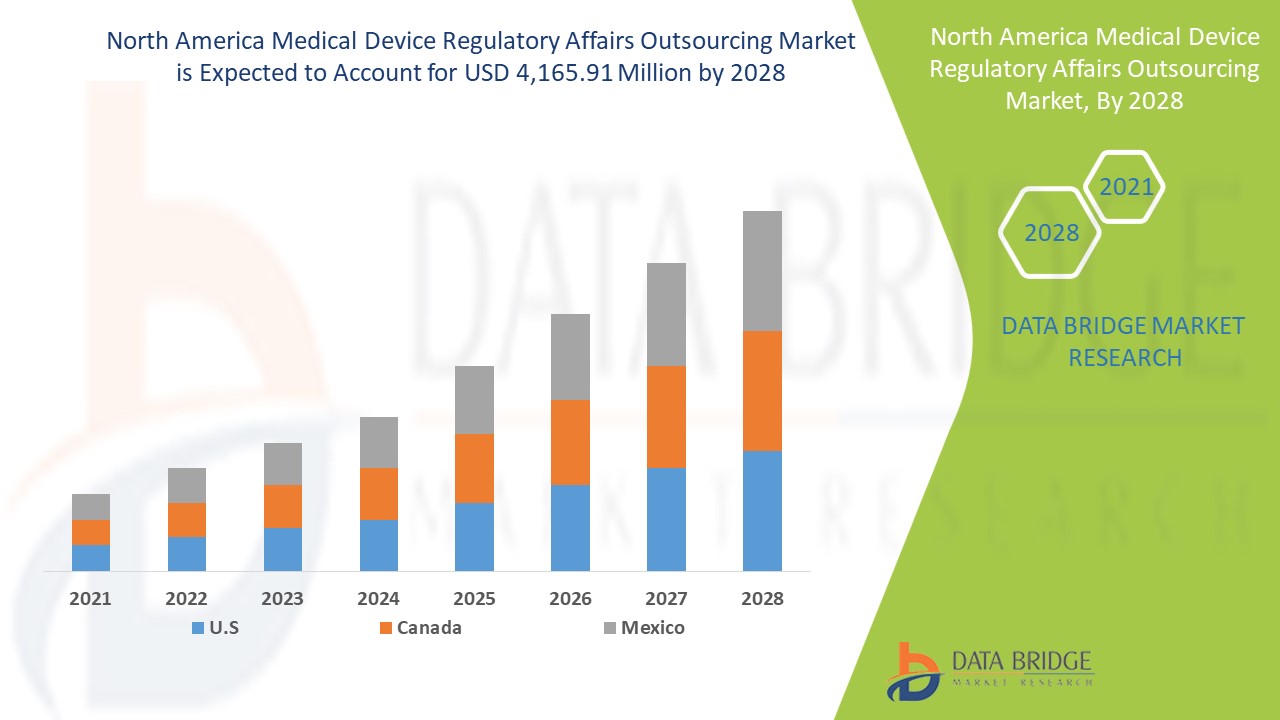
의료 기기 규제 업무 아웃소싱 시장은 2021년부터 2028년까지의 예측 기간 동안 시장 성장을 이룰 것으로 예상됩니다. Data Bridge Market Research는 시장이 2021년부터 2028년까지의 예측 기간 동안 12.4%의 CAGR로 성장하고 있으며 2028년까지 4,165.91백만 달러에 도달할 것으로 예상한다고 분석합니다. 지리적 확장을 위한 전략적 이니셔티브는 의료 기기 규제 업무 아웃소싱 시장의 성장을 촉진할 것으로 예상됩니다.
아웃소싱은 모든 제약 및 생명공학 기업의 연구 개발(R&D) 가치 사슬 에서 중요한 부분입니다 . 규제 업무 아웃소싱 서비스에는 전문 의료 저자, 품질 관리(QC) 감사원 및 고품질 임상 연구 프로젝트에 기여하는 출판사가 의료 저술 및 규제 문서를 발행하는 것이 포함됩니다. 규제 서비스 아웃소싱에 대한 수요는 신흥 경제에서 수행되는 임상 연구의 상당한 증가로 인해 촉진되었으며, 이 산업의 성장을 위한 건강한 플랫폼을 제공했습니다.
특허 만료 건수가 증가함에 따라 의료 기기 규제 업무 아웃소싱 시장에서 성장이 촉진되었습니다. 다양한 의료 기기 규제 업무 서비스 가격 변동은 의료 기기 규제 업무 아웃소싱 시장에서 성장이 제한되었습니다. 수상 및 인정은 의료 기기 규제 업무 아웃소싱 시장 성장에 탁월한 기회를 제공합니다. COVID-19의 팬데믹 발발은 의료 기기 규제 업무 아웃소싱 시장 성장에 도전이 되었습니다.
의료 기기 규제 업무 아웃소싱 시장 보고서는 시장 점유율, 새로운 개발 및 제품 파이프라인 분석, 국내 및 지역 시장 참여자의 영향, 새로운 수익 창출, 시장 규정의 변화, 제품 승인, 전략적 의사 결정, 제품 출시, 지리적 확장 및 시장의 기술 혁신 측면에서의 분석 기회에 대한 세부 정보를 제공합니다. 분석 및 의료 기기 규제 업무 아웃소싱 시장 시나리오를 이해하려면 Data Bridge Market Research에 연락하여 분석가 브리핑을 요청하세요. 당사 팀은 원하는 목표를 달성하기 위한 수익 영향 솔루션을 만드는 데 도움을 드립니다.
 의료 기기 규제 업무 아웃소싱 시장 범위 및 시장 규모
의료 기기 규제 업무 아웃소싱 시장 범위 및 시장 규모
의료 기기 규제 업무 아웃소싱 시장은 서비스, 제품, 기기 유형, 애플리케이션 및 최종 사용자를 기준으로 세분화됩니다. 세그먼트 간 성장은 틈새 성장 포켓과 시장에 접근하고 핵심 애플리케이션 영역과 타겟 시장의 차이점을 파악하기 위한 전략을 분석하는 데 도움이 됩니다.
- 서비스 기준으로 의료 기기 규제 업무 아웃소싱 시장은 규제 업무 서비스 , 품질 컨설팅 및 의료 문서 작성으로 세분화됩니다. 2021년에는 주요 의료 기기 회사에서 규제 업무 아웃소싱을 채택하는 경우가 늘어나 규제 업무 서비스 부문이 의료 기기 규제 업무 아웃소싱 시장을 지배할 것으로 예상됩니다.
- 제품 기준으로 의료 기기 규제 업무 아웃소싱 시장은 완제품, 전자 제품 및 원자재로 세분화됩니다. 2021년에는 주요 의료 기기 회사가 완제품에 대한 규제 업무 아웃소싱을 더 많이 도입함에 따라 완제품 세그먼트가 의료 기기 규제 업무 아웃소싱 시장을 지배할 것으로 예상됩니다.
- 의료 기기 규제 업무 아웃소싱 시장은 기기 유형에 따라 클래스 I, 클래스 II, 클래스 III로 세분화됩니다. 2021년에는 만성 질환 환자를 치료하기 위한 의료 기기에 대한 전 세계적 수요가 증가함에 따라 클래스 I 세그먼트가 의료 기기 규제 업무 아웃소싱 시장을 지배할 것으로 예상됩니다.
- 의료 기기 규제 업무 아웃소싱 시장은 응용 프로그램을 기준으로 심장학, 진단 영상 , 정형외과, IVD, 안과, 일반 및 성형외과, 약물 전달, 치과, 내시경, 당뇨병 관리 및 기타로 세분화됩니다. 2021년에는 주요 의료 기기 회사에서 3등급 의료 기기에 대한 규제 업무 아웃소싱을 채택하는 경우가 증가함에 따라 심장학 부문이 의료 기기 규제 업무 아웃소싱 시장을 지배할 것으로 예상됩니다.
- 최종 사용자를 기준으로 의료 기기 규제 업무 아웃소싱 시장은 소규모 의료 기기 회사, 중규모 의료 기기 회사, 대규모 의료 기기 회사로 세분화됩니다. 2021년에는 전 세계적으로 의료 기기에 대한 수요가 증가함에 따라 중규모 의료 기기 회사 부문이 의료 기기 규제 업무 아웃소싱 시장을 지배할 것으로 예상됩니다.
의료기기 규제 업무 아웃소싱 시장 국가 수준 분석
의료기기 규제 업무 아웃소싱 시장을 분석하고, 위에 언급된 국가, 서비스, 제품, 기기 유형, 애플리케이션 및 최종 사용자별로 시장 규모 정보를 제공합니다.
의료기기 규제 업무 아웃소싱에 적용되는 국가는 미국, 캐나다, 멕시코입니다.
미국은 북미 의료 기기 규제 업무 아웃소싱 시장을 주도하고 있으며 규제 업무 서비스 부문에 대한 아웃소싱 모델 채택 증가로 인해 2021년부터 2028년까지 예측 기간 동안 가장 높은 성장률을 기록할 것으로 예상됩니다.
보고서의 국가 섹션은 또한 개별 시장 영향 요인과 국내 시장의 현재 및 미래 트렌드에 영향을 미치는 규제 변화를 제공합니다. 신규 판매, 교체 판매, 국가 인구 통계, 규제 조치 및 수출입 관세와 같은 데이터 포인트는 개별 국가의 시장 시나리오를 예측하는 데 사용되는 주요 포인터 중 일부입니다. 또한 북미 브랜드의 존재 및 가용성과 지역 및 국내 브랜드와의 대규모 또는 희소한 경쟁으로 인해 직면한 과제, 판매 채널의 영향은 국가 데이터에 대한 예측 분석을 제공하는 동안 고려됩니다.
의료 기기 회사의 지리적 확장 활동 증가로 의료 기기 규제 업무 아웃소싱 시장 성장이 촉진되고 있습니다.
의료 기기 규제 업무 아웃소싱 시장은 또한 의료 기기 규제 업무 아웃소싱 산업의 모든 국가별 성장에 대한 자세한 시장 분석을 제공합니다. 게다가 의료 기기 규제 업무 아웃소싱 판매, 규제 시나리오의 영향 및 의료 기기 규제 업무 아웃소싱 시장과 관련된 추세 매개변수에 대한 자세한 정보를 제공합니다. 이 데이터는 2010년부터 2019년까지의 과거 기간에 대한 것입니다.
경쟁 환경 및 의료 기기 규제 업무 아웃소싱 시장 점유율 분석
의료 기기 규제 업무 아웃소싱 시장 경쟁 구도는 경쟁자별 세부 정보를 제공합니다. 포함된 세부 정보는 회사 개요, 회사 재무, 창출된 수익, 시장 잠재력, 연구 개발 투자, 새로운 시장 이니셔티브, 생산 현장 및 시설, 회사의 강점과 약점, 제품 출시, 제품 시험 파이프라인, 제품 승인, 특허, 제품 폭과 폭, 응용 프로그램 우세, 기술 수명선 곡선입니다. 위에 제공된 데이터 포인트는 의료 기기 규제 업무 아웃소싱 시장과 관련된 회사의 초점에만 관련이 있습니다.
보고서에서 다루는 주요 기업으로는 Parexel International Corporation, North American Science Associates, Inc., SGS SA, Pace Analytical Services, LLC, Trilogy Writing & Consulting GmbH, Creganna(TE Connectivity의 자회사), American Preclinical Services, LLC., Intertek Group plc, WuXi AppTec, Charles River Laboratories, Celestica Inc., Freyr, Cactus Communications, Eurofins Scientific, TÜV SÜD, Sterigenics US, LLC – Sotera Health 회사, TE Connectivity, FLEX LTD., Heraeus Holding, Integer Holdings Corporation, Nortech Systems, Inc., IQVIA, Covance, Plexus Corp., Sanmina Corporation, OMICS International, Tecomet, Inc., East West Manufacturing, Jabil Inc., Omron Corporation 등이 있습니다. DBMR 분석가는 경쟁 우위를 이해하고 각 경쟁자에 대한 경쟁 분석을 별도로 제공합니다.
또한 전 세계 여러 회사에서 많은 계약과 협정을 체결하면서 의료 기기 규제 업무 아웃소싱 시장도 가속화되고 있습니다.
예를 들어,
- 2020년 1월, Charles River Laboratories는 세포 치료를 전문으로 하는 회사인 HemaCare Corporation(HemaCare)을 인수했다고 발표했습니다. 이 회사의 전략적 인수로 초기 단계 연구 및 제조 지원 솔루션의 제품 포트폴리오가 확대되어 매출과 수익이 증가했습니다.
시장 참여자들의 협력, 제품 출시, 사업 확장, 수상 및 인정, 합작 투자 및 기타 전략을 통해 의료 기기 규제 업무 아웃소싱 시장에서 회사의 입지를 강화하고 있으며, 이는 조직의 수익 성장에도 도움이 됩니다.
SKU-
세계 최초의 시장 정보 클라우드 보고서에 온라인으로 접속하세요
- 대화형 데이터 분석 대시보드
- 높은 성장 잠재력 기회를 위한 회사 분석 대시보드
- 사용자 정의 및 질의를 위한 리서치 분석가 액세스
- 대화형 대시보드를 통한 경쟁자 분석
- 최신 뉴스, 업데이트 및 추세 분석
- 포괄적인 경쟁자 추적을 위한 벤치마크 분석의 힘 활용
연구 방법론
데이터 수집 및 기준 연도 분석은 대규모 샘플 크기의 데이터 수집 모듈을 사용하여 수행됩니다. 이 단계에는 다양한 소스와 전략을 통해 시장 정보 또는 관련 데이터를 얻는 것이 포함됩니다. 여기에는 과거에 수집한 모든 데이터를 미리 검토하고 계획하는 것이 포함됩니다. 또한 다양한 정보 소스에서 발견되는 정보 불일치를 검토하는 것도 포함됩니다. 시장 데이터는 시장 통계 및 일관된 모델을 사용하여 분석하고 추정합니다. 또한 시장 점유율 분석 및 주요 추세 분석은 시장 보고서의 주요 성공 요인입니다. 자세한 내용은 분석가에게 전화를 요청하거나 문의 사항을 드롭하세요.
DBMR 연구팀에서 사용하는 주요 연구 방법론은 데이터 마이닝, 시장에 대한 데이터 변수의 영향 분석 및 주요(산업 전문가) 검증을 포함하는 데이터 삼각 측량입니다. 데이터 모델에는 공급업체 포지셔닝 그리드, 시장 타임라인 분석, 시장 개요 및 가이드, 회사 포지셔닝 그리드, 특허 분석, 가격 분석, 회사 시장 점유율 분석, 측정 기준, 글로벌 대 지역 및 공급업체 점유율 분석이 포함됩니다. 연구 방법론에 대해 자세히 알아보려면 문의를 통해 업계 전문가에게 문의하세요.
사용자 정의 가능
Data Bridge Market Research는 고급 형성 연구 분야의 선두 주자입니다. 저희는 기존 및 신규 고객에게 목표에 맞는 데이터와 분석을 제공하는 데 자부심을 느낍니다. 보고서는 추가 국가에 대한 시장 이해(국가 목록 요청), 임상 시험 결과 데이터, 문헌 검토, 재생 시장 및 제품 기반 분석을 포함하도록 사용자 정의할 수 있습니다. 기술 기반 분석에서 시장 포트폴리오 전략에 이르기까지 타겟 경쟁업체의 시장 분석을 분석할 수 있습니다. 귀하가 원하는 형식과 데이터 스타일로 필요한 만큼 많은 경쟁자를 추가할 수 있습니다. 저희 분석가 팀은 또한 원시 엑셀 파일 피벗 테이블(팩트북)로 데이터를 제공하거나 보고서에서 사용 가능한 데이터 세트에서 프레젠테이션을 만드는 데 도움을 줄 수 있습니다.














