북미 레버 선천성 흑내장 시장 , 질병 유형(유아형, 청소년형 및 기타), 유형(치료 및 진단 ), 최종 사용자(병원, 전문 클리닉, 외래 수술 센터, 가정 의료 및 기타), 유통 채널(직접 입찰 및 소매 판매)별 산업 동향 및 2029년까지의 예측.
시장 분석 및 통찰력
북미 레버 선천성 아마우로시스 시장은 당뇨병 및 위장 장애와 같은 만성 질환 의 유병률 증가 , 신생 기업의 증가, 가정 간병 서비스에서 주입 펌프의 인기와 같은 요인에 의해 주도되며, 이는 수요를 증가시키고 연구 개발에 대한 투자 증가로 이어지며 시장 성장으로 이어집니다. 현재 다양한 연구 조사가 진행 중이며, 이는 제조업체가 새롭고 혁신적인 레버 선천성 아마우로시스 치료법과 요법을 개발할 수 있는 경쟁 우위를 창출할 것으로 예상되며, 이는 레버 선천성 아마우로시스 시장에서 다양한 다른 기회를 제공할 것으로 예상됩니다. 그러나 주입 요법 중 승인 및 장치 오류에 대한 엄격한 정부 규정은 성장을 방해할 것으로 예상됩니다.
북미 레버 레버 선천성 아마우로시스 시장 보고서는 시장 점유율, 새로운 개발 및 제품 파이프라인 분석, 국내 및 현지 시장 참여자의 영향, 새로운 수익 주머니, 시장 규정의 변화, 제품 승인, 전략적 의사 결정, 제품 출시, 지리적 확장 및 시장의 기술 혁신 측면에서의 분석 기회에 대한 세부 정보를 제공합니다. 분석 및 시장 시나리오를 이해하려면 분석가 브리핑을 위해 저희에게 연락하세요. 저희 팀은 보장된 지불과 제품의 높은 국제 판매로 인해 고객 사이에서 선호도가 높아지는 원하는 목표를 달성하기 위한 수익 영향 솔루션을 만드는 데 도움을 드릴 것입니다. 이는 예측 기간 동안 시장 수요를 촉진한 주요 요인입니다.
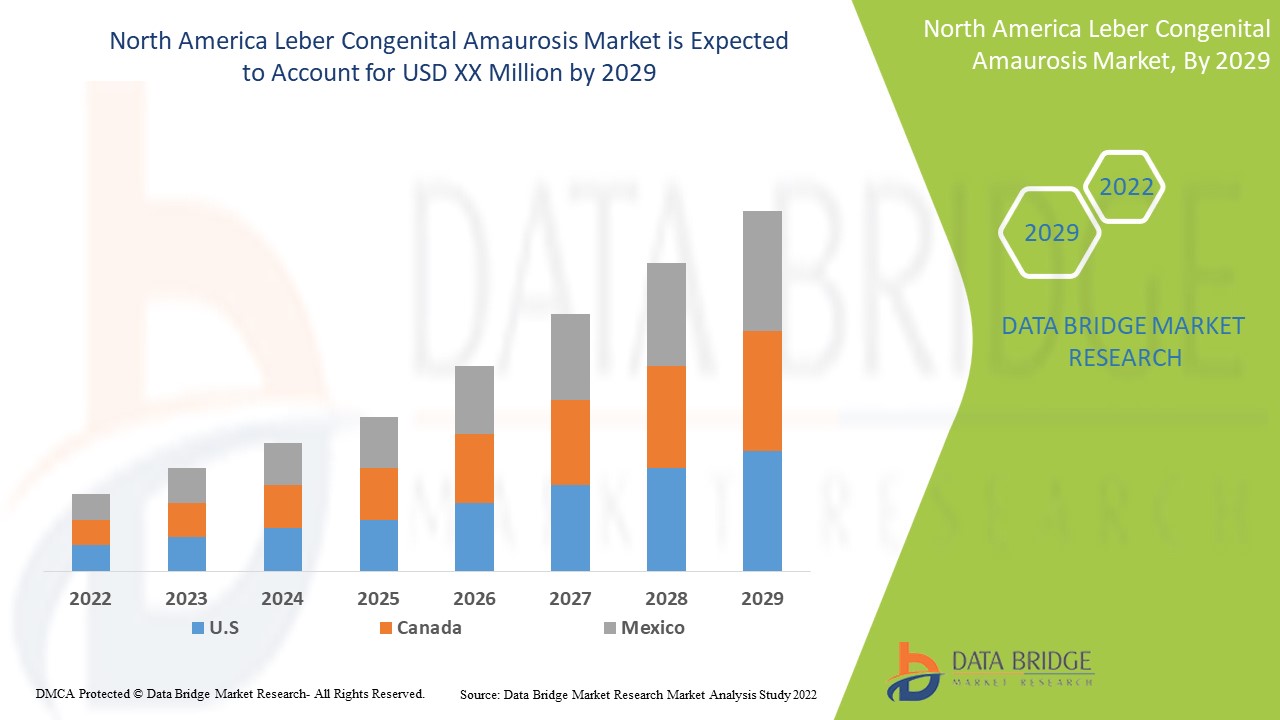
북미 레버 선천성 아마우로시스 시장은 지지적이며 질병의 진행을 줄이는 것을 목표로 합니다. Data Bridge Market Research는 북미 레버 선천성 아마우로시스 시장이 2022년에서 2029년까지의 예측 기간 동안 4.4%의 CAGR로 성장할 것이라고 분석합니다.
|
보고서 메트릭 |
세부 |
|
예측 기간 |
2022년부터 2029년까지 |
|
기준 연도 |
2021 |
|
역사적 연도 |
2020 (2019-2014까지 사용자 정의 가능) |
|
양적 단위 |
수익은 백만 달러, 가격은 미화로 표시됨 |
|
다루는 세그먼트 |
질병 유형(유아형, 청소년형 및 기타), 유형(치료, 진단), 최종 사용자(병원, 전문 클리닉, 외래 수술 센터, 가정 의료 및 기타), 유통 채널(직접 입찰 및 소매 판매)별 |
|
적용 국가 |
국가별(미국, 캐나다, 멕시코) |
|
시장 참여자 포함 |
Invitae Corporation, Johnson & Johnson Services, Inc., Spark Therapeutics, Inc., LKC TECHNOLOGIES, INC., Amarantus Bioscience Holdings, Inc., Sucampo Pharmaceuticals, Inc., Orphagen Pharmaceuticals, Inc. 등이 있습니다. |
북미 레버 선천성 아마우로시스 시장 동향
운전자
- 파이프라인 제품 및 임상 시험 증가
유전성 망막 질환과 레버 선천성 흑암시 장애의 치료를 위한 지속적인 혁신은 시장 규모가 증가함에 따라 시장에 긍정적인 영향을 미칠 가능성이 있으며, 이러한 제품이 북미 시장에서 승인을 받으면 더욱 그렇습니다. LCA에 대한 약리학적 치료법이 없기 때문에 주요 시장 참여자들은 지속적으로 치료법을 혁신하거나 치료법에 대한 R&D에 투자하고 있습니다. 다양한 진행 중인 임상 시험이 전 세계적으로 모집되어 질병의 치료 메커니즘을 찾고 있습니다.
- 주요 참여자의 전략적 이니셔티브 증가
협력, 계약, 판매 계약 체결과 같은 전략적 이니셔티브가 약리학적 치료법을 발명하고 혁신하기 위해 시장을 주도할 것으로 예상됩니다. 협회, 이 거래는 주요 플레이어가 세계의 다른 지역으로 시장을 확장하고 한 플레이어에서 다른 플레이어로 기술을 이전하여 제품 승인과 기관 연구를 늘리는 데 사용됩니다.
눈 질환 시장에서 회사의 입지를 확대하기 위한 파트너십 계약과 협력을 확대하는 것이 시장을 이끄는 원동력이 될 것입니다. 시장 참여자는 레버 선천성 흑암시 시장의 성장을 촉진할 다양한 전략적 이니셔티브를 거쳤습니다.
- 망막질환에서 rpe65 유전자 매개 발병률 및 유병률 증가
유전성 망막 이영양증(IRD)은 유전적으로 다양한 진행성 시력 저하 질환군을 나타냅니다. 65kDa 망막 색소 상피(RPE65) 유전자의 이형성 돌연변이로 인한 IRD로 인한 시력 상실이 있는 성인 및 소아 환자는 종종 임상적으로 망막 색소 변성증(RP) 및 레버 선천성 흑암시(LCA)로 진단됩니다.
레버 선천성 흑암시(LCA)는 주로 망막에 영향을 미치는 안구 질환입니다. LCA는 출생 시 또는 생후 몇 달 동안 심각한 시각 장애, 떠도는 눈 움직임 또는 안진, 동공 빛 반응 저하, 안구 디지털 신호(눈을 찌르거나 문지르거나 누르는 것), 감지할 수 없거나 심각하게 비정상적인 전체 시야 전기 망막도(ERG)를 특징으로 합니다. LCA는 일반적으로 상염색체 열성 유전 상태로 유전됩니다.
기회
- 레버 선천성 흑암시에 대한 정부 이니셔티브 증가
WHO에 따르면, 단일 유전자 IRD의 유병률은 북미 신생아 40,000명 중 약 1명에게 영향을 미칩니다. 이를 예방하기 위해 전 세계 여러 국가의 정부가 이니셔티브를 취했습니다.
전 세계 정부가 국민에게 최상의 치료를 제공하기 위해 취하는 다양한 프로그램과 결정은 수요를 증가시킬 것으로 예상되는 주요 요인이며, 북미 레버 선천성 흑암시 시장에 기회가 될 것입니다.
제약/도전
- 시술 및 치료와 관련된 높은 비용
제품의 비용은 시장에서 중요한 요소입니다. 유전성 망막 질환 시장에서 일반적으로 제품의 비용은 매우 정교하고 정확해야 하며 다른 사양은 제품의 비용을 증가시킵니다. 치료 비용이 높은 것은 치료의 다양한 체크포인트와 이러한 치료 절차를 수행하기 위한 하이테크 모달리티의 사용 때문입니다.
제품의 비용은 시장에서 중요한 요소입니다. 유전성 망막 질환 시장에서 일반적으로 제품의 비용은 매우 정교하고 정확해야 하며 다른 사양은 제품의 비용을 증가시키는 것으로 관찰됩니다. 게다가, 더 긴 기간 동안의 치료와 관련된 비용은 평균 소득을 가진 사람이 감당하기 매우 어렵습니다. 중환자 치료 및 집중 치료실 서비스의 활용은 전 세계적으로 증가하고 있으며, 그 비싼 비용은 현재 의료 시스템에서 주요 관심사입니다.
유전적 질환이 있는 환자는 일반적으로 빈번한 화폐화 및 기타 사용으로 인해 상당한 양의 의료 자원을 소모하는 장기 치료를 받아야 합니다. 이로 인해 장기적인 경제적 비용을 감당할 수 없는 대부분의 환자는 치료 초기 단계에서 퇴원합니다. 그러나 이로 인해 진단 시 새로운 합병증의 가능성과 취약성이 증가하여 추가적인 의료 자원과 치료가 필요합니다.
치료 비용이 높은 이유는 치료에 다양한 체크포인트를 사용하고 이러한 치료 과정을 시행하는 데 첨단 기술을 사용하기 때문입니다.
병원 치료는 레버 선천성 흑암시증의 비용을 더욱 증가시킵니다. 혁신적이고 진보된 제품의 비용이 높기 때문에 치료 비용도 비례적으로 상승하여 레버 선천성 흑암시증 치료 및 진단과 관련된 높은 비용이 레버 선천성 흑암시증 시장 성장을 억제할 것으로 예상됩니다.
최근 개발 사항
- Invitae Corporation은 2021년 4월 유전자 검사 서비스에 대한 증가하는 수요를 충족하기 위해 용량을 더욱 확장하기 위해 노스캐롤라이나에 새로운 실험실과 생산 시설을 열었습니다.
- 2017년 7월, Spark Therapeutics, Inc는 Voretigene Neparvovec의 제안된 상표명인 LUXTURNA에 대한 마케팅 허가 신청서(MAA)를 유럽 의약품 기관(EMA)에 제출했습니다. 이를 통해 회사의 매출과 제품 포트폴리오가 증가했습니다.
북미 레버 선천성 아마우로시스 시장 분할
북미 레버 선천성 아마우로시스 시장은 질병 유형, 유형, 최종 사용자 및 유통 채널로 분류됩니다. 세그먼트 간의 성장은 틈새 성장 포켓과 시장에 접근하고 핵심 응용 분야와 타겟 시장의 차이점을 파악하기 위한 전략을 분석하는 데 도움이 됩니다.
질병 유형
- 유아형
- 청소년형
- 기타
질병 유형을 기준으로 레버 선천성 아마우로시스 시장은 유아형, 청소년형 및 기타로 세분화됩니다.
유형
- 진단
- 요법
유형별로 레버 선천성 아마우로시스 시장은 진단과 치료로 세분화됩니다. 진단 세그먼트는 임상 진단과 유전적 진단으로 세분화됩니다. 임상 진단은 임상적 안구 검사, 시야 검사, 소매 영상 및 전기 생리학적 검사로 세분화됩니다. 임상 진단은 전기 생리학적 검사, 망막 영상, 시야 검사 및 임상적 안구 검사로 세분화됩니다. 전기 생리학 검사는 전체 시야 전기 망막 검사(ERG) 및 암흑 적응 검사(DA)로 세분화됩니다. 망막 영상은 광 간섭 단층 촬영(OCT), 안저 자가 형광 영상(FAF), 주사 레이저 검안경 검사(SLO) 및 적응 광학(AO) 영상 및 기존 컬러 안저 영상으로 세분화됩니다. 시야 검사는 컴퓨터화된 시야 검사와 수동 시야 검사로 세분화됩니다. 임상 검사는 슬릿 램프, 간접 검안경 검사, 굴절 검사 및 확장 검사로 세분화됩니다. 치료 세그먼트는 유전자 치료, 망막 치료, 신경 보호제 등으로 세분화됩니다. 유전자 치료는 룩스투르나 등으로 세분화됩니다. 신경 보호제는 비타민 A 팔미테이트, 도코사헥사엔산(DHA), 루테인 등으로 세분화됩니다.
최종 사용자
- 병원
- 전문 병원
- 외래 수술 센터
- 홈 헬스케어
- 기타
레버 선천성 흑암시 시장은 최종 사용자를 기준으로 병원, 전문 병원, 외래 수술 센터, 가정 의료 서비스 및 기타로 구분됩니다.
유통 채널
- 소매 판매
- 직접 입찰
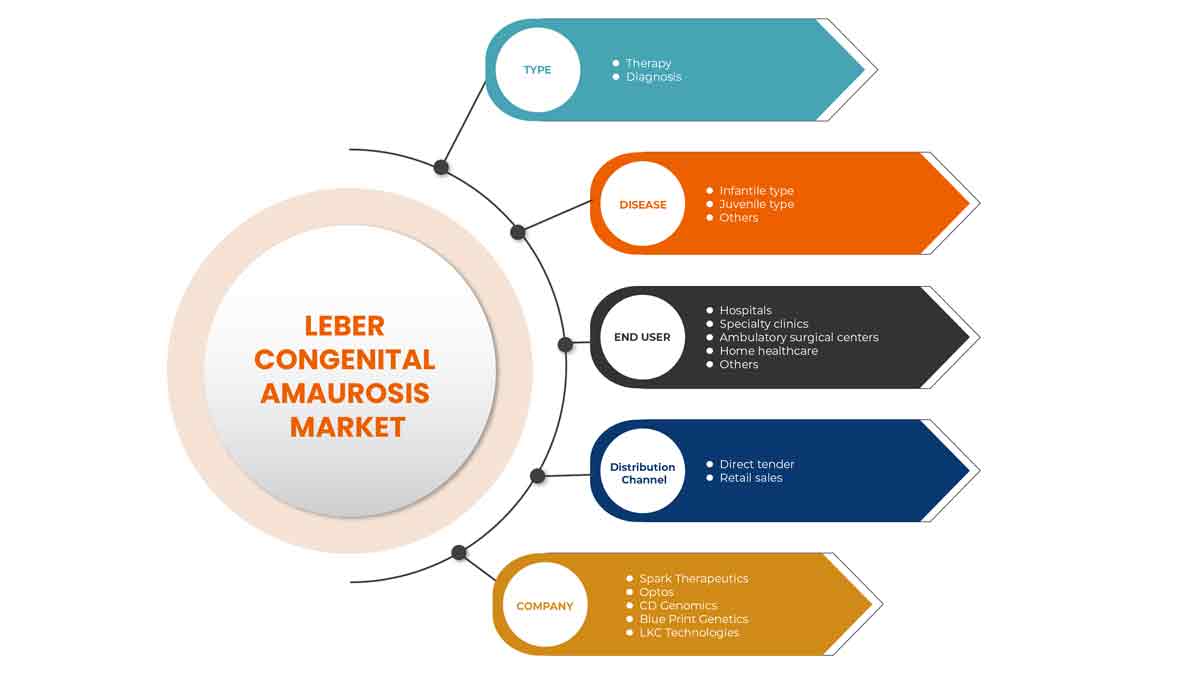
유통 채널을 기준으로 레버 선천성 흑암시 시장은 소매 판매, 직접 입찰로 세분화됩니다.
Leber 선천성 아마우로시스 시장 지역 분석/통찰력
레버 선천성 흑암시 시장을 분석하고, 위에 언급된 대로 국가, 질병 유형, 유형, 최종 사용자 및 유통 채널별로 시장 규모에 대한 통찰력과 추세를 제공합니다.
레버 선천성 흑암시 시장 보고서에서 다루는 국가는 미국, 캐나다, 멕시코입니다.
파이프라인 제품과 임상 시험이 늘어나면서 미국이 우위를 점할 것으로 예상됩니다.
보고서의 국가 섹션은 또한 현재 및 미래 시장 추세에 영향을 미치는 개별 시장 영향 요인과 시장 규제의 변화를 제공합니다. 다운스트림 및 업스트림 가치 사슬 분석, 기술 추세 및 포터의 5가지 힘 분석, 사례 연구와 같은 데이터 포인트는 개별 국가의 시장 시나리오를 예측하는 데 사용되는 몇 가지 포인터입니다. 또한 글로벌 브랜드의 존재 및 가용성과 지역 및 국내 브랜드와의 대규모 또는 희소한 경쟁으로 인해 직면한 과제, 국내 관세 및 무역 경로의 영향은 국가 데이터에 대한 예측 분석을 제공하는 동안 고려됩니다.
경쟁 환경 및 점유율 분석
북미 레버 선천성 아마우로시스 시장 경쟁 구도는 경쟁자에 대한 세부 정보를 제공합니다. 포함된 세부 정보는 회사 개요, 회사 재무, 창출된 수익, 시장 잠재력, 연구 개발 투자, 새로운 시장 이니셔티브, 북미 지역, 생산 현장 및 시설, 생산 용량, 회사의 강점과 약점, 제품 출시, 제품 폭과 범위, 응용 프로그램 우세입니다. 위에 제공된 데이터 포인트는 레버 선천성 아마우로시스 시장에 대한 회사의 초점과만 관련이 있습니다.
주요 기업으로는 Invitae Corporation, Johnson & Johnson Services, Inc., Spark Therapeutics, Inc., LKC TECHNOLOGIES, INC., Amarantus Bioscience Holdings, Inc., Sucampo Pharmaceuticals, Inc., Orphagen Pharmaceuticals, Inc. 등이 있습니다.
연구 방법론
데이터 수집 및 기준 연도 분석은 대규모 샘플 크기의 데이터 수집 모듈을 사용하여 수행됩니다. 시장 데이터는 시장 통계 및 일관된 모델을 사용하여 분석 및 추정됩니다. 또한 시장 점유율 분석 및 주요 추세 분석은 시장 보고서의 주요 성공 요인입니다. DBMR 연구팀에서 사용하는 주요 연구 방법론은 데이터 마이닝, 시장에 대한 데이터 변수의 영향 분석 및 기본(산업 전문가) 검증을 포함하는 데이터 삼각 측량입니다. 이 외에도 데이터 모델에는 공급업체 포지셔닝 그리드, 시장 타임라인 분석, 시장 개요 및 가이드, 회사 포지셔닝 그리드, 회사 시장 점유율 분석, 측정 표준, 북미 대 지역 및 공급업체 점유율 분석이 포함됩니다. 추가 문의 사항이 있는 경우 분석가 전화를 요청하십시오.
SKU-
세계 최초의 시장 정보 클라우드 보고서에 온라인으로 접속하세요
- 대화형 데이터 분석 대시보드
- 높은 성장 잠재력 기회를 위한 회사 분석 대시보드
- 사용자 정의 및 질의를 위한 리서치 분석가 액세스
- 대화형 대시보드를 통한 경쟁자 분석
- 최신 뉴스, 업데이트 및 추세 분석
- 포괄적인 경쟁자 추적을 위한 벤치마크 분석의 힘 활용
목차
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF NORTH AMERICA LEBER CONGENITAL AMAUROSIS MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 DISEASE TYPE LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 MARKET END USER COVERAGE GRID
2.11 VENDOR SHARE ANALYSIS
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PIPELINE ANALYSIS FOR NORTH AMERICA LEBER CONGENTIAL AMAUROSIS MARKET
4.2 NORTH AMERICA LEBER CONGENITAL AMAUROSIS MARKET: REGULATIONS
4.3 PESTEL ANALYSIS
4.4 PORTERS FIVE FORCES
5 EPIDEMIOLOGY
6 NORTH AMERICA LEBER CONGENITAL AMAUROSIS MARKET, MARKET OVERVIEW
6.1 DRIVERS
6.1.1 INCREASE IN INCIDENCE AND PREVALENCE OF RPE65 GENE-MEDIATED IN RETINAL DISEASES
6.1.2 INCREASE IN PIPELINE PRODUCTS AND CLINICAL TRIALS
6.1.3 INCREASE IN STRATEGIC INITIATIVE BY KEY PLAYER
6.2 RESTRAINTS
6.2.1 LACK OF QUALIFIED PROFESSIONALS
6.2.2 HIGH COSTS ASSOCIATED WITH TREATMENT AND PROCEDURE
6.3 OPPORTUNITIES
6.3.1 INCREASE IN GOVERNMENT INITIATIVES TOWARD LEBER CONGENITAL AMAUROSIS
6.3.2 INCREASE IN THE TREATMENT-SEEKING RATE
6.3.3 FAVOURABLE REIMBURSEMENT POLICIES FOR THE TREATMENT
6.4 CHALLENGES
6.4.1 RISKS ASSOCIATED WITH LEBER CONGENITAL AMAUROSIS GENE THERAPY
6.4.2 STRINGENT GOVERNMENT REGULATIONS FOR GENE THERAPY PRODUCTS
7 NORTH AMERICA LEBER CONGENITAL AMAUROSIS MARKET, BY DISEASE TYPE
7.1 OVERVIEW
7.2 INFANTILE TYPE
7.3 JUVENILE TYPE
7.4 OTHERS
8 NORTH AMERICA LEBER CONGENITAL AMAUROSIS MARKET, BY TYPE
8.1 OVERVIEW
8.2 THERAPY
8.2.1 GENE THERAPY
8.2.1.1 LUXTURNA
8.2.2 RETINAL PROSTHETIC
8.2.3 NEUROPROTECTIVE AGENTS
8.2.3.1 VITAMIN A PALMITATE
8.2.3.2 DOCOSAHEXAENOIC ACID
8.2.3.3 LUTEIN
8.2.3.4 OTHERS
8.2.4 OTHERS
8.3 DIAGNOSIS
8.3.1 CLINICAL DIAGNOSIS
8.3.1.1 ELECTROPHYSIOLOGICAL TESTS
8.3.1.1.1 FULL-FIELD ELECTRORETINOGRAM (ERG)
8.3.1.1.2 DARK ADAPTOMETRY (DA)
8.3.1.2 RETINAL IMAGING
8.3.1.2.1 OPTICAL COHERENCE TOMOGRAPHY (OCT)
8.3.1.2.2 FUNDUS AUTOFLUORESCENCE
8.3.1.2.3 SCANNING LASER OPHTHALMOSCOPY (SLO)
8.3.1.2.4 ADAPTIVE OPTICS (AO) IMAGING
8.3.1.2.5 CONVENTIONAL COLOR FUNDUS IMAGING
8.3.1.3 VISUAL FIELD TEST
8.3.1.3.1 COMPUTERIZED VISUAL FIELD TESTS
8.3.1.3.2 MANUAL FIELD TEST
8.3.1.4 CLINICAL EYE EXAMINATION
8.3.1.4.1 SLIT LAMP
8.3.1.4.2 INDIRECT OPHTHALMOSCOPY
8.3.1.4.3 REFRACTION TEST
8.3.1.4.4 DILATION EXAM
8.3.1.5 OTHERS
8.3.2 GENETIC DIAGNOSIS
9 NORTH AMERICA LEBER CONGENITAL AMAUROSIS MARKET, BY END USER
9.1 OVERVIEW
9.2 HOSPITALS
9.3 SPECIALITY CLINICS
9.4 AMBULATORY SURGICAL CENTERS
9.5 HOME HEALTHCARE
9.6 OTHERS
10 NORTH AMERICA LEBER CONGENITAL AMAUROSIS MARKET, BY DISTRIBUTION CHANNEL
10.1 OVERVIEW
10.2 RETAIL SALES
10.2.1.1 HOSPITAL PHARMACIES
10.2.1.2 RETAIL PHARMACIES
10.2.1.3 OTHERS
10.3 DIRECT TENDER
11 NORTH AMERICA LEBER CONGENITAL AMAUROSIS MARKET, BY REGION
11.1 NORTH AMERICA
11.1.1 U.S.
11.1.2 CANADA
11.1.3 MEXICO
12 NORTH AMERICA LEBER CONGENITAL AMAUROSIS MARKET: COMPANY LANDSCAPE
12.1 COMPANY SHARE ANALYSIS: NORTH AMERICA
13 SWOT ANALYSIS
14 COMPANY PROFILE
14.1 SPARK THERAPEUTICS, INC
14.1.1 COMPANY SNAPSHOT
14.1.2 COMPANY SHARE ANALYSIS
14.1.3 PRODUCT PORTFOLIO
14.1.4 RECENT DEVELOPMENT
14.1.4.1 EU AUTHORIZATION
14.2 OPTOS
14.2.1 COMPANY SNAPSHOT
14.2.2 COMPANY SHARE ANALYSIS
14.2.3 PRODUCT PORTFOLIO
14.2.4 RECENT DEVELOPMENT
14.2.4.1 PRODUCT APPROVAL
14.3 CD GENOMICS
14.3.1 COMPANY SNAPSHOT
14.3.2 COMPANY SHARE ANALYSIS
14.3.3 PRODUCT PORTFOLIO
14.3.4 RECENT DEVELOPMENT
14.4 BLUEPRINTS GENETICS OY (A SUBSIDIARY OF QUEST DIAGNOSTICS)
14.4.1 COMPANY SNAPSHOT
14.4.2 REVENUE ANALYSIS
14.4.3 COMPANY SHARE ANALYSIS
14.4.4 PRODUCT PORTFOLIO
14.4.5 RECENT DEVELOPMENTS
14.4.5.1 COLLABORATION
14.5 LKC TECHNOLOGIES, INC
14.5.1 COMPANY SNAPSHOT
14.5.2 COMPANY SHARE ANALYSIS
14.5.3 PRODUCT PORTFOLIO
14.5.4 RECENT DEVELOPMENT
14.5.4.1 PRODUCT APPROVAL
14.5.5 PRODUCT APPROVAL
14.6 AGTC
14.6.1 COMPANY SNAPSHOT
14.6.2 REVENUE ANALYSIS
14.6.3 PRODUCT PORTFOLIO
14.6.4 RECENT DEVELOPMENTS
14.6.4.1 AGREEMENT
14.7 ALLERGAN (A SUBSIDIARY OF ABBVIE INC.)
14.7.1 COMPANY SNAPSHOT
14.7.2 REVENUE ANALYSIS
14.7.3 PRODUCT PORTFOLIO
14.7.4 RECENT DEVELOPMENTS
14.8 ASTELLAS PHARMA INC.
14.8.1 COMPANY SNAPSHOT
14.8.2 REVENUE ANALYSIS
14.8.3 PRODUCT PORTFOLIO
14.8.4 RECENT DEVELOPMENT
14.9 ATSENA THERAPEUTICS
14.9.1 COMPANY SNAPSHOT
14.9.2 PRODUCT PORTFOLIO
14.9.3 RECENT DEVELOPMENT
14.1 CENTOGENE N.V.
14.10.1 COMPANY SNAPSHOT
14.10.2 REVENUE ANALYSIS
14.10.3 PRODUCT PORTFOLIO
14.10.4 RECENT DEVELOPMENT
14.10.4.1 PRODUCT LAUNCH
14.11 COAVE THERAPEUTICS
14.11.1 COMPANY SNAPSHOT
14.11.2 PRODUCT PORTFOLIO
14.11.3 RECENT DEVELOPMENT
14.12 EDITAS MEDICINE
14.12.1 COMPANY SNAPSHOT
14.12.2 REVENUE ANALYSIS
14.12.3 PRODUCT PORTFOLIO
14.12.4 RECENT DEVELOPMENTS
14.12.4.1 DATA & TRAIL DATA PRESENTATION
14.13 ELUMINEX BIOSCIENCES
14.13.1 COMPANY SNAPSHOT
14.13.2 PRODUCT PORTFOLIO
14.13.3 RECENT DEVELOPMENT
14.14 GYROSCOPE THERAPEUTICS LIMITED
14.14.1 COMPANY SNAPSHOT
14.14.2 PRODUCT PORTFOLIO
14.14.3 RECENT DEVELOPMENTS
14.15 INVITAE CORPORATION
14.15.1 COMPANY SNAPSHOT
14.15.2 REVENUE ANALYSIS
14.15.3 PRODUCT PORTFOLIO
14.15.4 RECENT DEVELOPMENTS
14.15.4.1 NEW FACILITY
14.15.4.2 EXPANSION
14.16 IVERIC BIO.
14.16.1 COMPANY SNAPSHOT
14.16.2 PRODUCT PORTFOLIO
14.16.3 RECENT DEVELOPMENT
14.17 KUBOTA PHARMACEUTICAL HOLDINGS CO., LTD.
14.17.1 COMPANY SNAPSHOT
14.17.2 REVENUE ANALYSIS
14.17.3 PRODUCT PORTFOLIO
14.17.4 RECENT DEVELOPMENTS
14.18 MEIRAGTX LIMITED
14.18.1 COMPANY SNAPSHOT
14.18.2 PRODUCT PORTFOLIO
14.18.3 RECENT DEVELOPMENT
14.19 METROVISION
14.19.1 COMPANY SNAPSHOT
14.19.2 PRODUCT PORTFOLIO
14.19.3 RECENT DEVELOPMENT
14.19.3.1 FDA CLEARANCE APPROVAL
14.2 OCUGEN INC.
14.20.1 COMPANY SNAPSHOT
14.20.2 PRODUCT PORTFOLIO
14.20.3 RECENT DEVELOPMENT
14.21 OKUVISION
14.21.1 COMPANY SNAPSHOT
14.21.2 PRODUCT PORTFOLIO
14.21.3 RECENT DEVELOPMENTS
14.21.3.1 APPROVAL
14.22 PROQR THERAPEUTICS
14.22.1 COMPANY SNAPSHOT
14.22.2 PRODUCT PORTFOLIO
14.22.3 RECENT DEVELOPMENTS
14.23 REGENXBIO INC.
14.23.1 COMPANY SNAPSHOT
14.23.2 REVENUE ANALYSIS
14.23.3 PRODUCT PORTFOLIO
14.23.4 RECENT DEVELOPMENTS
14.24 SPARINGVISION
14.24.1 COMPANY SNAPSHOT
14.24.2 PRODUCT PORTFOLIO
14.24.3 RECENT DEVELOPMENTS
15 QUESTIONNAIRE
16 RELATED REPORTS
표 목록
TABLE 1 NORTH AMERICA LEBER CONGENITAL AMAUROSIS MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 2 NORTH AMERICA INFANTILE TYPE IN LEBER CONGENITAL AMAUROSIS MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 3 NORTH AMERICA JUVENILE TYPE IN LEBER CONGENITAL AMAUROSIS MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 4 NORTH AMERICA OTHERS IN LEBER CONGENITAL AMAUROSIS MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 5 NORTH AMERICA LEBER CONGENITAL AMAUROSIS MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 6 NORTH AMERICA THERAPY IN LEBER CONGENITAL AMAUROSIS MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 7 NORTH AMERICA THERAPY IN LEBER CONGENITAL AMAUROSIS MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 8 NORTH AMERICA GENE THERAPY IN LEBER CONGENITAL AMAUROSIS MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 9 NORTH AMERICA NEUROPROTECTIVE AGENTS IN LEBER CONGENITAL AMAUROSIS MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 10 NORTH AMERICA DIAGNOSIS IN LEBER CONGENITAL AMAUROSIS MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 11 NORTH AMERICA DIAGNOSIS IN LEBER CONGENITAL AMAUROSIS MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 12 NORTH AMERICA CLINICAL DIAGNOSIS IN LEBER CONGENITAL AMAUROSIS MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 13 NORTH AMERICA ELECTROPHYSIOLOGICAL TESTS IN LEBER CONGENITAL AMAUROSIS MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 14 NORTH AMERICA RETINAL IMAGING IN LEBER CONGENITAL AMAUROSIS MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 15 NORTH AMERICA VISUAL FIELD TEST IN LEBER CONGENITAL AMAUROSIS MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 16 NORTH AMERICA CLINICAL EYE EXAMINATION IN LEBER CONGENITAL AMAUROSIS MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 17 NORTH AMERICA LEBER CONGENITAL AMAUROSIS MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 18 NORTH AMERICA HOSPITALS IN LEBER CONGENITAL AMAUROSIS MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 19 NORTH AMERICA SPECIALITY CLINICS IN LEBER CONGENITAL AMAUROSIS MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 20 NORTH AMERICA AMBULATORY SURGICAL CENTERS IN LEBER CONGENITAL AMAUROSIS MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 21 NORTH AMERICA HOME HEALTHCARE IN LEBER CONGENITAL AMAUROSIS MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 22 NORTH AMERICA OTHERS IN LEBER CONGENITAL AMAUROSIS MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 23 NORTH AMERICA LEBER CONGENITAL AMAUROSIS MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 24 NORTH AMERICA RETAIL SALES IN LEBER CONGENITAL AMAUROSIS MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 25 NORTH AMERICA RETAIL SALES IN LEBER CONGENITAL AMAUROSIS MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 26 NORTH AMERICA DIRECT TENDER IN LEBER CONGENITAL AMAUROSIS MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 27 NORTH AMERICA LEBER CONGENITAL AMAUROSIS MARKET, BY COUNTRY, 2022-2029 (USD MILLION)
TABLE 28 NORTH AMERICA LEBER CONGENITAL AMAUROSIS MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 29 NORTH AMERICA LEBER CONGENITAL AMAUROSIS MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 30 NORTH AMERICA THERAPY IN LEBER CONGENITAL AMAUROSIS MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 31 NORTH AMERICA GENE THERAPY IN LEBER CONGENITAL AMAUROSIS MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 32 NORTH AMERICA NEUROPROTECTIVE AGENTS IN LEBER CONGENITAL AMAUROSIS MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 33 NORTH AMERICA DIAGNOSIS IN LEBER CONGENITAL AMAUROSIS MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 34 NORTH AMERICA CLINICAL DIAGNOSIS IN LEBER CONGENITAL AMAUROSIS MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 35 NORTH AMERICA RETINAL IMAGING IN LEBER CONGENITAL AMAUROSIS MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 36 NORTH AMERICA ELECTROPHYSIOLOGICAL TESTS IN LEBER CONGENITAL AMAUROSIS MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 37 NORTH AMERICA VISUAL FIELD TEST IN LEBER CONGENITAL AMAUROSIS MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 38 NORTH AMERICA CLINICAL EYE EXAMINATION IN LEBER CONGENITAL AMAUROSIS MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 39 NORTH AMERICA LEBER CONGENITAL AMAUROSIS MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 40 NORTH AMERICA LEBER CONGENITAL AMAUROSIS MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 41 NORTH AMERICA RETAIL SALES IN LEBER CONGENITAL AMAUROSIS MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 42 U.S. LEBER CONGENITAL AMAUROSIS MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 43 U.S. LEBER CONGENITAL AMAUROSIS MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 44 U.S. THERAPY IN LEBER CONGENITAL AMAUROSIS MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 45 U.S. GENE THERAPY IN LEBER CONGENITAL AMAUROSIS MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 46 U.S. NEUROPROTECTIVE AGENTS IN LEBER CONGENITAL AMAUROSIS MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 47 U.S. DIAGNOSIS IN LEBER CONGENITAL AMAUROSIS MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 48 U.S. CLINICAL DIAGNOSIS IN LEBER CONGENITAL AMAUROSIS MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 49 U.S. RETINAL IMAGING IN LEBER CONGENITAL AMAUROSIS MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 50 U.S. ELECTROPHYSIOLOGICAL TESTS IN LEBER CONGENITAL AMAUROSIS MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 51 U.S. VISUAL FIELD TEST IN LEBER CONGENITAL AMAUROSIS MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 52 U.S. CLINICAL EYE EXAMINATION IN LEBER CONGENITAL AMAUROSIS MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 53 U.S. LEBER CONGENITAL AMAUROSIS MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 54 U.S. LEBER CONGENITAL AMAUROSIS MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 55 U.S. RETAIL SALES IN LEBER CONGENITAL AMAUROSIS MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 56 CANADA LEBER CONGENITAL AMAUROSIS MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 57 CANADA LEBER CONGENITAL AMAUROSIS MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 58 CANADA THERAPY IN LEBER CONGENITAL AMAUROSIS MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 59 CANADA GENE THERAPY IN LEBER CONGENITAL AMAUROSIS MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 60 CANADA NEUROPROTECTIVE AGENTS IN LEBER CONGENITAL AMAUROSIS MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 61 CANADA DIAGNOSIS IN LEBER CONGENITAL AMAUROSIS MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 62 CANADA CLINICAL DIAGNOSIS IN LEBER CONGENITAL AMAUROSIS MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 63 CANADA RETINAL IMAGING IN LEBER CONGENITAL AMAUROSIS MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 64 CANADA ELECTROPHYSIOLOGICAL TESTS IN LEBER CONGENITAL AMAUROSIS MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 65 CANADA VISUAL FIELD TEST IN LEBER CONGENITAL AMAUROSIS MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 66 CANADA CLINICAL EYE EXAMINATION IN LEBER CONGENITAL AMAUROSIS MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 67 CANADA LEBER CONGENITAL AMAUROSIS MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 68 CANADA LEBER CONGENITAL AMAUROSIS MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 69 CANADA RETAIL SALES IN LEBER CONGENITAL AMAUROSIS MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 70 MEXICO LEBER CONGENITAL AMAUROSIS MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 71 MEXICO LEBER CONGENITAL AMAUROSIS MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 72 MEXICO THERAPY IN LEBER CONGENITAL AMAUROSIS MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 73 MEXICO GENE THERAPY IN LEBER CONGENITAL AMAUROSIS MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 74 MEXICO NEUROPROTECTIVE AGENTS IN LEBER CONGENITAL AMAUROSIS MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 75 MEXICO DIAGNOSIS IN LEBER CONGENITAL AMAUROSIS MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 76 MEXICO CLINICAL DIAGNOSIS IN LEBER CONGENITAL AMAUROSIS MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 77 MEXICO RETINAL IMAGING IN LEBER CONGENITAL AMAUROSIS MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 78 MEXICO ELECTROPHYSIOLOGICAL TESTS IN LEBER CONGENITAL AMAUROSIS MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 79 MEXICO VISUAL FIELD TEST IN LEBER CONGENITAL AMAUROSIS MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 80 MEXICO CLINICAL EYE EXAMINATION IN LEBER CONGENITAL AMAUROSIS MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 81 MEXICO LEBER CONGENITAL AMAUROSIS MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 82 MEXICO LEBER CONGENITAL AMAUROSIS MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 83 MEXICO RETAIL SALES IN LEBER CONGENITAL AMAUROSIS MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
그림 목록
FIGURE 1 NORTH AMERICA LEBER CONGENITAL AMAUROSIS MARKET: SEGMENTATION
FIGURE 2 NORTH AMERICA LEBER CONGENITAL AMAUROSIS MARKET: DATA TRIANGULATION
FIGURE 3 NORTH AMERICA LEBER CONGENITAL AMAUROSIS MARKET: DROC ANALYSIS
FIGURE 4 NORTH AMERICA LEBER CONGENITAL AMAUROSIS MARKET: NORTH AMERICA VS REGIONAL MARKET ANALYSIS
FIGURE 5 NORTH AMERICA LEBER CONGENITAL AMAUROSIS MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 NORTH AMERICA LEBER CONGENITAL AMAUROSIS MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 NORTH AMERICA LEBER CONGENITAL AMAUROSIS MARKET: DBMR MARKET POSITION GRID
FIGURE 8 NORTH AMERICA LEBER CONGENITAL AMAUROSIS MARKET: END USER GRID
FIGURE 9 NORTH AMERICA LEBER CONGENITAL AMAUROSIS MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 NORTH AMERICA LEBER CONGENITAL AMAUROSIS MARKET: SEGMENTATION
FIGURE 11 RISING GOVERNMENT AWARENESS PROGRAMS ABOUT THE INHERITED RETINAL DISEASE IS EXPECTED TO DRIVE THE NORTH AMERICA LEBER CONGENITAL AMAUROSIS MARKET IN THE FORECAST PERIOD OF 2022 TO 2029
FIGURE 12 INFANTILE TYPE IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE NORTH AMERICA LEBER CONGENITAL AMAUROSIS MARKET IN 2022 & 2029
FIGURE 13 NORTH AMERICA IS EXPECTED TO DOMINATE THE NORTH AMERICA LEBER CONGENITAL AMAUROSIS MARKET IN THE FORECAST PERIOD OF 2022 TO 2029
FIGURE 14 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES FOR THE NORTH AMERICA LEBER CONGENITAL AMAUROSIS MARKET
FIGURE 15 PROPORTION OF RPE65 GENE MUTATION IN CLINICALLY DIAGNOSED LCA PATIENTS
FIGURE 16 NORTH AMERICA LEBER CONGENITAL AMAUROSIS MARKET: BY DISEASE TYPE, 2021
FIGURE 17 NORTH AMERICA LEBER CONGENITAL AMAUROSIS MARKET: BY DISEASE TYPE, 2022-2029 (USD MILLION)
FIGURE 18 NORTH AMERICA LEBER CONGENITAL AMAUROSIS MARKET: BY DISEASE TYPE, CAGR (2022-2029)
FIGURE 19 NORTH AMERICA LEBER CONGENITAL AMAUROSIS MARKET: BY DISEASE TYPE, LIFELINE CURVE
FIGURE 20 NORTH AMERICA LEBER CONGENITAL AMAUROSIS MARKET: BY TYPE, 2021
FIGURE 21 NORTH AMERICA LEBER CONGENITAL AMAUROSIS MARKET: BY TYPE, 2022-2029 (USD MILLION)
FIGURE 22 NORTH AMERICA LEBER CONGENITAL AMAUROSIS MARKET: BY TYPE, CAGR (2022-2029)
FIGURE 23 NORTH AMERICA LEBER CONGENITAL AMAUROSIS MARKET: BY TYPE, LIFELINE CURVE
FIGURE 24 NORTH AMERICA LEBER CONGENITAL AMAUROSIS MARKET: BY END USER, 2021
FIGURE 25 NORTH AMERICA LEBER CONGENITAL AMAUROSIS MARKET: BY END USER, 2022-2029 (USD MILLION)
FIGURE 26 NORTH AMERICA LEBER CONGENITAL AMAUROSIS MARKET: BY END USER, CAGR (2022-2029)
FIGURE 27 NORTH AMERICA LEBER CONGENITAL AMAUROSIS MARKET: BY END USER, LIFELINE CURVE
FIGURE 28 NORTH AMERICA LEBER CONGENITAL AMAUROSIS MARKET: BY DISTRIBUTION CHANNEL, 2021
FIGURE 29 NORTH AMERICA LEBER CONGENITAL AMAUROSIS MARKET: BY END USER, 2022-2029 (USD MILLION)
FIGURE 30 NORTH AMERICA LEBER CONGENITAL AMAUROSIS MARKET: BY END USER, CAGR (2022-2029)
FIGURE 31 NORTH AMERICA LEBER CONGENITAL AMAUROSIS MARKET: BY END USER, LIFELINE CURVE
FIGURE 32 NORTH AMERICA LEBER CONGENITAL AMAUROSIS MARKET: SNAPSHOT (2021)
FIGURE 33 NORTH AMERICA LEBER CONGENITAL AMAUROSIS MARKET: BY COUNTRY (2021)
FIGURE 34 NORTH AMERICA LEBER CONGENITAL AMAUROSIS MARKET: BY COUNTRY (2022 & 2029)
FIGURE 35 NORTH AMERICA LEBER CONGENITAL AMAUROSIS MARKET: BY COUNTRY (2021 & 2029)
FIGURE 36 NORTH AMERICA LEBER CONGENITAL AMAUROSIS MARKET: BY DISEASE TYPE (2022-2029)
FIGURE 37 NORTH AMERICA LEBER CONGENITAL AMAUROSIS MARKET: COMPANY SHARE 2021 (%)

연구 방법론
데이터 수집 및 기준 연도 분석은 대규모 샘플 크기의 데이터 수집 모듈을 사용하여 수행됩니다. 이 단계에는 다양한 소스와 전략을 통해 시장 정보 또는 관련 데이터를 얻는 것이 포함됩니다. 여기에는 과거에 수집한 모든 데이터를 미리 검토하고 계획하는 것이 포함됩니다. 또한 다양한 정보 소스에서 발견되는 정보 불일치를 검토하는 것도 포함됩니다. 시장 데이터는 시장 통계 및 일관된 모델을 사용하여 분석하고 추정합니다. 또한 시장 점유율 분석 및 주요 추세 분석은 시장 보고서의 주요 성공 요인입니다. 자세한 내용은 분석가에게 전화를 요청하거나 문의 사항을 드롭하세요.
DBMR 연구팀에서 사용하는 주요 연구 방법론은 데이터 마이닝, 시장에 대한 데이터 변수의 영향 분석 및 주요(산업 전문가) 검증을 포함하는 데이터 삼각 측량입니다. 데이터 모델에는 공급업체 포지셔닝 그리드, 시장 타임라인 분석, 시장 개요 및 가이드, 회사 포지셔닝 그리드, 특허 분석, 가격 분석, 회사 시장 점유율 분석, 측정 기준, 글로벌 대 지역 및 공급업체 점유율 분석이 포함됩니다. 연구 방법론에 대해 자세히 알아보려면 문의를 통해 업계 전문가에게 문의하세요.
사용자 정의 가능
Data Bridge Market Research는 고급 형성 연구 분야의 선두 주자입니다. 저희는 기존 및 신규 고객에게 목표에 맞는 데이터와 분석을 제공하는 데 자부심을 느낍니다. 보고서는 추가 국가에 대한 시장 이해(국가 목록 요청), 임상 시험 결과 데이터, 문헌 검토, 재생 시장 및 제품 기반 분석을 포함하도록 사용자 정의할 수 있습니다. 기술 기반 분석에서 시장 포트폴리오 전략에 이르기까지 타겟 경쟁업체의 시장 분석을 분석할 수 있습니다. 귀하가 원하는 형식과 데이터 스타일로 필요한 만큼 많은 경쟁자를 추가할 수 있습니다. 저희 분석가 팀은 또한 원시 엑셀 파일 피벗 테이블(팩트북)로 데이터를 제공하거나 보고서에서 사용 가능한 데이터 세트에서 프레젠테이션을 만드는 데 도움을 줄 수 있습니다.