North America Glioblastoma Multiforme Treatment Market, By Type (Primary (De Novo), Secondary), Treatment (Surgery, Radiotherapy, Medications), Patient Type (Adult, Geriatric, Child), Drug Type (Generics, Branded), Route of Administration (Parenteral, Oral, Others), End User (Hospitals, Clinics, Home Healthcare, Others), Distribution Channel (Hospital Pharmacy, Retail Pharmacy, Online Pharmacy, Others) - Industry Trends and Forecast to 2029.
Market Analysis and Insights
Glioblastoma multiforme (GBM) is a grade IV WHO malignant tumor with astrocytic differentiation. As one of the most common clinically diagnosed central nervous system (CNS) oncological entries, there have been a wide variety of historical reports of the description and evolution of ideas regarding these tumors. The first recorded reports of gliomas were given in British scientific reports, by Berns in 1800 and in 1804 by Abernety, with the first comprehensive histomorphological description being given in 1865 by Rudolf Virchow. In 1926 Percival Bailey and Harvey Cushing gave the base for the modern classification of gliomas. Between 1934 and 1941 the most prolific researcher in glioma research was Hans-Joachim Scherer, who postulated some of the clinico-morphological aspects of GBM. With the introduction of molecular and genetic tests the true multifomity of GBM has been established, with different genotypes bearing the same histomorphological and IHC picture, as well as some of the aspects of gliomagenesis. For a GBM to develop, a specific trigger mutation needs to occur in a GBM stem cell - primary GBM, or a slow aggregation of individual mutations, without a distinct trigger mutation - secondary GBM. Knowledge of GBM has been closely related to general medical knowledge of the CNS since these malignancies were first described more than 200 years ago. Several great leaps have been made in that time, in the footsteps of both CNS and advancements in general medical knowledge. The demand for glioblastoma multiforme treatment is increasing, for which manufacturers are involved in the new product launches, increasing pipeline products and event participation in the market. These decisions are ultimately enhancing the growth of the market.

교모세포종 다형성 치료 시장 보고서는 시장 점유율, 새로운 개발, 국내 및 지역 시장 참여자의 영향에 대한 세부 정보를 제공하고, 새로운 수익 주머니, 시장 규정의 변화, 제품 승인, 전략적 결정, 제품 출시, 지리적 확장 및 시장의 기술 혁신 측면에서 기회를 분석합니다. 분석과 시장 시나리오를 이해하려면 분석가 브리핑을 위해 저희에게 연락하세요. 저희 팀은 원하는 목표를 달성하기 위한 수익 영향 솔루션을 만드는 데 도움을 드릴 것입니다. 약리학적 치료법을 발명하고 혁신하기 위한 협력, 계약 및 판매 계약 체결과 같은 전략적 이니셔티브는 예측 기간 동안 시장 수요를 촉진한 주요 원동력입니다.
교모세포종 다형성 치료 시장은 지지적이며 질병의 진행을 줄이는 것을 목표로 합니다. Data Bridge Market Research는 교모세포종 다형성 치료 시장이 2022년에서 2029년의 예측 기간 동안 8.5%의 CAGR로 성장할 것이라고 분석합니다.
|
보고서 메트릭 |
세부 |
|
예측 기간 |
2022년부터 2029년까지 |
|
기준 연도 |
2021 |
|
역사적 연도 |
2020 (2019-2014까지 사용자 정의 가능) |
|
양적 단위 |
수익은 백만 달러, 가격은 미화로 표시됨 |
|
다루는 세그먼트 |
유형별(1차(De Novo), 2차), 치료(수술, 방사선 치료, 약물), 환자 유형(성인, 노인, 소아), 약물 유형(제네릭, 브랜드), 투여 경로(비경구, 경구, 기타), 최종 사용자(병원, 진료소, 가정 의료, 기타), 유통 채널(병원 약국, 소매 약국, 온라인 약국, 기타) |
|
적용 국가 |
미국, 캐나다, 멕시코 |
|
시장 참여자 포함 |
F. Hoffmann-La Roche AG, Amgen Inc., Merck & Co., Inc., Pfizer Inc., Varian Medical Systems, Inc.(Siemens Healthcare의 자회사), ZEISS International, Amneal Pharmaceuticals LLC, Elekta, Sun Pharmaceutical Industries Ltd, Teva Pharmaceutical Industries Ltd., Eckert & Ziegler, Accord Healthcare, Angiochem, ANI Pharmaceuticals, Inc., Arbor Pharmaceuticals, LLC.(Azurity Pharmaceuticals, Inc.의 자회사), AstraZeneca, Cantex Pharmaceuticals, Inc., CELON LABS, Diffusion Pharmaceuticals Inc., EnGeneIC, ERC.SA., Genenta science, Jazz Pharmaceuticals, Inc., Loxo Oncology(Eli Lilly의 자회사), Novartis AG, VBL THERAPEUTICS, Viatris Inc., 및 Zydus Pharmaceuticals, Inc. 등이 있습니다. |
시장 정의
영어: Glioblastoma multiforme(GBM)는 가장 흔하고 공격적인 원발성 악성 뇌종양으로 성인 뇌종양의 60%를 차지합니다. GBM은 뇌에서 새로 발생하거나 저등급 성상세포종에서 진화할 수 있습니다. 성인의 경우 GBM은 대뇌 반구, 특히 뇌의 전두엽과 측두엽에서 가장 자주 발생합니다. Glioblastoma multiforme에서 많은 유전적 및 환경적 요인이 연구되었지만 GBM의 상당 부분을 차지하는 위험 요인은 확인되지 않았습니다. 따라서 다른 많은 암과 마찬가지로 GBM은 산발적이지만 일부 연구에 따르면 GBM 환자 중에서 사전 치료적 방사선 조사의 유병률(17%)이 높습니다. 방사선 조사와 GBM 발병 사이의 잠복기는 수년에서 수십 년까지 다양합니다. 흡연, 음주, 약물 사용 또는 N-Nitroso 화합물 노출과 같은 라이프스타일 요인과 GBM의 연관성에 대한 실질적인 증거는 없습니다. 연구에 따르면 휴대전화 사용은 GBM 발병 위험을 증가시키지 않습니다. 그러나 장기간 사용과의 연관성은 추가 확인이 필요합니다.
다형성 교모세포종 치료 시장 동향
운전자
- 다형성교모세포종의 유병률 증가
영어: Glioblastoma multiforme(GBM)는 가장 흔히 발생하는 악성 원발성 뇌종양으로, 모든 중추신경계(CNS) 원발성 악성 종양의 77%-81%를 차지합니다. 세계보건기구에서는 이를 4등급 확산성 성상세포성 및 과립교세포성 종양으로 분류했습니다. 원발성 GBM 발병의 평균 연령은 62세이고, 중앙 생존기간은 약 14.6개월입니다. GBM과 관련된 예후가 좋지 않다는 것은 잘 알려져 있는 반면, 의학 및 외과적 발전에도 불구하고 생존율은 실망스럽게도 낮습니다. 연구에 따르면, 국제 연구에서는 10만 명당 연간 발병률이 약 0.59~5명인 것으로 나타났지만, 연구에서는 발병률이 증가하고 있음을 나타냅니다. Miranda-Filho 등은 2017년에 남미, 동유럽, 남유럽 국가에서 CNS 및 뇌암 발병률이 증가하는 반면, 일본에서만 발병률이 감소했다고 보고했습니다. Dobes 등은 2011년에도 호주의 다기관 연구 두 건에서 GBM 종양의 발생률이 증가하는 것을 발견했으며, 특히 전두엽과 측두엽 GBM 종양이 증가했습니다. 다형성 교모세포종의 발생률이 증가함에 따라 최신 기술을 활용하여 조기에 발견하고 진단해야 한다는 요구가 커져 글로벌 다형성 교모세포종 치료 시장이 성장하고 있습니다. 전 세계적으로 교모세포종의 발생률이 증가함에 따라 다형성 교모세포종 치료에 대한 수요가 가속화될 것으로 예상됩니다. 따라서 다형성 교모세포종의 발생률이 증가함에 따라 시장 성장이 촉진될 것으로 예상됩니다.
- 연구개발(R&D) 확대
분자 생명공학 및 암 및 관련 질병에 대한 유전자 치료 분야의 연구 개발(R&D) 활동이 증가함에 따라 다양한 생물학적 약물의 개발이 용이해졌습니다. 이러한 약물은 기존 치료 방법의 부작용을 줄이는 데 도움이 되어 환자 사이에서 더 폭넓은 수용을 이끌어냅니다. 종양 이질성과 환자 간 치료 접근 방식의 변화로 인해 교모세포종을 관리하기 위한 개인화된 치료 접근 방식에 대한 수요가 증가할 것으로 예상됩니다. 새로운 치료법이 승인되면 교모세포종 환자의 기대 수명이 늘어날 것으로 예상됩니다. 또한 FDA가 조사 약물에 부여한 특별 지정으로 새로운 치료법의 승인 절차와 상용화가 촉진될 것으로 예상됩니다. 연구자와 시장 참여자 간의 협업이 증가함에 따라 교모세포종에 대한 새롭고 효과적인 치료 옵션의 개발이 촉진될 것으로 예상됩니다. 새로운 치료법과 병용 치료법에 대한 승인이 증가함에 따라 교모세포종 치료 시장이 성장할 것으로 예상됩니다.
기회
- 약물 승인 증가
교모세포종 치료에 대한 수요가 증가함에 따라 관련 약물에 대한 규제 승인이 더 많아질 것입니다. 관련 약물 및 재조합 제품에 대한 규제 승인이 증가함에 따라 향후 몇 년 동안 교모세포종 치료 시장 가치가 증가할 것입니다. 약물 규제 기관의 인정을 촉진하기 위한 범미 보건 기구(PAHO) 이니셔티브의 틀에서 ANMAT의 평가 프로세스는 2009년 12월 11일에 완료되었습니다. 교모세포종 치료 산업은 최근 몇 년 동안 질병의 사망률 증가에 힘입어 수많은 약물 승인을 목격했습니다. 약물 승인이 증가함에 따라 교모세포종 치료 시장 수요가 증가할 것입니다.
제약/도전
다형성교모세포종 치료 비용이 높다
교모세포종 다형성 진단 검사에는 기술적으로 매우 진보된 제품이 포함됩니다. 이러한 제품을 개발하려면 개발하는 플레이어의 엄격한 연구 및 개발이 필요합니다. 따라서 제품 비용은 높게 유지되며, 그에 비례하여 검사 비용도 증가합니다.
다형성교모세포종 진단에 사용되는 진단 도구 및 기술은 다음과 같습니다.
방사선 요법, 화학 요법 등이 있습니다. GBM의 초기 단계는 일반적으로 증상이 거의 없거나 전혀 없습니다. 따라서 GBM은 종종 진행된 단계에서 진단되어 예후가 나쁩니다. 따라서 첨단 모달리티와 기술 제품을 사용하여 다형성 교모세포종을 치료하는 데 드는 높은 비용은 글로벌 다형성 교모세포종 치료 시장 성장에 주요 제약 요인으로 작용할 것입니다.
최근 개발 사항
- 2022년 4월, Elekta와 GE Healthcare는 방사선 종양학 분야에서 글로벌 상업적 협력 계약을 체결했다고 발표했습니다. 이를 통해 방사선 요법이 필요한 암 환자를 대상으로 영상 및 치료 전반에 걸쳐 종합적인 서비스를 병원에 제공할 수 있게 되었습니다. 이 파트너십을 통해 두 회사는 각 암 센터의 요구에 맞는 솔루션을 공동으로 홍보할 수 있습니다.
- 2019년 7월, Amgen과 Allergan plc는 Avastin(bevacizumab)의 바이오시밀러인 MVASI(bevacizumab-awwb)가 미국(US)에서 출시되었다고 발표했습니다. 이번 출시로 해당 지역에서의 제품 판매가 늘어날 것입니다.
다형성 교모세포종 치료 시장 범위
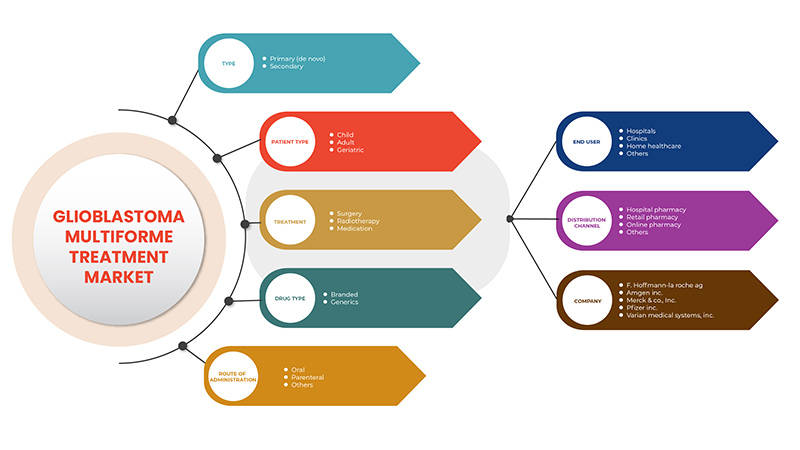
교모세포종 다형성 치료 시장은 유형, 치료, 환자 유형, 약물 유형, 투여 경로, 최종 사용자 및 유통 채널을 기준으로 하는 7개의 주요 세그먼트로 분류됩니다. 세그먼트 간의 성장은 틈새 성장 포켓과 시장에 접근하고 핵심 응용 분야와 타겟 시장의 차이점을 파악하기 위한 전략을 분석하는 데 도움이 됩니다.
유형
- 기본 (De Novo)
- 반성
교모세포종 치료 시장은 유형을 기준으로 1차(De Novo) 및 2차로 구분됩니다.
치료
- 수술
- 방사선 치료
- 약물
치료를 기준으로 볼 때, 다형성교모세포종 치료 시장은 수술, 방사선 치료, 약물 치료로 구분됩니다.
환자 유형
- 성인
- 노인
- 어린이
환자 유형을 기준으로, 다형성교모세포종 치료 시장은 성인, 노인, 소아로 구분됩니다.
약물 유형
- 브랜드화
- 제네릭
약물 유형을 기준으로 볼 때, 다형성교모세포종 치료 시장은 제네릭과 브랜드 제품으로 구분됩니다.
투여 경로
- 경구
- 비경구적
- 기타
투여 경로를 기준으로 볼 때, 다형성교모세포종 치료 시장은 비경구, 경구 및 기타로 구분됩니다.
최종 사용자
- 병원
- 클리닉
- 홈 헬스케어
- 기타
최종 사용자를 기준으로 볼 때, 다형성교모세포종 치료 시장은 병원, 진료소, 가정 건강 관리 및 기타로 구분됩니다.
유통 채널
- 병원 약국
- 소매 약국
- 온라인 약국
- 기타
유통 채널을 기준으로 볼 때, 다형성교모세포종 치료 시장은 병원 약국, 소매 약국 및 기타로 구분됩니다.
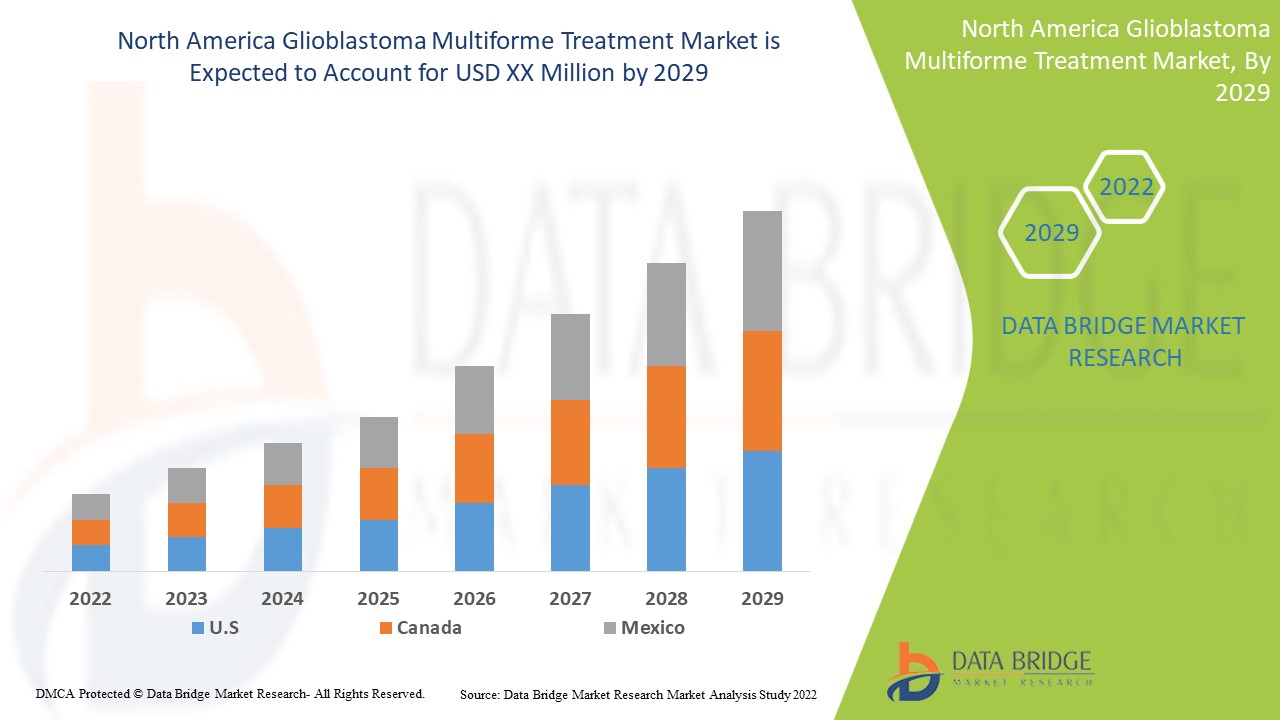
글로벌 교모세포종 다형성 치료 시장 지역 분석/통찰력
다형성교모세포종 치료 시장을 분석하고, 위에 언급된 대로 유형, 치료, 환자 유형, 약물 유형, 투여 경로, 최종 사용자 및 유통 채널별로 시장 규모에 대한 통찰력과 추세를 제공합니다.
다형성교모세포종 치료 시장 보고서에서 다루는 지역은 미국, 캐나다, 멕시코입니다.
북미에서는 해당 지역에서 질병의 유병률이 증가하고 있어 미국이 시장을 지배할 것으로 예상됩니다.
보고서의 국가 섹션은 또한 현재 및 미래 시장 추세에 영향을 미치는 개별 시장 영향 요인과 시장 규제의 변화를 제공합니다. 다운스트림 및 업스트림 가치 사슬 분석, 기술 추세 및 포터의 5가지 힘 분석, 사례 연구와 같은 데이터 포인트는 개별 국가의 시장 시나리오를 예측하는 데 사용되는 몇 가지 포인터입니다. 또한 글로벌 브랜드의 존재 및 가용성과 지역 및 국내 브랜드와의 대규모 또는 희소한 경쟁으로 인해 직면한 과제, 국내 관세 및 무역 경로의 영향은 국가 데이터에 대한 예측 분석을 제공하는 동안 고려됩니다.
경쟁 환경 및 다형성교모세포종 치료 시장 점유율 분석
교모세포종 다형성 치료 시장 경쟁 구도는 경쟁자들의 세부 정보를 제공합니다. 포함된 세부 정보에는 회사 개요, 회사 재무, 창출된 수익, 시장 잠재력, 연구 개발 투자, 새로운 시장 이니셔티브, 글로벌 입지, 생산 현장 및 시설, 생산 용량, 회사의 강점과 약점, 제품 출시, 제품 폭과 범위, 응용 분야 우위가 있습니다. 제공된 위의 데이터 포인트는 교모세포종 다형성 치료 시장에 대한 회사의 초점과만 관련이 있습니다.
시장의 주요 기업으로는 F. Hoffmann-La Roche AG, Amgen Inc., Merck & Co., Inc., Pfizer Inc., Varian Medical Systems, Inc.(Siemens Healthcare의 자회사), ZEISS International, Amneal Pharmaceuticals LLC, Elekta, Sun Pharmaceutical Industries Ltd, Teva Pharmaceutical Industries Ltd., Eckert & Ziegler, Accord Healthcare, Angiochem, ANI Pharmaceuticals, Inc., Arbor Pharmaceuticals, LLC.(Azurity Pharmaceuticals, Inc.의 자회사), AstraZeneca, Cantex Pharmaceuticals, Inc., CELON LABS, Diffusion Pharmaceuticals Inc., EnGeneIC, ERC.SA., Genenta science, Jazz Pharmaceuticals, Inc., Loxo Oncology(Eli Lilly의 자회사), Novartis AG, VBL THERAPEUTICS, Viatris Inc., 및 Zydus Pharmaceuticals, Inc. 등이 있습니다.
연구 방법론
데이터 수집 및 기준 연도 분석은 대규모 샘플 크기의 데이터 수집 모듈을 사용하여 수행됩니다. 시장 데이터는 시장 통계 및 일관된 모델을 사용하여 분석 및 추정됩니다. 또한 시장 점유율 분석 및 주요 추세 분석은 시장 보고서의 주요 성공 요인입니다. DBMR 연구팀에서 사용하는 주요 연구 방법론은 데이터 마이닝, 시장에 대한 데이터 변수의 영향 분석 및 기본(산업 전문가) 검증을 포함하는 데이터 삼각 측량입니다. 이 외에도 데이터 모델에는 공급업체 포지셔닝 그리드, 시장 타임라인 분석, 시장 개요 및 가이드, 회사 포지셔닝 그리드, 회사 시장 점유율 분석, 측정 표준, 글로벌 대 지역 및 공급업체 점유율 분석이 포함됩니다. 추가 문의 사항이 있는 경우 분석가 전화를 요청하십시오.
SKU-
세계 최초의 시장 정보 클라우드 보고서에 온라인으로 접속하세요
- 대화형 데이터 분석 대시보드
- 높은 성장 잠재력 기회를 위한 회사 분석 대시보드
- 사용자 정의 및 질의를 위한 리서치 분석가 액세스
- 대화형 대시보드를 통한 경쟁자 분석
- 최신 뉴스, 업데이트 및 추세 분석
- 포괄적인 경쟁자 추적을 위한 벤치마크 분석의 힘 활용
목차
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 TYPE LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 MARKET END USER COVERAGE GRID
2.11 VENDOR SHARE ANALYSIS
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL
4.2 PORTER'S FIVE FORCES MODEL
5 EPIDEMIOLOGY
6 PIPELINE ANALYSIS
7 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: REGULATORY SCENARIO
8 MARKET OVERVIEW
8.1 DRIVERS
8.1.1 GROWING PREVALENCE OF GLIOBLASTOMA MULTIFORME
8.1.2 INCREASING RESEARCH AND DEVELOPMENT (R&D)
8.1.3 PRESENCE OF A STRONG PIPELINE
8.1.4 GROWING GERIATRIC POPULATION
8.2 RESTRAINTS
8.2.1 HIGH COST OF GLIOBLASTOMA MULTIFORME TREATMENT
8.2.2 ADVERSE SIDE-EFFECTS OF GLIOBLASTOMA MULTIFORME TREATMENT
8.3 OPPORTUNITIES
8.3.1 INCREASING DRUG APPROVALS
8.3.2 PARTNERSHIP AND AGREEMENT BY MAJOR PLAYERS
8.3.3 INCREASING SUPPORT OF PRIVATE AND GOVERNMENT AGENCIES FOR TREATMENT
8.4 CHALLENGES
8.4.1 LACK OF NEW TREATMENT
8.4.2 ADVERSE EFFECTS AND RISKS ASSOCIATED WITH CANCER TREATMENT DRUGS
8.4.3 LACK OF EARLY DETECTION
9 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TYPE
9.1 OVERVIEW
9.2 PRIMARY (DE NOVO)
9.3 SECONDARY
10 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT
10.1 OVERVIEW
10.2 SURGERY
10.3 RADIOTHERAPY
10.3.1 BRACHYTHERAPY
10.3.2 FRACTIONATED STEREOTACTIC RT (FSRT)
10.3.3 CONFORMAL OR INTENSITY-MODULATED RT
10.3.4 RADIOSURGERY
10.4 MEDICATIONS
10.4.1 TEMOZOLOMIDE
10.4.1.1 ORAL
10.4.1.1 INTRAVENOUS
10.4.2 NITROSOUREAS DRUGS
10.4.2.1 CARMUSTINE
10.4.2.1.1 PARENTERAL
10.4.2.1.2 IMPLANTABLE WAFERS
10.4.2.2 LOMUSTINE
10.4.2.3 NIMUSTINE
10.4.2.4 FOTEMUSTINE
10.4.3 TARGETED THERAPY
10.4.3.1 BEVACIZUMAB
10.4.3.2 OTHERS
10.4.4 ANTI-EPILEPTICS
10.4.4.1 LEVETIRACETAM
10.4.4.2 PHENYTOIN
10.4.4.3 CARBAMAZEPINE
10.4.5 CORTICOSTEROIDS
10.4.5.1 METHYLPREDNISOLONE
10.4.5.2 PREDNISONE
10.4.5.3 OTHERS
11 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY PATIENT TYPE
11.1 OVERVIEW
11.2 ADULT
11.2.1 MALE
11.2.2 FEMALE
11.3 GERIATRIC
11.3.1 MALE
11.3.2 FEMALE
11.4 CHILD
12 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY DRUG TYPE
12.1 OVERVIEW
12.2 GENERICS
12.3 BRANDED
13 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY ROUTE OF ADMINISTRATION
13.1 OVERVIEW
13.2 PARENTERAL
13.3 ORAL
13.3.1 CAPSULES
13.3.2 TABLETS
13.3.3 POWDERS
13.4 OTHERS
14 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY END USER
14.1 OVERVIEW
14.2 HOSPITAL
14.3 CLINICS
14.4 HOME HEALTHCARE
14.5 OTHERS
15 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY DISTRIBUTION CHANNEL
15.1 OVERVIEW
15.2 HOSPITAL PHARMACY
15.3 RETAIL PHARMACY
15.4 ONLINE PHARMACY
15.5 OTHERS
16 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION
16.1 NORTH AMERICA
16.1.1 U.S.
16.1.2 CANADA
16.1.3 MEXICO
17 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: COMPANY LANDSCAPE
17.1 COMPANY SHARE ANALYSIS: NORTH AMERICA
18 SWOT ANALYSIS
19 COMPANY PROFILE
19.1 F.HOFFMAN-LA ROCHE
19.1.1 COMPANY SNAPSHOT
19.1.2 REVENUE ANALYSIS
19.1.3 COMPANY SHARE ANALYSIS
19.1.4 PRODUCT PORTFOLIO
19.1.5 RECENT DEVELOPMENT
19.2 AMGEN INC.
19.2.1 COMPANY SNAPSHOT
19.2.2 REVENUE ANALYSIS
19.2.3 COMPANY SHARE ANALYSIS
19.2.4 PRODUCT PORTFOLIO
19.2.5 RECENT DEVELOPMENTS
19.2.5.1 PRODUCT APPROVAL
19.3 MERCK & CO., INC
19.3.1 COMPANY SNAPSHOT
19.3.2 REVENUE ANALYSIS
19.3.3 COMPANY SHARE ANALYSIS
19.3.4 PRODUCT PORTFOLIO
19.3.5 RECENT DEVELOPMENTS
19.3.5.1 STRATETIC COLLABORATION
19.3.5.2 EVENTS
19.4 PFIZER INC.
19.4.1 COMPANY SNAPSHOT
19.4.2 REVENUE ANALYSIS
19.4.3 COMPANY SHARE ANALYSIS
19.4.4 PRODUCT PORTFOLIO
19.4.5 RECENT DEVELOPMENT
19.4.5.1 MERGER
19.5 VARIAN MEDICAL SYSTEMS, INC. (A SUBSIDIARY OF SIEMENS HEALTHCARE)
19.5.1 COMPANY SNAPSHOT
19.5.2 REVENUE ANALYSIS
19.5.3 COMPANY SHARE ANALYSIS
19.5.4 PRODUCT PORTFOLIO
19.5.5 RECENT DEVELOPMENT
19.5.5.1 PARTNERSHIP
19.5.5.2 ACQUISITION
19.6 ZEISS INTERNATIONAL
19.6.1 COMPANY SNAPSHOT
19.6.2 REVENUE ANALYSIS
19.6.3 PRODUCT PORTFOLIO
19.6.4 RECENT DEVELOPMENTS
19.6.4.1 PRODUCT EXPANSION
19.7 AMNEAL PHARMACEUTICALS LLC
19.7.1 COMPANY SNAPSHOT
19.7.2 REVENUE ANALYSIS
19.7.3 PRODUCT PORTFOLIO
19.7.4 RECENT DEVELOPMENTS
19.7.4.1 EVENT
19.7.4.2 LAUNCH
19.7.4.3 ACQUISITION
19.8 ELEKTA
19.8.1 COMPANY SNAPSHOT
19.8.2 REVENUE ANALYSIS
19.8.3 PRODUCT PORTFOLIO
19.8.4 RECENT DEVELOPMENTS
19.8.4.1 PARTNERSHIP
19.9 SUN PHARMACEUTICAL INDUSTRIES LTD
19.9.1 COMPANY SNAPSHOT
19.9.2 REVENUE ANALYSIS
19.9.3 PRODUCT PORTFOLIO
19.9.4 RECENT DEVELOPMENT
19.9.4.1 AGREEMENT
19.1 TEVA PHARMACEUTICAL INDUSTRIES LTD
19.10.1 COMPANY SNAPSHOT
19.10.2 REVENUE ANALYSIS
19.10.3 PRODUCT PORTFOLIO
19.10.4 RECENT DEVELOPMENT
19.11 ECKERT & ZIEGLER
19.11.1 COMPANY SNAPSHOT
19.11.2 REVENUE ANALYSIS
19.11.3 PRODUCT PORTFOLIO
19.11.4 RECENT DEVELOPMENT
19.12 ACCORD HEALTHCARE
19.12.1 COMPANY SNAPSHOT
19.12.2 PRODUCT PORTFOLIO
19.12.3 RECENT DEVELOPMENT
19.13 ANGIOCHEM
19.13.1 COMPANY SNAPSHOT
19.13.2 PRODUCT PORTFOLIO
19.13.3 RECENT DEVELOPMENT
19.13.3.1 AGREMEENT
19.14 ANI PHARMACEUTICALS, INC.
19.14.1 COMPANY SNAPSHOT
19.14.2 REVENUE ANALYSIS
19.14.3 PRODUCT PORTFOLIO
19.14.4 RECENT DEVELOPMENTS
19.14.4.1 ACQUISITION
19.15 ARBOR PHARMACEUTICALS, LLC. A SUBSIDIARY OF AZURITY PHARMACEUTICALS, INC.
19.15.1 COMPANY SNAPSHOT
19.15.2 PRODUCT PORTFOLIO
19.15.3 RECENT DEVELOPMENT
19.15.3.1 ACQUISITION
19.15.3.2 PRODUCT APPROVAL
19.16 ASTRAZENECA
19.16.1 COMPANY SNAPSHOT
19.16.2 REVENUE ANALYSIS
19.16.3 PRODUCT PORTFOLIO
19.16.4 RECENT DEVELOPMENT
19.16.4.1 AGREEMENT
19.17 CANTEX PHARMACEUTICALS, INC.
19.17.1 COMPANY SNAPSHOT
19.17.2 PRODUCT PORTFOLIO
19.17.3 RECENT DEVELOPMENT
19.18 CELON LABS
19.18.1 COMPANY SNAPSHOT
19.18.2 PRODUCT PORTFOLIO
19.18.3 RECENT DEVELOPMENT
19.19 DIFFUSION PHARMACEUTICAL
19.19.1 COMPANY SNAPSHOT
19.19.2 SERVICES PORTFOLIO
19.19.3 RECENT DEVELOPMENT
19.2 ERC.SA
19.20.1 COMPANY SNAPSHOT
19.20.2 PRODUCT PORTFOLIO
19.20.3 RECENT DEVELOPMENT
19.20.3.1 PIPELINE UPDATE
19.21 ENGENEIC
19.21.1 COMPANY SNAPSHOT
19.21.2 PRODUCT PORTFOLIO
19.21.3 RECENT DEVELOPMENTS
19.21.3.1 AWARDS
19.22 GENENTA SCIENCE
19.22.1 COMPANY SNAPSHOT
19.22.2 PRODUCT PORTFOLIO
19.22.3 RECENT DEVELOPMENT
19.22.3.1 EVENT
19.23 JAZZ PHARMACEUTICALS, INC.
19.23.1 COMPANY SNAPSHOT
19.23.2 REVENUE ANALYSIS
19.23.3 PRODUCT PORTFOLIO
19.23.4 RECENT DEVELOPMENT
19.23.4.1 ACQUISITION
19.24 LOXO ONCOLOGY (A SUBSIDIARY OF ELI LILLY)
19.24.1 COMPANY SNAPSHOT
19.24.2 PRODUCT PORTFOLIO
19.24.3 RECENT DEVELOPMENT
19.25 NOVARTIS AG
19.25.1 COMPANY SNAPSHOT
19.25.2 REVENUE ANALYSIS
19.25.3 PRODUCT PORTFOLIO
19.25.4 RECENT DEVELOPMENT
19.26 VBL THERAPEUTICS
19.26.1 COMPANY SNAPSHOT
19.26.2 PRODUCT PORTFOLIO
19.26.3 RECENT DEVELOPMENT
19.26.3.1 EVENT
19.26.3.2 AWARD
19.27 VIATRIS INC
19.27.1 COMPANY SNAPSHOT
19.27.2 REVENUE ANALYSIS
19.27.3 PRODUCT PORTFOLIO
19.27.4 RECENT DEVELOPMENT
19.27.4.1 AGREEMENT
19.28 ZYDUS PHARMACEUTICALS, INC.
19.28.1 COMPANY SNAPSHOT
19.28.2 PRODUCT PORTFOLIO
19.28.3 RECENT DEVELOPMENTS
20 QUESTIONNAIRE
21 RELATED REPORTS
표 목록
TABLE 1 PIPELINE ANALYSIS FOR GLIOBLASTOMA MULTIFORME TREATMENT MARKET
TABLE 2 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 3 NORTH AMERICA PRIMARY (DE NOVO) IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 4 NORTH AMERICA SECONDARY IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 5 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 6 NORTH AMERICA SURGERY IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 7 NORTH AMERICA RADIOTHERAPY IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 8 NORTH AMERICA RADIOTHERAPY IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 9 NORTH AMERICA MEDICATIONS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 10 NORTH AMERICA MEDICATIONS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 11 NORTH AMERICA TEMOZOLOMIDE IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 12 NORTH AMERICA NITROSOUREAS DRUGS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 13 NORTH AMERICA CARMUSTINE IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 14 NORTH AMERICA TARGETED THERAPY IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 15 NORTH AMERICA ANTI-EPILEPTICS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 16 NORTH AMERICA CORTICOSTEROIDS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 17 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 18 NORTH AMERICA ADULTS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 19 NORTH AMERICA ADULT IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 20 NORTH AMERICA GERIATRIC IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 21 NORTH AMERICA GERIATRIC IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 22 NORTH AMERICA CHILD IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 23 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY DRUG TYPE, 2020-2029 (USD MILLION)
TABLE 24 NORTH AMERICA GENERICS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 25 NORTH AMERICA BRANDED IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 26 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD MILLION)
TABLE 27 NORTH AMERICA PARENTERAL IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 28 NORTH AMERICA ORAL IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 29 NORTH AMERICA ORAL IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD MILLION)
TABLE 30 NORTH AMERICA OTHERS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 31 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 32 NORTH AMERICA HOSPITAL IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 33 NORTH AMERICA CLINICS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 34 NORTH AMERICA HOME HEALTHCARE IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 35 NORTH AMERICA OTHERS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 36 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 37 NORTH AMERICA HOSPITAL PHARMACY IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 38 NORTH AMERICA RETAIL PHARMACY IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 39 NORTH AMERICA ONLINE PHARMACY IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 40 NORTH AMERICA OTHERS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 41 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY COUNTRY, 2020-2029 (USD MILLION)
TABLE 42 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 43 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 44 NORTH AMERICA RADIOTHERAPY IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 45 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 46 NORTH AMERICA TEMOZOLOMIDE IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 47 NORTH AMERICA NITROSOUREAS DRUGS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 48 NORTH AMERICA CARMUSTINE IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 49 NORTH AMERICA TARGETED THERAPY IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 50 NORTH AMERICA ANTI-EPILEPTICS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 51 NORTH AMERICA CORTICOSTEROIDS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 52 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 53 NORTH AMERICA ADULT IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 54 NORTH AMERICA GERIATRIC IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 55 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY DRUG TYPE, 2020-2029 (USD MILLION)
TABLE 56 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD MILLION)
TABLE 57 NORTH AMERICA ORAL IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD MILLION)
TABLE 58 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 59 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 60 U.S. GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 61 U.S. GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 62 U.S. RADIOTHERAPY IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 63 U.S. GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 64 U.S. TEMOZOLOMIDE IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 65 U.S. NITROSOUREAS DRUGS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 66 U.S. CARMUSTINE IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 67 U.S. TARGETED THERAPY IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 68 U.S. ANTI-EPILEPTICS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 69 U.S. CORTICOSTEROIDS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 70 U.S. GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 71 U.S. ADULT IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 72 U.S. GERIATRIC IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 73 U.S. GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY DRUG TYPE, 2020-2029 (USD MILLION)
TABLE 74 U.S. GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD MILLION)
TABLE 75 U.S. ORAL IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD MILLION)
TABLE 76 U.S. GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 77 U.S. GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 78 CANADA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 79 CANADA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 80 CANADA RADIOTHERAPY IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 81 CANADA MEDICATIONS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 82 CANADA TEMOZOLOMIDE IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 83 CANADA NITROSOUREAS DRUGS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 84 CANADA CARMUSTINE IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 85 CANADA TARGETED THERAPY IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 86 CANADA ANTI-EPILEPTICS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 87 CANADA CORTICOSTEROIDS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 88 CANADA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 89 CANADA ADULT IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 90 CANADA GERIATRIC IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 91 CANADA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY DRUG TYPE, 2020-2029 (USD MILLION)
TABLE 92 CANADA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD MILLION)
TABLE 93 CANADA ORAL IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD MILLION)
TABLE 94 CANADA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 95 CANADA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 96 MEXICO GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 97 MEXICO GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 98 MEXICO RADIOTHERAPY IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 99 MEXICO MEDICATIONS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 100 MEXICO TEMOZOLOMIDE IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 101 MEXICO NITROSOUREAS DRUGS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 102 MEXICO CARMUSTINE IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 103 MEXICO TARGETED THERAPY IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 104 MEXICO ANTI-EPILEPTICS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 105 MEXICO CORTICOSTEROIDS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 106 MEXICO GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 107 MEXICO ADULT IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 108 MEXICO GERIATRIC IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 109 MEXICO GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY DRUG TYPE, 2020-2029 (USD MILLION)
TABLE 110 MEXICO GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD MILLION)
TABLE 111 MEXICO ORAL IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD MILLION)
TABLE 112 MEXICO GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 113 MEXICO GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
그림 목록
FIGURE 1 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: SEGMENTATION
FIGURE 2 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET : DATA TRIANGULATION
FIGURE 3 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: DROC ANALYSIS
FIGURE 4 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: NORTH AMERICA VS REGIONAL MARKET ANALYSIS
FIGURE 5 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: MULTIVARIATE MODELLING
FIGURE 7 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 8 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: DBMR MARKET POSITION GRID
FIGURE 9 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: MARKET END USER COVERAGE GRID
FIGURE 10 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: VENDOR SHARE ANALYSIS
FIGURE 11 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: SEGMENTATION
FIGURE 12 NORTH AMERICA IS EXPECTED TO DOMINATE AND ASIA-PACIFIC IS GROWING AT THE FASTEST PACE IN NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET IN THE FORECAST PERIOD OF 2022 TO 2029
FIGURE 13 INCREASE IN THE PREVALENCE OF GLIOBLASTOMA MULTIFORME AND INCREASE IN PIPELINE PRODUCTS ARE DRIVING THE NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET IN THE FORECAST PERIOD OF 2022 TO 2029
FIGURE 14 PRIMARY (DE NOVO) SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET IN 2022 & 2029
FIGURE 15 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF THE NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET
FIGURE 16 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY TYPE, 2021
FIGURE 17 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY TYPE, 2022-2029 (USD MILLION)
FIGURE 18 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY TYPE, CAGR (2022-2029)
FIGURE 19 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY TYPE, LIFELINE CURVE
FIGURE 20 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY TREATMENT, 2021
FIGURE 21 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY TREATMENT, 2022-2029 (USD MILLION)
FIGURE 22 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT: BY TREATMENT, CAGR (2022-2029)
FIGURE 23 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT: BY TREATMENT, LIFELINE CURVE
FIGURE 24 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY PATIENT TYPE, 2021
FIGURE 25 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY PATIENT TYPE, 2022-2029 (USD MILLION)
FIGURE 26 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY PATIENT TYPE, CAGR (2022-2029)
FIGURE 27 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY PATIENT TYPE, LIFELINE CURVE
FIGURE 28 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY DRUG TYPE, 2021
FIGURE 29 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY DRUG TYPE, 2022-2029 (USD MILLION)
FIGURE 30 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY DRUG TYPE, CAGR (2022-2029)
FIGURE 31 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY DRUG TYPE, LIFELINE CURVE
FIGURE 32 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY ROUTE OF ADMINISTRATION, 2021
FIGURE 33 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY ROUTE OF ADMINISTRATION, 2022-2029 (USD MILLION)
FIGURE 34 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY ROUTE OF ADMINISTRATION, CAGR (2022-2029)
FIGURE 35 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY ROUTE OF ADMINISTRATION, LIFELINE CURVE
FIGURE 36 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY END USER, 2021
FIGURE 37 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY END USER, 2022-2029 (USD MILLION)
FIGURE 38 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY END USER, CAGR (2022-2029)
FIGURE 39 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY END USER, LIFELINE CURVE
FIGURE 40 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY DISTRIBUTION CHANNEL, 2021
FIGURE 41 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY DISTRIBUTION CHANNEL, 2022-2029 (USD MILLION)
FIGURE 42 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY DISTRIBUTION CHANNEL, CAGR (2022-2029)
FIGURE 43 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 44 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: SNAPSHOT (2021)
FIGURE 45 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY COUNTRY (2021)
FIGURE 46 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY COUNTRY (2022 & 2029)
FIGURE 47 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY COUNTRY (2021 & 2029)
FIGURE 48 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY TYPE (2022-2029)
FIGURE 49 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: COMPANY SHARE 2021 (%)

연구 방법론
데이터 수집 및 기준 연도 분석은 대규모 샘플 크기의 데이터 수집 모듈을 사용하여 수행됩니다. 이 단계에는 다양한 소스와 전략을 통해 시장 정보 또는 관련 데이터를 얻는 것이 포함됩니다. 여기에는 과거에 수집한 모든 데이터를 미리 검토하고 계획하는 것이 포함됩니다. 또한 다양한 정보 소스에서 발견되는 정보 불일치를 검토하는 것도 포함됩니다. 시장 데이터는 시장 통계 및 일관된 모델을 사용하여 분석하고 추정합니다. 또한 시장 점유율 분석 및 주요 추세 분석은 시장 보고서의 주요 성공 요인입니다. 자세한 내용은 분석가에게 전화를 요청하거나 문의 사항을 드롭하세요.
DBMR 연구팀에서 사용하는 주요 연구 방법론은 데이터 마이닝, 시장에 대한 데이터 변수의 영향 분석 및 주요(산업 전문가) 검증을 포함하는 데이터 삼각 측량입니다. 데이터 모델에는 공급업체 포지셔닝 그리드, 시장 타임라인 분석, 시장 개요 및 가이드, 회사 포지셔닝 그리드, 특허 분석, 가격 분석, 회사 시장 점유율 분석, 측정 기준, 글로벌 대 지역 및 공급업체 점유율 분석이 포함됩니다. 연구 방법론에 대해 자세히 알아보려면 문의를 통해 업계 전문가에게 문의하세요.
사용자 정의 가능
Data Bridge Market Research는 고급 형성 연구 분야의 선두 주자입니다. 저희는 기존 및 신규 고객에게 목표에 맞는 데이터와 분석을 제공하는 데 자부심을 느낍니다. 보고서는 추가 국가에 대한 시장 이해(국가 목록 요청), 임상 시험 결과 데이터, 문헌 검토, 재생 시장 및 제품 기반 분석을 포함하도록 사용자 정의할 수 있습니다. 기술 기반 분석에서 시장 포트폴리오 전략에 이르기까지 타겟 경쟁업체의 시장 분석을 분석할 수 있습니다. 귀하가 원하는 형식과 데이터 스타일로 필요한 만큼 많은 경쟁자를 추가할 수 있습니다. 저희 분석가 팀은 또한 원시 엑셀 파일 피벗 테이블(팩트북)로 데이터를 제공하거나 보고서에서 사용 가능한 데이터 세트에서 프레젠테이션을 만드는 데 도움을 줄 수 있습니다.