중동 및 아프리카 IVD 규제 업무 아웃소싱 시장, 서비스별(규제 작성 및 제출, 규제 등록 및 임상 시험 신청, 규제 컨설팅, 법률 대리, 데이터 관리 서비스, 화학 제조 및 제어(CMC) 서비스 및 기타), 적응증(종양학, 신경학 , 심장학, 임상 화학 및 면역 검정, 정밀 의학 , 감염성 질환, 당뇨병, 유전자 검사, HIV/AIDS, 혈액학, 약물 검사/약리유전체학, 수혈, 진료 시점 및 기타), 배포 모델(클라우드 및 온프레미스), 조직 규모(중소기업(SMES) 및 대기업), 단계(임상, 전임상 및 PMA(시판 후 허가)), 클래스(클래스 I, 클래스 II 및 클래스 III), 최종 사용자(제약 회사, 의료 기기 회사, 생명 공학 회사 및 기타), 국가(남아프리카, 사우디 아라비아, UAE, 이집트, 이스라엘, 기타 중동 및 아프리카 지역) 산업 동향 및 2029년 전망.
시장 분석 및 통찰력 : 중동 및 아프리카 IVD 규제 업무 아웃소싱 시장
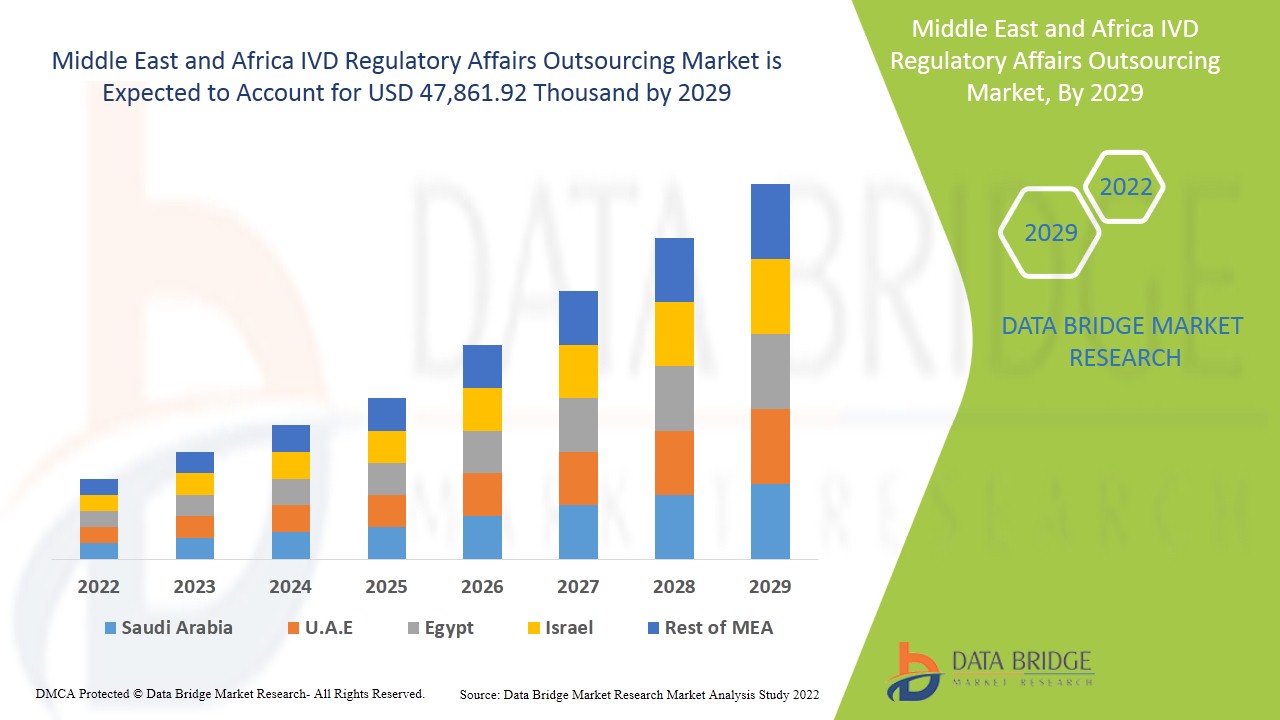
중동 및 아프리카 IVD 규제 업무 아웃소싱 시장은 2022년부터 2029년까지의 예측 기간 동안 시장 성장을 이룰 것으로 예상됩니다. Data Bridge Market Research는 시장이 2022년부터 2029년까지의 예측 기간 동안 11.6%의 CAGR로 성장하고 있으며 2029년까지 47,861.92천 달러에 도달할 것으로 예상한다고 분석합니다.
- 체외 진단 제품은 질병이나 기타 상태를 진단하는 데 사용되는 시약, 장치 및 시스템으로, 질병을 치료, 완화, 치료 또는 예방하기 위해 건강 상태를 판단하는 것을 포함합니다. 이러한 제품은 인체 표본을 수집, 준비 및 검사하는 데 사용하도록 의도되었습니다. 규제 업무는 체외진단 장치(IVD) 및 의료 기기 산업에서 중요한 역할을 합니다. 규제 업무 아웃소싱 서비스에는 임상 연구 프로젝트를 위한 고품질 문서 제작에 기여하는 전문가가 의료 문서를 작성하고 규제 문서를 발행하는 것이 포함됩니다. 신흥 경제권에서 수행되는 임상 연구에서 규제 서비스 아웃소싱에 대한 수요가 크게 증가하고 있어 이 산업의 성장을 위한 건강한 플랫폼을 제공합니다.
IVD 규제 업무 아웃소싱 시장 성장을 주도하는 주요 요인은 지역 전체의 만성 질환 유병률 증가와 다양한 체외 진단 기기의 기술 발전입니다. 조직 간의 전략적 인수 및 파트너십 증가는 시장 성장의 기회를 창출하고 있습니다. 다양한 지역의 의료 기기에 대한 규정 변경은 IVD 규제 업무 아웃소싱 시장의 주요 제약으로 작용하고 있습니다. 의료 서비스 인프라 부족은 시장 성장의 주요 과제로 작용하고 있습니다.
이 IVD 규제 업무 아웃소싱 시장 보고서는 시장 점유율, 새로운 개발 및 제품 파이프라인 분석, 국내 및 지역 시장 참여자의 영향, 새로운 수익 주머니, 시장 규정의 변화, 제품 승인, 전략적 의사 결정, 제품 출시, 지리적 확장 및 시장의 기술 혁신 측면에서의 분석 기회에 대한 세부 정보를 제공합니다. 분석 및 시장 시나리오를 이해하려면 분석가 브리핑을 위해 저희에게 연락하세요. 저희 팀은 원하는 목표를 달성하기 위한 수익 영향 솔루션을 만드는 데 도움을 드릴 것입니다.
중동 및 아프리카 IVD 규제 업무 아웃소싱 시장 범위 및 시장 규모
중동 및 아프리카 IVD 규제 업무 아웃소싱 시장은 서비스, 적응증, 배포 모드, 조직 규모, 단계, 계층 및 최종 사용자를 기준으로 7개의 주요 세그먼트로 구분됩니다.
- 중동 및 아프리카 IVD 규제 업무 아웃소싱 시장은 서비스 기준으로 규제 작성 및 제출, 규제 등록 및 임상 시험 신청, 규제 컨설팅, 법률 대리, 데이터 관리 서비스, 화학 제조 및 통제(CMC) 서비스 등으로 세분화됩니다. 2022년에는 규제 작성 및 제출이 비용 효율적이고 IVD 규제에서 클라우드 컴퓨팅 기술의 솔루션 유연한 특성이 IVD 시스템 거래 조직에 최대 출력을 제공하는 안정적인 인프라를 제공하기 때문에 지배적일 것으로 예상됩니다.
- 중동 및 아프리카 IVD 규제 업무 아웃소싱 시장은 지표에 따라 종양학, 신경학, 심장학, 임상 화학 및 면역 검사, 정밀 의학, 감염성 질환, 당뇨병, 유전자 검사, HIV/AIDS, 혈액학, 약물 검사/약리유전체학, 수혈, 진료 시점 및 기타로 세분화됩니다. 2022년에는 종양학 부문이 종양학 약물 개발 프로세스의 예측 가능성을 개선하고 환자의 치료 선택과 관련하여 종양학자에게 중요한 도구가 되므로 지배적일 것으로 예상됩니다.
- 배포 모드를 기준으로 중동 및 아프리카 IVD 규제 업무 아웃소싱 시장은 클라우드와 온프레미스로 세분화됩니다. 2022년에는 클라우드 세그먼트가 지배적일 것으로 예상됩니다. 클라우드 컴퓨팅 기술의 유연한 특성은 IVD 규제에서 비용 효율적이며 IVD 시스템 거래 조직에 최대 출력을 제공하는 안정적인 인프라를 제공하기 때문입니다.
- 조직 규모에 따라 중동 및 아프리카 IVD 규제 업무 아웃소싱 시장은 중소기업(SMES)과 대기업으로 세분화됩니다. 2022년에는 조직 간 전략적 인수 및 파트너십이 증가함에 따라 대기업 부문이 우세할 것으로 예상됩니다.
- 중동 및 아프리카 IVD 규제 업무 아웃소싱 시장은 단계에 따라 임상, 전임상 및 PMA(시판 후 허가)로 세분화됩니다. 2022년에는 다양한 효율적인 기술 서비스와 표준이 등장하면서 임상 부문이 시장을 지배할 것으로 예상됩니다.
- 클래스 기준으로 중동 및 아프리카 IVD 규제 업무 아웃소싱 시장은 클래스 I, 클래스 II, 클래스 III로 세분화됩니다. 2022년에는 클래스 I 세그먼트가 시장을 지배할 것으로 예상되며, 이는 공공 보건 위험이 없거나 개인 위험이 낮고 규제가 가장 낮기 때문입니다. 이러한 세그먼트는 수요가 높고 의료 기기의 47%가 이 범주에 속합니다.
- 최종 사용자를 기준으로 중동 및 아프리카 IVD 규제 업무 아웃소싱 시장은 제약 회사, 의료 기기 회사, 생명 공학 회사 등으로 세분화됩니다. 2022년에는 의료 기기 회사가 시장을 지배할 것으로 예상되며, 의료 산업은 연구 개발에 지출하는 금액 면에서 모든 산업 중 2위를 차지합니다. 증가하는 의료 지출로 인해 연구 개발 기회가 더 잘 제공되었습니다.
중동 및 아프리카 IVD 규제 업무 아웃소싱 시장 국가 수준 분석
중동 및 아프리카 IVD 규제 업무 아웃소싱 시장을 분석하고, 국가, 서비스, 적응증, 배포 모드, 조직 규모, 단계, 계층 및 최종 사용자별로 시장 규모 정보를 제공합니다.
중동 및 아프리카 IVD 규제 업무 아웃소싱 시장 보고서에서 다루는 국가는 남아프리카공화국, 사우디아라비아, UAE, 이집트, 이스라엘, 기타 중동 및 아프리카 국가입니다.
남아프리카공화국은 중동과 아프리카 지역 시장을 장악하고 있으며, 이 지역 전역의 헬스케어 부문과 연구개발 활동에서 기술이 크게 발전했습니다.
보고서의 국가 섹션은 또한 개별 시장 영향 요인과 국내 시장의 현재 및 미래 트렌드에 영향을 미치는 규제 변화를 제공합니다. 신규 판매, 교체 판매, 국가 인구 통계, 규제 조치 및 수출입 관세와 같은 데이터 포인트는 개별 국가의 시장 시나리오를 예측하는 데 사용되는 주요 포인터 중 일부입니다. 또한 글로벌 브랜드의 존재 및 가용성과 지역 및 국내 브랜드와의 대규모 또는 희소한 경쟁으로 인해 직면한 과제, 판매 채널의 영향은 국가 데이터에 대한 예측 분석을 제공하는 동안 고려됩니다.
IVD 규제 업무 아웃소싱에 대한 수요 증가
중동 및 아프리카 IVD 규제 업무 아웃소싱 시장은 또한 모든 국가에 대한 자세한 시장 분석을 제공하여 산업의 매출, 구성 요소 매출, IVD 규제 업무 아웃소싱의 기술 개발 영향 및 IVD 규제 업무 아웃소싱 시장에 대한 지원과 함께 규제 시나리오의 변화를 제공합니다. 이 데이터는 2012년부터 2020년까지의 과거 기간에 대해 제공됩니다.
경쟁 환경 및 중동 및 아프리카 IVD 규제 업무 아웃소싱 시장 점유율 분석
중동 및 아프리카 IVD 규제 업무 아웃소싱 시장 경쟁 구도는 경쟁자별 세부 정보를 제공합니다. 포함된 세부 정보에는 회사 개요, 회사 재무, 창출된 수익, 시장 잠재력, 연구 개발 투자, 새로운 시장 이니셔티브, 글로벌 입지, 생산 현장 및 시설, 회사의 강점과 약점, 제품 출시, 제품 시험 파이프라인, 제품 승인, 특허, 제품 폭과 폭, 응용 분야 우세, 기술 수명선 곡선이 있습니다. 위에 제공된 데이터 포인트는 중동 및 아프리카 IVD 규제 업무 아웃소싱 시장과 관련된 회사의 초점에만 관련이 있습니다.
보고서에서 활동하는 주요 업체 중 일부는 IVD 규제 업무 아웃소싱 시장으로 Freyr Solutions, PPD Inc.(Thremofisher Scientific Inc.의 자회사), EMERGO, ICON, Parexel International Corporation, CRITERIUM, INC., Groupe ProductLife SA, Labcorp Drug Development, WuXi AppTec, Genpact, Medpace, Dor Pharmaceutical Services, Qserve 등이 있습니다. DBMR 분석가는 경쟁 우위를 이해하고 각 경쟁사에 대한 경쟁 분석을 별도로 제공합니다.
전 세계 여러 회사에서 많은 제품 개발을 시작하면서 중동과 아프리카의 IVD 규제 업무 아웃소싱 시장 성장도 가속화되고 있습니다.
예를 들어,
- 2021년 11월, USA-9 Technology Magazine은 Freyr를 "2021년 최고의 기술 솔루션 공급업체 10곳"에 선정했습니다. USA-9.com은 선도적인 글로벌 규제 솔루션 및 서비스 공급업체인 Freyr Solutions를 "2021년 최고의 기술 솔루션 공급업체 10곳"으로 선정한 기술 잡지로, Fryer는 혁신적인 소프트웨어 솔루션을 설계하고 고객이 각자의 규정 준수 목표를 달성할 수 있도록 지원합니다. 이를 통해 회사는 인기를 높일 수 있었습니다.
- 2021년 12월, Labcorp Drug Development는 동급 최고의 비임상 시험 서비스를 제공하는 계약 연구 기관인 Toxikon Corporation을 인수했습니다. Labcorp Drug Development에 Toxikon이 추가되면서 Labcorp의 강력한 비임상 개발 포트폴리오가 강화되었고, 보스턴, 매사추세츠 지역의 제약 및 생명공학 고객과 협력할 수 있는 전략적 발자취가 생겼습니다. 이를 통해 회사는 사업을 확장할 수 있었습니다.
파트너십, 합작 투자 및 기타 전략은 적용 범위와 입지를 확대하여 회사 시장 점유율을 높입니다. 또한 조직이 규모 범위를 확대하여 IVD 규제 업무 아웃소싱에 대한 제안을 개선할 수 있는 이점도 제공합니다.
SKU-
세계 최초의 시장 정보 클라우드 보고서에 온라인으로 접속하세요
- 대화형 데이터 분석 대시보드
- 높은 성장 잠재력 기회를 위한 회사 분석 대시보드
- 사용자 정의 및 질의를 위한 리서치 분석가 액세스
- 대화형 대시보드를 통한 경쟁자 분석
- 최신 뉴스, 업데이트 및 추세 분석
- 포괄적인 경쟁자 추적을 위한 벤치마크 분석의 힘 활용
목차
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF MIDDLE EAST & AFRICA IVD REGULATORY AFFAIRS OUTSOURCING MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATIONS
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 DBMR TRIPOD DATA VALIDATION MODEL
2.5 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.6 DBMR MARKET POSITION GRID
2.7 VENDOR SHARE ANALYSIS
2.8 MULTIVARIATE MODELING
2.9 SERVICE TIMELINE CURVE
2.1 MARKET END USER COVERAGE GRID
2.11 SECONDARY SOURCES
2.12 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 MIDDLE EAST & AFRICA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, REGULATORY SCENARIO
4.1.1 THE U.S.
4.1.2 REGULATIONS IN EUROPE
4.1.3 REGULATIONS IN ASIA
4.1.3.1 CHINA
4.1.3.2 SOUTH KOREA
4.1.3.3 MALAYSIA
4.1.3.4 THAILAND
5 MARKET OVERVIEW
5.1 DRIVERS
5.1.1 RISE IN PREVALENCE OF CHRONIC DISEASES ACROSS THE REGION
5.1.2 TECHNOLOGICAL ADVANCEMENT IN DEVELOPING VARIOUS IN VITRO DIAGNOSTIC DEVICES
5.1.3 DEVELOPMENT OF PROJECT-BASED SUPPORT LEADS TO LONG TERM OUTSOURCING AGREEMENT AMONG ORGANIZATION
5.1.4 INCREASE IN PRODUCT REGISTRATION NUMBERS AND CLINICAL TRIAL APPROVALS ACROSS THE REGION
5.2 RESTRAINTS
5.2.1 STRINGENT REGULATIONS REGARDING MEDICAL DEVICES IN DIFFERENT REGIONS
5.2.2 HIGHER COST RELATED TO MAINTENANCE AND OUTSOURCING OF IVD
5.3 OPPORTUNITIES
5.3.1 RISE IN STRATEGIC ACQUISITION & PARTNERSHIP AMONG ORGANIZATION
5.3.2 EMERGENCE OF VARIOUS EFFICIENT TECHNOLOGICAL SERVICES AND STANDARDS
5.3.3 INCREASE IN R&D ACTIVITIES BY COMPANIES ACROSS THE REGION
5.4 CHALLENGES
5.4.1 LACK OF INFRASTRUCTURE IN HEALTHCARE SERVICE
5.4.2 SHORTAGE OF SKILLED PERSONNEL FOR HANDLING IN VITRO DIAGNOSTIC DEVICES
6 MIDDLE EAST & AFRICA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE
6.1 OVERVIEW
6.2 REGULATORY WRITING & SUBMISSIONS
6.3 LEGAL REPRESENTATION
6.4 REGULATORY CONSULTING
6.5 REGULATORY REGISTRATION & CLINICAL TRIAL APPLICATIONS
6.6 CHEMISTRY MANUFACTURING AND CONTROLS (CMC) SERVICES
6.7 DATA MANAGEMENT SERVICES
6.8 OTHERS
7 MIDDLE EAST & AFRICA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY INDICATION
7.1 OVERVIEW
7.2 CLINICAL CHEMISTRY AND IMMUNOASSAYS
7.3 INFECTIOUS DISEASES
7.3.1 VIROLOGY
7.3.2 MICROBIOLOGY AND MYCOLOGY
7.3.3 BACTERIOLOGY
7.3.4 SEPSIS
7.3.5 HEPATITIS B
7.3.6 HEPATITIS C
7.3.7 MALARIA
7.3.8 TUBERCULOSIS
7.3.9 SYPHILIS
7.3.10 HUMAN PAPILLOMAVIRUS (HPV) INFECTION
7.3.11 OTHERS
7.4 HAEMATOLOGY
7.5 DRUG TESTING/PHARMACOGENOMICS
7.6 PRECISION MEDICINE
7.7 DIABETES
7.8 BLOOD TRANSFUSION
7.9 CARDIOLOGY
7.1 POINT OF CARE
7.10.1 WAIVED TEST
7.10.2 AT HOME TESTS
7.11 ONCOLOGY
7.12 NEUROLOGY
7.13 HIV/AIDS
7.14 GENETIC TESTING
7.15 OTHERS
8 MIDDLE EAST & AFRICA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY CLASS
8.1 OVERVIEW
8.2 CLASS I
8.3 CLASS III
8.4 CLASS II
9 MIDDLE EAST & AFRICA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY DEPLOYMENT MODE
9.1 OVERVIEW
9.2 CLOUD
9.3 ON-PREMISES
10 MIDDLE EAST & AFRICA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE
10.1 OVERVIEW
10.2 LARGE ENTERPRISES
10.3 SMALL & MEDIUM ENTERPRISES (SMES)
11 MIDDLE EAST & AFRICA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY STAGE
11.1 OVERVIEW
11.2 CLINICAL
11.3 PRECLINICAL
11.4 PMA (POST MARKET AUTHORIZATION)
12 MIDDLE EAST & AFRICA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY END USER
12.1 OVERVIEW
12.2 MEDICAL DEVICE COMPANIES
12.2.1 BY ORGANIZATION SIZE
12.2.1.1 LARGE ENTERPRISES
12.2.1.2 SMALL & MEDIUM ENTERPRISES (SMES)
12.2.2 BY SERVICE
12.2.2.1 REGULATORY WRITING & SUBMISSIONS
12.2.2.2 LEGAL REPRESENTATION
12.2.2.3 REGULATORY CONSULTING
12.2.2.4 REGULATORY REGISTRATION & CLINICAL TRIAL APPLICATIONS
12.2.2.5 CHEMISTRY MANUFACTURING AND CONTROLS (CMC) SERVICES
12.2.2.6 DATA MANAGEMENT SERVICES
12.2.2.7 OTHERS
12.3 PHARMACEUTICAL COMPANIES
12.3.1 BY ORGANIZATION SIZE
12.3.1.1 LARGE ENTERPRISES
12.3.1.2 SMALL & MEDIUM ENTERPRISES (SMES)
12.3.2 BY SERVICE
12.3.2.1 REGULATORY WRITING & SUBMISSIONS
12.3.2.2 LEGAL REPRESENTATION
12.3.2.3 REGULATORY CONSULTING
12.3.2.4 REGULATORY REGISTRATION & CLINICAL TRIAL APPLICATIONS
12.3.2.5 CHEMISTRY MANUFACTURING AND CONTROLS (CMC) SERVICES
12.3.2.6 DATA MANAGEMENT SERVICES
12.3.2.7 OTHERS
12.4 BIOTECHNOLOGY COMPANIES
12.4.1 BY ORGANIZATION SIZE
12.4.1.1 LARGE ENTERPRISES
12.4.1.2 SMALL & MEDIUM ENTERPRISES (SMES)
12.4.2 BY SERVICE
12.4.2.1 REGULATORY WRITING & SUBMISSIONS
12.4.2.2 LEGAL REPRESENTATION
12.4.2.3 REGULATORY CONSULTING
12.4.2.4 REGULATORY REGISTRATION & CLINICAL TRIAL APPLICATIONS
12.4.2.5 CHEMISTRY MANUFACTURING AND CONTROLS (CMC) SERVICES
12.4.2.6 DATA MANAGEMENT SERVICES
12.4.2.7 OTHERS
12.5 OTHERS
13 MIDDLE EAST & AFRICA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION
13.1 MIDDLE EAST & AFRICA
13.1.1 SOUTH AFRICA
13.1.2 SAUDI ARABIA
13.1.3 U.A.E
13.1.4 EGYPT
13.1.5 ISRAEL
13.1.6 REST OF MIDDLE EAST & AFRICA
14 MIDDLE EAST & AFRICA IVD REGULATORY AFFAIRS OUTSOURCING MARKET: COMPANY LANDSCAPE
14.1 COMPANY SHARE ANALYSIS: MIDDLE EAST & AFRICA
15 SWOT ANALYSIS
16 COMPANY PROFILE
16.1 ICON PLC
16.1.1 COMPANY SNAPSHOT
16.1.2 REVENUE ANALYSIS
16.1.3 COMPANY SHARE ANALYSIS
16.1.4 PRODUCT PORTFOLIO
16.1.5 RECENT DEVELOPMENTS
16.2 MEDPACE
16.2.1 COMPANY SNAPSHOT
16.2.2 REVENUE ANALYSIS
16.2.3 COMPANY SHARE ANALYSIS
16.2.4 PRODUCT PORTFOLIO
16.2.5 RECENT DEVELOPMENTS
16.3 PAREXEL INTERNATIONAL CORPORATION
16.3.1 COMPANY SNAPSHOT
16.3.2 COMPANY SHARE ANALYSIS
16.3.3 PRODUCT PORTFOLIO
16.3.4 RECENT DEVELOPMENTS
16.4 LABCORP DRUG DEVELOPMENT
16.4.1 COMPANY SNAPSHOT
16.4.2 REVENUE ANALYSIS
16.4.3 COMPANY SHARE ANALYSIS
16.4.4 PRODUCT PORTFOLIO
16.4.5 RECENT DEVELOPMENT
16.5 PPD INC. (A SUBSIDIARY OF THERMOFISHER SCIENTIFIC INC.)
16.5.1 COMPANY SNAPSHOT
16.5.2 COMPANY SHARE ANALYSIS
16.5.3 PRODUCT PORTFOLIO
16.5.4 RECENT DEVELOPMENTS
16.6 CHARLES RIVER LABORATORIES
16.6.1 COMPANY SNAPSHOT
16.6.2 REVENUE ANALYSIS
16.6.3 PRODUCT PORTFOLIO
16.6.4 RECENT DEVELOPMENTS
16.7 FREYR
16.7.1 COMPANY SNAPSHOT
16.7.2 PRODUCT PORTFOLIO
16.7.3 RECENT DEVELOPMENTS
16.8 ASSENT COMPLIANCE INC.
16.8.1 COMPANY SNAPSHOT
16.8.2 PRODUCT PORTFOLIO
16.8.3 RECENT DEVELOPMENT
16.9 ANDAMAN MEDICAL
16.9.1 COMPANY SNAPSHOT
16.9.2 PRODUCT PORTFOLIO
16.9.3 RECENT DEVELOPMENT
16.1 ASIA ACTUAL
16.10.1 COMPANY SNAPSHOT
16.10.2 SERVICE PORTFOLIO
16.10.3 RECENT DEVELOPMENTS
16.11 AXSOURCE
16.11.1 COMPANY SNAPSHOT
16.11.2 PRODUCT PORTFOLIO
16.11.3 RECENT DEVELOPMENTS
16.12 CRITERIUM, INC.
16.12.1 COMPANY SNAPSHOT
16.12.2 PRODUCT PORTFOLIO
16.12.3 RECENT DEVELOPMENT
16.13 DOR PHARMACEUTICAL SERVICES
16.13.1 COMPANY SNAPSHOT
16.13.2 SERVICE PORTFOLIO
16.13.3 RECENT DEVELOPMENTS
16.14 EMERGO BY UL
16.14.1 COMPANY SNAPSHOT
16.14.2 PRODUCT PORTFOLIO
16.14.3 RECENT DEVELOPMENTS
16.15 GENPACT
16.15.1 COMPANY SNAPSHOT
16.15.2 REVENUE ANALYSIS
16.15.3 PRODUCT PORTFOLIO
16.15.4 RECENT DEVELOPMENTS
16.16 GROUPE PRODUCTLIFE S.A.
16.16.1 COMPANY SNAPSHOT
16.16.2 PRODUCT PORTFOLIO
16.16.3 RECENT DEVELOPMENTS
16.17 LORENZ LIFE SCIENCES GROUP
16.17.1 COMPANY SNAPSHOT
16.17.2 PRODUCT PORTFOLIO
16.17.3 RECENT DEVELOPMENTS
16.18 MAKROCARE
16.18.1 COMPANY SNAPSHOT
16.18.2 PRODUCT PORTFOLIO
16.18.3 RECENT DEVELOPMENTS
16.19 MARACA INTERNATIONAL BVBA
16.19.1 COMPANY SNAPSHOT
16.19.2 SERVICE PORTFOLIO
16.19.3 RECENT DEVELOPMENTS
16.2 MDICONSULTANTS, INC.
16.20.1 COMPANY SNAPSHOT
16.20.2 PRODUCT PORTFOLIO
16.20.3 RECENT DEVELOPMENTS
16.21 PBC BIOMED
16.21.1 COMPANY SNAPSHOT
16.21.2 PRODUCT PORTFOLIO
16.21.3 RECENT DEVELOPMENT
16.22 PROMEDICA INTERNATIONAL, A CALIFORNIA CORPORATION
16.22.1 COMPANY SNAPSHOT
16.22.2 PRODUCT PORTFOLIO
16.22.3 RECENT DEVELOPMENT
16.23 PROPHARMA GROUP
16.23.1 COMPANY SNAPSHOT
16.23.2 PRODUCT PORTFOLIO
16.23.3 RECENT DEVELOPMENTS
16.24 QSERVE
16.24.1 COMPANY SNAPSHOT
16.24.2 SERVICE PORTFOLIO
16.24.3 RECENT DEVELOPMENTS
16.25 REGULATORY COMPLIANCE ASSOCIATES INC.
16.25.1 COMPANY SNAPSHOT
16.25.2 PRODUCT PORTFOLIO
16.25.3 RECENT DEVELOPMENT
16.26 RMQ+
16.26.1 COMPANY SNAPSHOT
16.26.2 PRODUCT PORTFOLIO
16.26.3 RECENT DEVELOPMENT
16.27 SARACA SOLUTIONS PRIVATE LIMITED
16.27.1 COMPANY SNAPSHOT
16.27.2 PRODUCT PORTFOLIO
16.27.3 RECENT DEVELOPMENT
16.28 VCLS
16.28.1 COMPANY SNAPSHOT
16.28.2 PRODUCT PORTFOLIO
16.28.3 RECENT DEVELOPMENTS
16.29 WUXI APPTEC
16.29.1 COMPANY SNAPSHOT
16.29.2 REVENUE ANALYSIS
16.29.3 PRODUCT PORTFOLIO
16.29.4 RECENT DEVELOPMENTS
17 QUESTIONNAIRE
18 RELATED REPORTS
표 목록
TABLE 1 MIDDLE EAST & AFRICA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 2 MIDDLE EAST & AFRICA REGULATORY WRITING & SUBMISSIONS IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 3 MIDDLE EAST & AFRICA LEGAL REPRESENTATION IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 4 MIDDLE EAST & AFRICA REGULATORY CONSULTING IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 5 MIDDLE EAST & AFRICA REGULATORY REGISTRATION & CLINICAL TRIAL APPLICATIONS IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 6 MIDDLE EAST & AFRICA CHEMISTRY MANUFACTURING AND CONTROLS (CMC) SERVICES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 7 MIDDLE EAST & AFRICA DATA MANAGEMENT SERVICES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY MATERIAL, 2020-2029 (USD THOUSAND)
TABLE 8 MIDDLE EAST & AFRICA OTHERS IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 9 MIDDLE EAST & AFRICA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY INDICATION, 2020-2029 (USD THOUSAND)
TABLE 10 MIDDLE EAST & AFRICA CLINICAL CHEMISTRY AND IMMUNOASSAYS IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 11 MIDDLE EAST & AFRICA INFECTIOUS DISEASES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION,2020-2029 (THOUSAND)
TABLE 12 MIDDLE EAST & AFRICA INFECTIOUS DISEASES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY TYPE, 2020-2029 (USD THOUSAND)
TABLE 13 MIDDLE EAST & AFRICA HAEMATOLOGY IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 14 MIDDLE EAST & AFRICA DRUG TESTING/PHARMACOGENOMICS IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (THOUSAND)
TABLE 15 MIDDLE EAST & AFRICA PRECISION MEDICINE IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (THOUSAND)
TABLE 16 MIDDLE EAST & AFRICA DIABETES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (THOUSAND)
TABLE 17 MIDDLE EAST & AFRICA BLOOD TRANSFUSION IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (THOUSAND)
TABLE 18 MIDDLE EAST & AFRICA CARDIOLOGY IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (THOUSAND)
TABLE 19 MIDDLE EAST & AFRICA POINT OF CARE IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (THOUSAND)
TABLE 20 MIDDLE EAST & AFRICA POINT OF CARE IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY TYPE, 2020-2029 (USD THOUSAND)
TABLE 21 MIDDLE EAST & AFRICA ONCOLOGY IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (THOUSAND)
TABLE 22 MIDDLE EAST & AFRICA NEUROLOGY IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (THOUSAND)
TABLE 23 MIDDLE EAST & AFRICA HIV/AIDS IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (THOUSAND)
TABLE 24 MIDDLE EAST & AFRICA GENETIC TESTING IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (THOUSAND)
TABLE 25 MIDDLE EAST & AFRICA OTHERS IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (THOUSAND)
TABLE 26 MIDDLE EAST & AFRICA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY CLASS, 2020-2029 (USD THOUSAND)
TABLE 27 MIDDLE EAST & AFRICA CLASS I IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 28 MIDDLE EAST & AFRICA CLASS III IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (THOUSAND)
TABLE 29 MIDDLE EAST & AFRICA CLASS II IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 30 MIDDLE EAST & AFRICA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY DEPLOYMENT MODE, 2020-2029 (USD THOUSAND)
TABLE 31 MIDDLE EAST & AFRICA CLOUD IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 32 MIDDLE EAST & AFRICA ON-PREMISES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 33 MIDDLE EAST & AFRICA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY DEPLOYMENT MODE, 2020-2029 (USD THOUSAND)
TABLE 34 MIDDLE EAST & AFRICA LARGE ENTERPRISES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 35 MIDDLE EAST & AFRICA SMALL & MEDIUM ENTERPRISES (SMES) IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 36 MIDDLE EAST & AFRICA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY STAGE, 2020-2029 (USD THOUSAND)
TABLE 37 MIDDLE EAST & AFRICA CLINICAL IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 38 MIDDLE EAST & AFRICA PRECLINICAL IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 39 MIDDLE EAST & AFRICA PMA (POST MARKET AUTHORIZATION) IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 40 MIDDLE EAST & AFRICA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY END USER, 2020-2029 (USD THOUSAND)
TABLE 41 MIDDLE EAST & AFRICA MEDICAL DEVICE COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 42 MIDDLE EAST & AFRICA MEDICAL DEVICE COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 43 MIDDLE EAST & AFRICA MEDICAL DEVICE COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 44 MIDDLE EAST & AFRICA PHARMACEUTICAL COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 45 MIDDLE EAST & AFRICA PHARMACEUTICAL COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 46 MIDDLE EAST & AFRICA PHARMACEUTICAL COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 47 MIDDLE EAST & AFRICA BIOTECHNOLOGY COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 48 MIDDLE EAST & AFRICA BIOTECHNOLOGY COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 49 MIDDLE EAST & AFRICA BIOTECHNOLOGY COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 50 MIDDLE EAST & AFRICA OTHERS IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 51 MIDDLE EAST AND AFRICA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY COUNTRY, 2020-2029 (USD THOUSAND)
TABLE 52 MIDDLE EAST AND AFRICA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 53 MIDDLE EAST AND AFRICA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY INDICATION, 2020-2029 (USD THOUSAND)
TABLE 54 MIDDLE EAST AND AFRICA INFECTIOUS DISEASES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY TYPE, 2020-2029 (USD THOUSAND)
TABLE 55 MIDDLE EAST AND AFRICA POINT OF CARE IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BYTYPE, 2020-2029 (USD THOUSAND)
TABLE 56 MIDDLE EAST AND AFRICA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY DEPLOYMENT MODE, 2020-2029 (USD THOUSAND)
TABLE 57 MIDDLE EAST AND AFRICA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 58 MIDDLE EAST AND AFRICA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY STAGE, 2020-2029 (USD THOUSAND)
TABLE 59 MIDDLE EAST AND AFRICA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY CLASS, 2020-2029 (USD THOUSAND)
TABLE 60 MIDDLE EAST AND AFRICA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY END USER, 2020-2029 (USD THOUSAND)
TABLE 61 MIDDLE EAST AND AFRICA MEDICAL DEVICE COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 62 MIDDLE EAST AND AFRICA MEDICAL DEVICE COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 63 MIDDLE EAST AND AFRICA PHARMACEUTICAL COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 64 MIDDLE EAST AND AFRICA PHARMACEUTICAL COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 65 MIDDLE EAST AND AFRICA BIOTECHNOLOGY COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 66 MIDDLE EAST AND AFRICA BIOTECHNOLOGY COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 67 SOUTH AFRICA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 68 SOUTH AFRICA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY INDICATION, 2020-2029 (USD THOUSAND)
TABLE 69 SOUTH AFRICA INFECTIOUS DISEASES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY TYPE, 2020-2029 (USD THOUSAND)
TABLE 70 SOUTH AFRICA POINT OF CARE IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BYTYPE, 2020-2029 (USD THOUSAND)
TABLE 71 SOUTH AFRICA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY DEPLOYMENT MODE, 2020-2029 (USD THOUSAND)
TABLE 72 SOUTH AFRICA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 73 SOUTH AFRICA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY STAGE, 2020-2029 (USD THOUSAND)
TABLE 74 SOUTH AFRICA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY CLASS, 2020-2029 (USD THOUSAND)
TABLE 75 SOUTH AFRICA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY END USER, 2020-2029 (USD THOUSAND)
TABLE 76 SOUTH AFRICA MEDICAL DEVICE COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 77 SOUTH AFRICA MEDICAL DEVICE COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 78 SOUTH AFRICA PHARMACEUTICAL COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 79 SOUTH AFRICA PHARMACEUTICAL COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 80 SOUTH AFRICA BIOTECHNOLOGY COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 81 SOUTH AFRICA BIOTECHNOLOGY COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 82 SAUDI ARABIA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 83 SAUDI ARABIA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY INDICATION, 2020-2029 (USD THOUSAND)
TABLE 84 SAUDI ARABIA INFECTIOUS DISEASES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY TYPE, 2020-2029 (USD THOUSAND)
TABLE 85 SAUDI ARABIA POINT OF CARE IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BYTYPE, 2020-2029 (USD THOUSAND)
TABLE 86 SAUDI ARABIA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY DEPLOYMENT MODE, 2020-2029 (USD THOUSAND)
TABLE 87 SAUDI ARABIA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 88 SAUDI ARABIA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY STAGE, 2020-2029 (USD THOUSAND)
TABLE 89 SAUDI ARABIA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY CLASS, 2020-2029 (USD THOUSAND)
TABLE 90 SAUDI ARABIA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY END USER, 2020-2029 (USD THOUSAND)
TABLE 91 SAUDI ARABIA MEDICAL DEVICE COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 92 SAUDI ARABIA MEDICAL DEVICE COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 93 SAUDI ARABIA PHARMACEUTICAL COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 94 SAUDI ARABIA PHARMACEUTICAL COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 95 SAUDI ARABIA BIOTECHNOLOGY COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 96 SAUDI ARABIA BIOTECHNOLOGY COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 97 U.A.E IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 98 U.A.E IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY INDICATION, 2020-2029 (USD THOUSAND)
TABLE 99 U.A.E INFECTIOUS DISEASES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY TYPE, 2020-2029 (USD THOUSAND)
TABLE 100 U.A.E POINT OF CARE IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BYTYPE, 2020-2029 (USD THOUSAND)
TABLE 101 U.A.E IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY DEPLOYMENT MODE, 2020-2029 (USD THOUSAND)
TABLE 102 U.A.E. IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 103 U.A.E IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY STAGE, 2020-2029 (USD THOUSAND)
TABLE 104 U.A.E IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY CLASS, 2020-2029 (USD THOUSAND)
TABLE 105 U.A.E IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY END USER, 2020-2029 (USD THOUSAND)
TABLE 106 U.A.E MEDICAL DEVICE COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 107 U.A.E MEDICAL DEVICE COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 108 U.A.E PHARMACEUTICAL COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 109 U.A.E PHARMACEUTICAL COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 110 U.A.E BIOTECHNOLOGY COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 111 U.A.E BIOTECHNOLOGY COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 112 EGYPT IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 113 EGYPT IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY INDICATION, 2020-2029 (USD THOUSAND)
TABLE 114 EGYPT INFECTIOUS DISEASES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY TYPE, 2020-2029 (USD THOUSAND)
TABLE 115 EGYPT POINT OF CARE IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BYTYPE, 2020-2029 (USD THOUSAND)
TABLE 116 EGYPT IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY DEPLOYMENT MODE, 2020-2029 (USD THOUSAND)
TABLE 117 EGYPT IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 118 EGYPT IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY STAGE, 2020-2029 (USD THOUSAND)
TABLE 119 EGYPT IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY CLASS, 2020-2029 (USD THOUSAND)
TABLE 120 EGYPT IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY END USER, 2020-2029 (USD THOUSAND)
TABLE 121 EGYPT MEDICAL DEVICE COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 122 EGYPT MEDICAL DEVICE COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 123 EGYPT PHARMACEUTICAL COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 124 EGYPT PHARMACEUTICAL COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 125 EGYPT BIOTECHNOLOGY COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 126 EGYPT BIOTECHNOLOGY COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 127 ISRAEL IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 128 ISRAEL IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY INDICATION, 2020-2029 (USD THOUSAND)
TABLE 129 ISRAEL INFECTIOUS DISEASES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY TYPE, 2020-2029 (USD THOUSAND)
TABLE 130 ISRAEL POINT OF CARE IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BYTYPE, 2020-2029 (USD THOUSAND)
TABLE 131 ISRAEL IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY DEPLOYMENT MODE, 2020-2029 (USD THOUSAND)
TABLE 132 ISRAEL IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 133 ISRAEL IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY STAGE, 2020-2029 (USD THOUSAND)
TABLE 134 ISRAEL IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY CLASS, 2020-2029 (USD THOUSAND)
TABLE 135 ISRAEL IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY END USER, 2020-2029 (USD THOUSAND)
TABLE 136 ISRAEL MEDICAL DEVICE COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 137 ISRAEL MEDICAL DEVICE COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 138 ISRAEL PHARMACEUTICAL COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 139 ISRAEL PHARMACEUTICAL COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 140 ISRAEL BIOTECHNOLOGY COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 141 ISRAEL BIOTECHNOLOGY COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 142 REST OF MIDDLE EAST AND AFRICA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
그림 목록
FIGURE 1 MIDDLE EAST & AFRICA IVD REGULATORY AFFAIRS OUTSOURCING MARKET: SEGMENTATION
FIGURE 2 MIDDLE EAST & AFRICA IVD REGULATORY AFFAIRS OUTSOURCING MARKET: DATA TRIANGULATION
FIGURE 3 MIDDLE EAST & AFRICA IVD REGULATORY AFFAIRS OUTSOURCING MARKET: DROC ANALYSIS
FIGURE 4 MIDDLE EAST & AFRICA IVD REGULATORY AFFAIRS OUTSOURCING MARKET: MIDDLE EAST & AFRICA VS REGIONAL MARKET ANALYSIS
FIGURE 5 MIDDLE EAST & AFRICA IVD REGULATORY AFFAIRS OUTSOURCING MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 MIDDLE EAST & AFRICA IVD REGULATORY AFFAIRS OUTSOURCING MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 MIDDLE EAST & AFRICA IVD REGULATORY AFFAIRS OUTSOURCING MARKET: DBMR MARKET POSITION GRID
FIGURE 8 MIDDLE EAST & AFRICA IVD REGULATORY AFFAIRS OUTSOURCING MARKET: VENDOR SHARE ANALYSIS
FIGURE 9 MIDDLE EAST & AFRICA IVD REGULATORY AFFAIRS OUTSOURCING MARKET: END USER COVERAGE GRID
FIGURE 10 MIDDLE EAST & AFRICA IVD REGULATORY AFFAIRS OUTSOURCING MARKET: SEGMENTATION
FIGURE 11 RISE IN THE PREVALENCE OF CHRONIC DISEASES ACROSS IS EXPECTED TO DRIVE MIDDLE EAST & AFRICA IVD REGULATORY AFFAIRS OUTSOURCING MARKET IN THE FORECAST PERIOD OF 2022 TO 2029
FIGURE 12 SERVICE SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF MIDDLE EAST & AFRICA IVD REGULATORY AFFAIRS OUTSOURCING MARKET IN 2022 & 2029
FIGURE 13 ASIA-PACIFIC IS EXPECTED TO DOMINATE AND IS THE FASTEST GROWING REGION IN THE MIDDLE EAST & AFRICA IVD REGULATORY AFFAIRS OUTSOURCING MARKET IN THE FORECAST PERIOD OF 2022 TO 2029
FIGURE 14 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES FOR MIDDLE EAST & AFRICA IVD REGULATORY AFFAIRS OUTSOURCING MARKET
FIGURE 15 MULTIPLE CHRONIC CONDITIONS AMONG PEOPLE AGED 65 AND ABOVE IN EUROPEAN REGION (2017)
FIGURE 16 MIDDLE EAST & AFRICA IVD REGULATORY AFFAIRS OUTSOURCING MARKET: BY SERVICE, 2021
FIGURE 17 MIDDLE EAST & AFRICA IVD REGULATORY AFFAIRS OUTSOURCING MARKET: BY INDICATION, 2021
FIGURE 18 MIDDLE EAST & AFRICA IVD REGULATORY AFFAIRS OUTSOURCING MARKET: BY CLASS, 2021
FIGURE 19 MIDDLE EAST & AFRICA IVD REGULATORY AFFAIRS OUTSOURCING MARKET: BY DEPLOYMENT MODE, 2021
FIGURE 20 MIDDLE EAST & AFRICA IVD REGULATORY AFFAIRS OUTSOURCING MARKET: BY ORGANIZATION SIZE, 2021
FIGURE 21 MIDDLE EAST & AFRICA IVD REGULATORY AFFAIRS OUTSOURCING MARKET: BY STAGE, 2021
FIGURE 22 MIDDLE EAST & AFRICA IVD REGULATORY AFFAIRS OUTSOURCING MARKET: BY END USER, 2021
FIGURE 23 MIDDLE EAST & AFRICA IVD REGULATORY AFFAIRS OUTSOURCING MARKET: SNAPSHOT (2021)
FIGURE 24 MIDDLE EAST & AFRICA IVD REGULATORY AFFAIRS OUTSOURCING MARKET: BY COUNTRY (2021)
FIGURE 25 MIDDLE EAST & AFRICA IVD REGULATORY AFFAIRS OUTSOURCING MARKET: BY COUNTRY (2022 & 2029)
FIGURE 26 MIDDLE EAST & AFRICA IVD REGULATORY AFFAIRS OUTSOURCING MARKET: BY COUNTRY (2021 & 2029)
FIGURE 27 MIDDLE EAST & AFRICA IVD REGULATORY AFFAIRS OUTSOURCING MARKET: BY SERVICE (2022-2029)
FIGURE 28 MIDDLE EAST & AFRICA IVD REGULATORY AFFAIRS OUTSOURCING MARKET: COMPANY SHARE 2021 (%)

연구 방법론
데이터 수집 및 기준 연도 분석은 대규모 샘플 크기의 데이터 수집 모듈을 사용하여 수행됩니다. 이 단계에는 다양한 소스와 전략을 통해 시장 정보 또는 관련 데이터를 얻는 것이 포함됩니다. 여기에는 과거에 수집한 모든 데이터를 미리 검토하고 계획하는 것이 포함됩니다. 또한 다양한 정보 소스에서 발견되는 정보 불일치를 검토하는 것도 포함됩니다. 시장 데이터는 시장 통계 및 일관된 모델을 사용하여 분석하고 추정합니다. 또한 시장 점유율 분석 및 주요 추세 분석은 시장 보고서의 주요 성공 요인입니다. 자세한 내용은 분석가에게 전화를 요청하거나 문의 사항을 드롭하세요.
DBMR 연구팀에서 사용하는 주요 연구 방법론은 데이터 마이닝, 시장에 대한 데이터 변수의 영향 분석 및 주요(산업 전문가) 검증을 포함하는 데이터 삼각 측량입니다. 데이터 모델에는 공급업체 포지셔닝 그리드, 시장 타임라인 분석, 시장 개요 및 가이드, 회사 포지셔닝 그리드, 특허 분석, 가격 분석, 회사 시장 점유율 분석, 측정 기준, 글로벌 대 지역 및 공급업체 점유율 분석이 포함됩니다. 연구 방법론에 대해 자세히 알아보려면 문의를 통해 업계 전문가에게 문의하세요.
사용자 정의 가능
Data Bridge Market Research는 고급 형성 연구 분야의 선두 주자입니다. 저희는 기존 및 신규 고객에게 목표에 맞는 데이터와 분석을 제공하는 데 자부심을 느낍니다. 보고서는 추가 국가에 대한 시장 이해(국가 목록 요청), 임상 시험 결과 데이터, 문헌 검토, 재생 시장 및 제품 기반 분석을 포함하도록 사용자 정의할 수 있습니다. 기술 기반 분석에서 시장 포트폴리오 전략에 이르기까지 타겟 경쟁업체의 시장 분석을 분석할 수 있습니다. 귀하가 원하는 형식과 데이터 스타일로 필요한 만큼 많은 경쟁자를 추가할 수 있습니다. 저희 분석가 팀은 또한 원시 엑셀 파일 피벗 테이블(팩트북)로 데이터를 제공하거나 보고서에서 사용 가능한 데이터 세트에서 프레젠테이션을 만드는 데 도움을 줄 수 있습니다.