Middle East and Africa Genetic Testing Market, By Type (Carrier Testing, Diagnostic Testing, New-Born Screening, Predictive and Presymptomatic Testing, Prenatal Testing, Other Types), Technology (DNA Sequencing (NGS-Based Testing), Polymerase Chain Reaction, Microarrays, Whole Genome Sequencing, Fluorescence In Situ Hybridization (FISH), and Others), Diseases (Rare Genetic Disorder, Cancer, Cystic Fibrosis, Reproductive Genetic Testing, Health & Wellness Genetic Testing, Sickle Cell Anemia, Duchenne Muscular Dystrophy, Thalassemia, Huntington's Disease, Fragile X Syndrome, and Other), End User (Hospitals, Clinics, Diagnostic Centers, Private Clinics, Laboratory Service Providers, and Private Laboratories) Industry Trends and Forecast to 2030.
Middle East and Africa Genetic Testing Market Analysis and Insights
Middle East and Africa genetic testing market is driven by the factors such as the high prevalence of genetic disorders, growing technological advancements in the genetic testing market, which enhance its demand, as well as increasing investment in research and development, which leads to market growth. Currently, healthcare expenditure has increased across developed and emerging countries, which is expected to create a competitive advantage for manufacturers to develop new and innovative genetic testing markets.
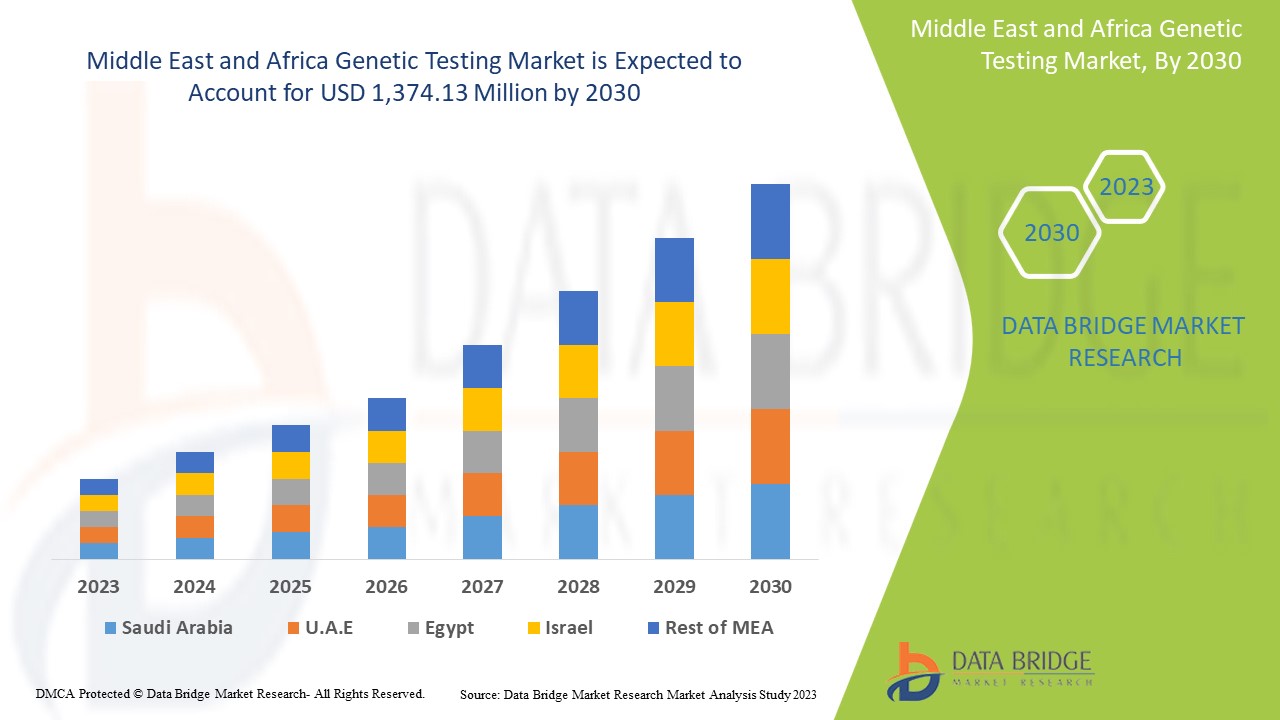
Middle East and Africa genetic testing market is expected to gain market growth in the forecast period of 2023 to 2030. Data Bridge Market Research analyses that the market is growing with a CAGR of 13.6% in the forecast period of 2023 to 2030 and is expected to reach USD 1,374.13 million by 2030.
The Middle East and Africa genetic testing market report provides details of market share, new developments, and product pipeline analysis, the impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, product approvals, strategic decisions, product launches, geographic expansions, and technological innovations in the market. To understand the analysis and the market scenario, contact us for an analyst brief, our team will help you create a revenue-impact solution to achieve your desired goal.
|
Report Metric |
Details |
|
Forecast Period |
2023 to 2030 |
|
Base Year |
2022 |
|
Historic Years |
2021 (Customizable to 2020-2015) |
|
Quantitative Units |
Revenue in USD Million |
|
Segments Covered |
유형별(보균자 검사, 진단 검사, 신생아 선별 검사, 예측 및 증상 전 검사, 산전 검사, 기타 유형), 기술별(DNA 시퀀싱(NGS 기반 검사), 중합효소 연쇄 반응, 마이크로어레이, 전장 유전체 시퀀싱, 형광 현장 교잡법(FISH) 및 기타), 질병별(희귀 유전 질환, 암, 낭포성 섬유증, 생식 유전자 검사, 건강 및 웰빙 유전자 검사, 겸상 적혈구 빈혈, 듀센 근이영양증, 지중해 빈혈, 헌팅턴병, 연약 X 증후군 및 기타), 최종 사용자별(병원, 진료소, 진단 센터, 개인 진료소, 검사 서비스 제공업체 및 개인 실험실). |
|
적용 국가 |
남아프리카공화국, 사우디아라비아, UAE, 이집트, 이스라엘, 기타 중동 및 아프리카 국가. |
|
시장 참여자 포함 |
Abbott, 23ANDME, Inc., Danaher, Myriad Genetics, Inc., OCP Medical Centre, LLC, Healthchecks360, Bayer AG, BioReference Health, LLC(OPKO Health, Inc.의 자회사), FREIBURG MEDICAL LABORATORY MIDDLE EAST(LLC), Thermo Fisher Scientific Inc., Bio-Rad Laboratories, Inc., Biocartis, Eurofins Scientific, PerkinElmer Inc., ELITechGroup, F. Hoffmann-La Roche Ltd., Illumina, Inc., QIAGEN, BIO-HELIX, PacBio 등이 있습니다. |
시장 정의
유전자 검사는 유전자, 염색체 또는 단백질의 변화를 식별하는 일종의 의료 검사입니다. 유전자 검사 결과는 의심되는 유전적 상태를 확인하거나 배제하거나 유전적 장애를 발병하거나 유전될 가능성을 결정하는 데 도움이 될 수 있습니다. 현재 77,000개 이상의 유전자 검사가 사용 중이며 다른 검사도 개발 중입니다.
혁신과 기술이 증가하고 시장에 참여하는 기업 수가 늘어나며, 새로운 제품이 출시됨에 따라 중동과 아프리카 유전자 검사 시장도 성장하고 있습니다.
중동 및 아프리카 유전자 검사 시장 동향
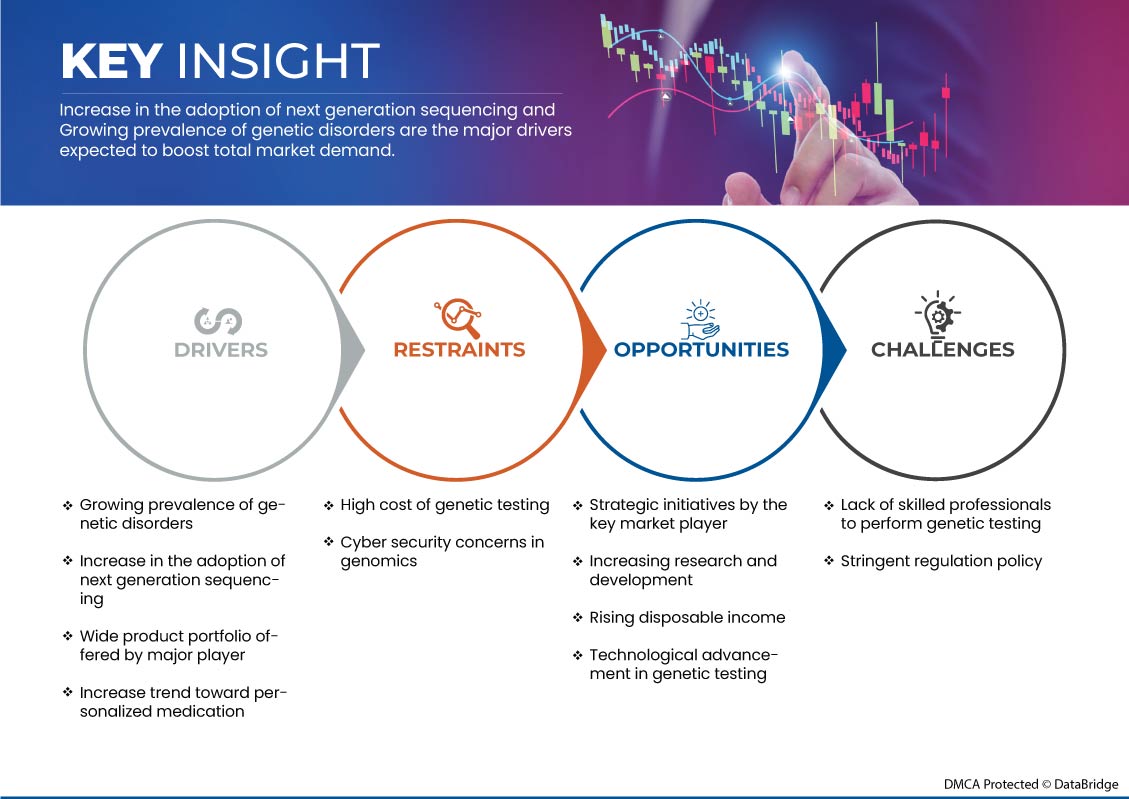
이 섹션에서는 시장 동인, 이점, 기회, 제약 및 과제를 이해하는 것을 다룹니다. 이 모든 내용은 아래에서 자세히 설명합니다.
드라이버/기회
- 유전적 질환의 유병률 증가
유전적 질환은 생명과 양립할 수 없는 심각한 건강 문제를 일으킬 수 있습니다. 가장 심각한 경우, 이러한 상태는 영향을 받은 배아 또는 태아의 유산을 일으킬 수 있습니다. 유전적 질환과 선천적 결함의 유병률이 증가함에 따라 유전자 검사 시장에 대한 수요가 증가합니다.
- 세계보건기구의 기사인 유전적 질환과 선천적 이상: 이 지역의 부담을 줄이기 위한 전략, 2022에 따르면, 유전적 및 선천적 질환은 이 지역의 많은 국가에서 주산기 및 신생아 사망률의 상당 부분을 차지합니다. 선천적 결함은 현재 아랍에미리트에서 유아 사망률의 주요 원인으로 간주되고 바레인, 쿠웨이트, 오만, 카타르에서는 두 번째 원인으로 간주됩니다. 사우디아라비아의 보고서에 따르면 두 병원에서 발생한 산전 사망의 약 25-35%가 선천적 결함 때문이었습니다.
따라서 유전자 검사 시장에 대한 수요가 증가하고 있습니다.
- 차세대 시퀀싱 도입 증가
유전체학에 초점을 맞춘 약리학이 다양한 만성 질환, 특히 암 치료에 점점 더 큰 역할을 함에 따라 차세대 시퀀싱(NGS)은 개별 종양과 특정 수용체의 분자적 기초에 대한 더 깊고 정확한 통찰력을 제공하는 강력한 도구로 발전하고 있습니다.
NGS는 종양학 분야에 상당한 영향을 미칠 수 있는 기존 방법에 비해 정확도, 민감도, 속도 면에서 이점을 제공합니다. NGS는 단일 검사에서 여러 유전자를 평가할 수 있으므로 원인 돌연변이를 식별하기 위해 여러 검사를 주문할 필요가 없습니다.
따라서 이는 유전자 검사 시장 성장을 촉진하는 원동력이 될 것으로 기대됩니다.
- 가처분 소득 증가
한 국가가 의료에 사용하는 돈의 비용과 시간이 지남에 따른 성장률은 재정 조달 방식과 의료 시스템 조직의 구조를 포함한 다양한 경제적, 사회적 요인에 따라 기울어집니다. 특히, 한 국가의 전체 소득 수준과 그 국가의 인구가 의료에 지출하는 금액 사이에는 강력한 연관성이 있습니다.
또한 주요 시장 참여자가 취하는 전략적 이니셔티브는 2023~2030년 예측 기간 동안 유전자 검사 시장에 구조적 무결성과 미래 기회를 제공할 것입니다.
제약/도전
- 유전자 검사 비용이 높다
유전자 검사는 비쌀 수 있으며 일부 건강 보험 플랜에서는 보장되지 않을 수 있습니다. 수많은 유전자 검사는 검사 대상 질병에 따라 비용이 다릅니다.
- Breastcancer.org에 따르면 암 유전자 검사 비용은 크게 다를 수 있으며 300달러에서 5,000달러 사이가 될 수 있습니다. 유전자 검사 비용은 검사 유형과 검사의 복잡성에 따라 달라질 수 있습니다.
- 유전자 검사 비용은 검사의 성격과 복잡성에 따라 100달러에서 2,000달러 이상까지 다양합니다. 두 개 이상의 검사가 필요하거나 많은 가족 구성원이 유의미한 결과를 얻기 위해 검사를 받아야 하는 경우 비용이 증가합니다. 신생아 검진 비용은 주마다 다릅니다.
따라서 유전자 검사의 높은 비용이 시장 성장을 제한할 수도 있다.
- 엄격한 규제 정책
규제 업무(RA)는 의료 기기 산업에서 중요한 역할을 하는데, 이는 의료 제품 수명 주기와 관련이 있기 때문입니다. 이는 전술적, 전략적, 운영적 경로를 제공하며, 전 세계 인구에게 안전하고 효과적인 암 침 검사 장치와 제품의 제공 및 개발을 촉진하기 위해 규정 내에서 기능하는 데 도움을 줍니다. 규제 업무의 역할은 규제 전략을 수립하고 실행하는 것입니다.
많은 의료 기관과 정부 기관이 암 침 검사 장치와 제품의 출시 및 승인에 대한 규제 정책을 제공하고 있습니다. 지역 규제 기관의 제품 승인은 약물 개발 팀에 필수적인 역할을 합니다. 승인은 회사의 약물 개발 활동을 보장하고 회사를 경쟁사와 차별화합니다.
COVID-19 이후 중동 및 아프리카 유전자 검사 시장에 미치는 영향
유전자 검사 시장은 COVID-19로 인해 큰 타격을 입었습니다. 병원 입원은 필수적이지 않은 치료에 국한되었고, 진료소는 팬데믹 기간 동안 일시적으로 문을 닫았습니다. 사회적 거리두기 시행, 인구 차단, 진료소 접근 제한은 시장에 큰 영향을 미쳤습니다. 환자 흐름과 추천의 둔화도 시장 성장에 영향을 미쳤습니다. 그러나 이전에 부과된 제한이 완화되어 팬데믹 이후 기간에도 시장은 계속 성장할 것입니다.
제조업체들은 COVID-19 이후 회복하기 위해 다양한 전략적 결정을 내리고 있습니다. 업체들은 유전자 검사 시장에 관련된 기술과 검사 결과를 개선하기 위해 여러 R&D 활동과 제품 출시, 전략적 파트너십을 진행하고 있습니다.
최근 개발
- 2022년 11월 10일, 유전자 검사 및 정밀 의학 분야의 선두주자인 Myriad Genetics, Inc.는 전립선암 치료의 모든 단계를 포괄하는 포괄적인 유전적 위험 평가 검사인 UroSuite를 발표했습니다. UroSuite에는 Myriad의 Prolaris 전립선암 검사, MyRisk 유전성 암 검사, BRACAnalysis CDx, Precise Tumor Molecular Profile 검사가 포함됩니다. 이러한 검사를 조합하면 통합된 유전적 개요를 제공하고 환자의 치료 및 임상 시험 선택을 용이하게 합니다.
- 2022년 9월, DNA 시퀀싱 및 어레이 기반 기술 분야의 세계적 리더인 Illumina, Inc.는 NovaSeq X-Series(NovaSeq X 및 NovaSeq X Plus) 출시를 발표했습니다. 이는 더 빠르고, 더 효율적이며, 더 지속 가능한 시퀀싱을 가능하게 하여 게놈 의학의 경계를 넓히는 새로운 생산 규모 시퀀서입니다. 혁신적인 신기술을 탑재한 NovaSeq X Plus는 연간 20,000개 이상의 전체 게놈을 생성할 수 있습니다. 이는 이전 시퀀서의 성능보다 2.5배 더 높은 수치로, 게놈 발견과 임상적 통찰력을 획기적으로 가속화하여 질병을 이해하고 궁극적으로 환자의 삶을 변화시킵니다.
중동 및 아프리카 유전자 검사 시장 범위
중동 및 아프리카 유전자 검사 시장은 유형, 기술, 질병 및 최종 사용자로 세분화됩니다. 이러한 세그먼트 간의 성장은 산업의 성장 세그먼트를 분석하고 측정하고 사용자에게 핵심 시장 응용 프로그램을 식별하기 위한 전략적 결정을 내릴 수 있는 귀중한 시장 개요와 시장 통찰력을 제공하는 데 도움이 됩니다.
유형
- 진단 테스트
- 태아 검사
- 신생아 검진
- 예측 및 증상 전 검사
- 캐리어 테스트
- 기타 유형
중동 및 아프리카 유전자 검사 시장은 유형을 기준으로 진단 검사, 산전 검사, 신생아 선별 검사, 예측 및 증상 전 검사, 보균자 검사 및 기타 유형으로 구분됩니다.
기술
- 중합효소 연쇄 반응
- DNA 시퀀싱(NGS 기반 테스트)
- 전체 게놈 시퀀싱
- 마이크로어레이
- 형광 현장 교잡화(FISH)
- 기타
중동 및 아프리카 유전자 검사 시장은 기술을 기준으로 DNA 시퀀싱, 중합효소 연쇄 반응, 마이크로어레이, 전체 게놈 시퀀싱, 형광 현장 교잡법(FISH) 및 기타로 구분됩니다.
질병
- 암
- 낫적혈구빈혈
- 지중해 빈혈
- 희귀 유전질환
- 연약 X 증후군
- 듀센 근이영양증
- 헌팅턴병
- 낭포성 섬유증
- 생식 유전자 검사
- 건강 및 웰빙 유전자 검사
- 기타
질병을 기준으로 중동 및 아프리카 유전자 검사 시장은 희귀 유전적 질환 , 암, 낭포성 섬유증, 낫적혈구 빈혈, 듀센 근이영양증, 지중해 빈혈, 헌팅턴병, 연약 X 증후군, 생식 유전자 검사, 건강 및 웰빙 유전자 검사 및 기타로 구분됩니다.
최종 사용자
- 병원
- 클리닉
- 진단 센터
- 개인 병원
- 실험실 서비스 제공자
- 개인 연구실
중동 및 아프리카 유전자 검사 시장은 최종 사용자를 기준으로 병원, 진료소, 진단 센터, 사립 진료소, 실험실 서비스 제공업체, 사립 실험실로 구분됩니다.
중동 및 아프리카 유전자 검사 시장 지역 분석/통찰력
중동 및 아프리카 유전자 검사 시장을 분석하고, 유형, 기술, 질병 및 최종 사용자별로 시장 규모 정보를 제공합니다.
이 시장 보고서에서 다루는 국가는 남아프리카공화국, 사우디아라비아, UAE, 이집트, 이스라엘, 그리고 나머지 중동 및 아프리카 국가입니다.
2023년에는 남아프리카공화국이 R&D 투자 증가로 시장 성장을 촉진할 것으로 예상되어 시장을 주도할 것으로 예상됩니다.
보고서의 국가 섹션은 또한 개별 시장 영향 요인과 국내 시장의 현재 및 미래 트렌드에 영향을 미치는 규제 변화를 제공합니다. 신규 판매, 교체 판매, 국가 인구 통계, 규제 조치 및 수출입 관세와 같은 데이터 포인트는 개별 국가의 시장 시나리오를 예측하는 데 사용되는 주요 포인터 중 일부입니다. 또한 중동 및 아프리카 브랜드의 존재 및 가용성과 지역 및 국내 브랜드와의 대규모 또는 희소한 경쟁으로 인해 직면한 과제와 판매 채널의 영향이 국가 데이터에 대한 예측 분석을 제공하는 동안 고려됩니다.
경쟁 환경 및 중동 및 아프리카 유전자 검사 시장 점유율 분석
중동 및 아프리카 유전자 검사 시장 경쟁 구도는 경쟁업체의 세부 정보를 제공합니다. 포함된 세부 정보에는 회사 개요, 회사 재무, 창출된 수익, 시장 잠재력, R&D 투자, 새로운 시장 이니셔티브, 생산 현장 및 시설, 회사의 강점과 약점, 제품 출시, 제품 시험 파이프라인, 제품 승인, 특허, 제품 폭 및 호흡, 응용 프로그램 우세, 기술 수명선 곡선이 있습니다. 위에 제공된 데이터 포인트는 중동 및 아프리카 유전자 검사 시장에 대한 회사의 초점과만 관련이 있습니다.
중동 및 아프리카 유전자 검사 시장의 주요 기업으로는 Abbott, 23ANDME, Inc., Danaher, Myriad Genetics, Inc., OCP Medical Centre, LLC, Healthchecks360, Bayer AG, BioReference Health, LLC(OPKO Health, Inc.의 자회사), FREIBURG MEDICAL LABORATORY MIDDLE EAST (LLC), Thermo Fisher Scientific Inc., Bio-Rad Laboratories, Inc., Biocartis, Eurofins Scientific, PerkinElmer Inc., ELITechGroup, F. Hoffmann-La Roche Ltd., Illumina, Inc., QIAGEN, BIO-HELIX, PacBio 등이 있습니다.
데이터 수집 및 기준 연도 분석은 대규모 샘플 크기의 데이터 수집 모듈을 사용하여 수행됩니다. 시장 데이터는 시장 통계 및 일관된 모델을 사용하여 분석 및 추정됩니다. 또한 시장 점유율 분석 및 주요 추세 분석은 시장 보고서의 주요 성공 요인입니다. DBMR 연구팀에서 사용하는 주요 연구 방법론은 데이터 마이닝, 시장에 대한 데이터 변수의 영향 분석 및 기본(산업 전문가) 검증을 포함하는 데이터 삼각 측량입니다. 이 외에도 데이터 모델에는 공급업체 포지셔닝 그리드, 시장 타임라인 분석, 시장 개요 및 가이드, 회사 포지셔닝 그리드, 회사 시장 점유율 분석, 측정 표준, 중동 및 아프리카 대 지역, 공급업체 점유율 분석이 포함됩니다. 추가 문의 사항이 있는 경우 분석가에게 전화를 요청하십시오.
SKU-
세계 최초의 시장 정보 클라우드 보고서에 온라인으로 접속하세요
- 대화형 데이터 분석 대시보드
- 높은 성장 잠재력 기회를 위한 회사 분석 대시보드
- 사용자 정의 및 질의를 위한 리서치 분석가 액세스
- 대화형 대시보드를 통한 경쟁자 분석
- 최신 뉴스, 업데이트 및 추세 분석
- 포괄적인 경쟁자 추적을 위한 벤치마크 분석의 힘 활용
목차
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF THE MIDDLE EAST AND AFRICA GENETIC TESTING MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 YEARS CONSIDERED FOR THE STUDY
2.3 CURRENCY AND PRICING
2.4 DBMR TRIPOD DATA VALIDATION MODEL
2.5 MULTIVARIATE MODELLING
2.6 TYPE LIFELINE CURVE
2.7 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.8 DBMR MARKET POSITION GRID
2.9 MARKET APPLICATION COVERAGE GRID
2.1 VENDOR SHARE ANALYSIS
2.11 SECONDARY SOURCES
2.12 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL’S MODEL
4.2 PORTER'S FIVE FORCES MODEL
4.3 STRATEGIC INITIATIVES:
5 INDUSTRY INSIGHTS
5.1 DEMOGRAPHIC TRENDS: IMPACTS ON ALL INCIDENCE RATES
5.2 CANCER GENETICS RISK ASSESSMENT AND COUNSELING
5.3 GENETIC TESTS PRICING AND KEY PRICING STRATEGIES
5.3.1 LAUNCH OF NEW TECHNOLOGY GENETIC TESTING KITS
5.3.2 PARTNERSHIPS WITH MARKET PLAYERS
5.3.3 PRENATAL PRICING STRATEGY
5.4 KEY PATIENT ENROLLMENT STRATEGIES
5.4.1 AWARENESS OF THE PUBLIC TOWARDS GENETIC TESTING TECHNOLOGY
5.4.2 GENETIC COUNSELLORS' SCOPE OF PRACTICE
5.4.3 EDUCATION AND COMMUNICATION
6 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: REGULATIONS
7 EPIDEMIOLOGY
8 MARKET OVERVIEW
8.1 DRIVERS
8.1.1 GROWING PREVALENCE OF GENETIC DISORDERS
8.1.2 INCREASE IN THE ADOPTION OF NEXT GENERATION SEQUENCING
8.1.3 WIDE PRODUCT PORTFOLIO OFFERED BY MAJOR PLAYER
8.1.4 INCREASE TREND TOWARD PERSONALIZED MEDICATION
8.2 RESTRAINT
8.2.1 HIGH COST OF GENETIC TESTING
8.2.2 CYBER SECURITY CONCERN IN GENOMICS
8.3 OPPORTUNITIES
8.3.1 STRATEGIC INITIATIVES BY THE KEY MARKET PLAYER
8.3.2 TECHNOLOGICAL ADVANCEMENTS IN GENETIC TESTING
8.3.3 INCREASING RESEARCH AND DEVELOPMENT
8.3.4 RISING DISPOSABLE INCOME
8.4 CHALLENGES
8.4.1 LACK OF SKILLED PROFESSIONALS TO PERFORM GENETIC TESTING
8.4.2 STRINGENT REGULATION POLICY
9 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET, BY TYPE
9.1 OVERVIEW
9.2 DIAGNOSTIC TESTING
9.3 PRENATAL TESTING
9.3.1 NON-INVASIVE SCREENING
9.3.1.1 BY SCREENING METHOD
9.3.1.1.1 WHOLE GENOME SEQUENCING
9.3.1.1.2 COUNTING OF CFDNA FRAGMENTS
9.3.1.1.3 OTHERS
9.3.1.2 BY CONDITION
9.3.1.2.1 TRISOMY 21
9.3.1.2.2 KLINEFELTER SYNDROME
9.3.1.2.3 JACOBS SYNDROME
9.3.1.2.4 CYSTIC FIBROSIS
9.3.1.2.5 TURNER SYNDROME
9.3.1.2.6 TRISOMY 18
9.3.1.2.7 HEMOPHILIA
9.3.1.2.8 TRISOMY 13
9.3.1.2.9 MICRODELETION SYNDROME
9.3.1.2.10 FETAL GENDER
9.3.1.2.11 OTHERS
9.3.1.3 BY SCREENING TYPE
9.3.1.3.1 CARRIER SEQUENCING
9.3.1.3.2 SEQUENTIAL SEQUENCING
9.3.2 MATERNAL SERUM QUAD SCREENING
9.4 NEW BORN SCREENING
9.4.1 SICKLE CELL DISEASE
9.4.2 CONGENITAL HYPOTHYROIDISM
9.4.3 PHENYLKETONURINA (PKU)
9.4.4 GALACTOSEMIA
9.4.5 MAPLE SYRUP URINE DISEASE
9.4.6 OTHERS
9.5 PREDICTIVE AND PRESYMPTOMATIC TESTING
9.6 CARRIER TESTING
9.6.1 BY TEST TYPE
9.6.1.1 MOLECULAR SCREENING TEST
9.6.1.2 BIOCHEMICAL SCREENING TEST
9.6.2 BY TYPE
9.6.2.1 EXPANDED CARRIER SCREENING
9.6.2.1.1 PREDESIGNED PANEL TESTING
9.6.2.1.2 CUSTOM-MADE PANEL TESTING
9.6.2.2 TARGETED DISEASE CARRIER SCREENING
9.6.3 BY MEDICAL CONDITION
9.6.3.1 HEMATOLOGICAL CONDITIONS
9.6.3.2 PULMONARY CONDITIONS
9.6.3.3 NEUROLOGICAL CONDITIONS
9.6.3.4 OTHER CONDITIONS
9.7 OTHER TYPES
10 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET, BY TECHNOLOGY
10.1 OVERVIEW
10.2 POLYMERASE CHAIN REACTION
10.2.1 REAL-TIME PCR (QPCR)
10.2.2 DIGITAL PCR (DPCR)
10.2.3 REVERSE TRANSCRIPTION PCR (RT-PCR)
10.2.4 HOT-START PCR
10.2.5 MULTIPLEX PCR
10.2.6 OTHER PCR
10.3 DNA SEQUENCING (NGS-BASED TESTING)
10.3.1 NEXT GENERATION SEQUENCING (NGS)
10.3.2 SANGER SEQUENCING (SINGLE GENE)
10.3.3 OTHER
10.4 WHOLE GENOME SEQUENCING
10.5 MICROARRAYS
10.5.1 DNA MICROARRAYS
10.5.2 PROTEIN MICROARRAYS
10.5.3 OTHER MICROARRAYS
10.6 FLUORESCENCE IN SITU HYBRIDIZATION (FISH)
10.7 OTHERS
11 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET, BY DISEASES
11.1 OVERVIEW
11.2 CANCER
11.2.1 BREAST
11.2.2 COLON
11.2.3 LUNG
11.2.4 PROSTATE
11.2.5 OTHERS
11.3 REPRODUCTIVE GENETIC TESTING
11.4 HEALTH AND WELLNESS GENETIC TESTING
11.5 SICKLE CELL ANEMIA
11.6 THALASSEMIA
11.7 RARE GENETIC DISORDER
11.7.1 TRISOMY 21
11.7.2 MONOSOMY X
11.7.3 TRISOMY 13
11.7.4 MICRODELETION SYNDROME
11.7.5 TRISOMY 18
11.7.6 OTHERS
11.8 FRAGILE X SYNDROME
11.9 DUCHENNE MUSCULAR DYSTROPHY
11.1 HUNTINGTON'S DISEASE
11.11 CYSTIC FIBROSIS
11.12 OTHERS
12 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET, BY END USER
12.1 OVERVIEW
12.2 HOSPITALS
12.3 CLINICS
12.4 DIAGNOSTIC CENTERS
12.5 PRIVATE CLINICS
12.6 LABORATORY SERVICE PROVIDERS
12.7 PRIVATE LABORATORIES
13 SUMMARY WRITE-UP (MIDDLE EAST AND AFRICA)
13.1 OVERVIEW
13.2 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET, BY COUNTRY
13.2.1 SOUTH AFRICA
13.2.2 SAUDI ARABIA
13.2.3 U.A.E.
13.2.4 ISRAEL
13.2.5 EGYPT
13.2.6 REST OF MIDDLE EAST AND AFRICA
14 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET, COMPANY LANDSCAPE
14.1 COMPANY SHARE ANALYSIS: MIDDLE EAST AND AFRICA
15 SWOT ANALYSIS
16 COMPANY PROFILE
16.1 BAYER AG
16.1.1 COMPANY SNAPSHOT
16.1.2 REVENUE ANALYSIS
16.1.3 PRODUCT PORTFOLIO
16.1.4 RECENT DEVELOPMENTS
16.2 ABBOTT
16.2.1 COMPANY SNAPSHOT
16.2.2 REVENUE ANALYSIS
16.2.3 PRODUCT PORTFOLIO
16.2.4 RECENT DEVELOPMENTS
16.3 ILLUMINA, INC.
16.3.1 COMPANY SNAPSHOT
16.3.2 REVENUE ANALYSIS
16.3.3 PRODUCT PORTFOLIO
16.3.4 RECENT DEVELOPMENTS
16.4 THERMO FISHER SCIENTIFIC INC.
16.4.1 COMPANY SNAPSHOT
16.4.2 REVENUE ANALYSIS
16.4.3 PRODUCT PORTFOLIO
16.4.4 RECENT DEVELOPMENTS
16.5 F.HOFFAMANN-LA ROCHE LTD.
16.5.1 COMPANY SNAPSHOT
16.5.2 REVENUE ANALYSIS
16.5.3 PRODUCT PORTFOLIO
16.5.4 RECENT DEVELOPMENTS
16.6 BIOREFERNCE HEALTH INC. (SUBSIDIARY OPKO HEALTH, INC.)
16.6.1 COMPANY SNAPSHOT
16.6.2 REVENUE ANALYSIS
16.6.3 PRODUCT PORTFOLIO
16.6.4 RECENT DEVELOPMENTS
16.7 ELITECHGROUP
16.7.1 COMPANY SNAPSHOT
16.7.2 PRODUCT PORTFOLIO
16.7.3 RECENT DEVELOPMENTS
16.8 23ANDME, INC.
16.8.1 COMPANY SNAPSHOT
16.8.2 REVENUE ANALYSIS
16.8.3 PRODUCT PORTFOLIO
16.8.4 RECENT DEVELOPMENTS
16.9 BIOCARTIS
16.9.1 COMPANY SNAPSHOT
16.9.2 REVENUE ANALYSIS
16.9.3 PRODUCT PORTFOLIO
16.9.4 RECENT DEVELOPMENTS
16.1 BIO-HELIX
16.10.1 COMPANY SNAPSHOT
16.10.2 PRODUCT PORTFOLIO
16.10.3 RECENT DEVELOPMENTS
16.11 BIO-RAD LABORATORIES, INC.
16.11.1 COMPANY SNAPSHOT
16.11.2 REVENUE ANALYSIS
16.11.3 PRODUCT PORTFOLIO
16.11.4 RECENT DEVELOPMENTS
16.12 DANAHER
16.12.1 COMPANY SNAPSHOT
16.12.2 REVENUE ANALYSIS
16.12.3 PRODUCT PORTFOLIO
16.12.4 RECENT DEVELOPMENTS
16.13 EUROFINS SCIENTIFIC
16.13.1 COMPANY SNAPSHOT
16.13.2 REVENUE ANALYSIS
16.13.3 PRODUCT PORTFOLIO
16.13.4 RECENT DEVELOPMENTS
16.14 FREIBURG MEDICAL LABORATORY MIDDLE EAST (L.L.C)
16.14.1 COMPANY SNAPSHOT
16.14.2 PRODUCT PORTFOLIO
16.14.3 RECENT DEVELOPMENTS
16.15 HEALTHCHECKS360
16.15.1 COMPANY SNAPSHOT
16.15.2 PRODUCT PORTFOLIO
16.15.3 RECENT DEVELOPMENTS
16.16 MYRIAD GENETICS, INC.
16.16.1 COMPANY SNAPSHOT
16.16.2 REVENUE ANALYSIS
16.16.3 PRODUCT PORTFOLIO
16.16.4 RECENT DEVELOPMENTS
16.17 OCP MEDICAL CENTER L.L.C
16.17.1 COMPANY SNAPSHOT
16.17.2 PRODUCT PORTFOLIO
16.17.3 RECENT DEVELOPMENTS
16.18 PACBIO
16.18.1 COMPANY SNAPSHOT
16.18.2 REVENUE ANALYSIS
16.18.3 PRODUCT PORTFOLIO
16.18.4 RECENT DEVELOPMENTS
16.19 PERKINELMER ONC.
16.19.1 COMPANY SNAPSHOT
16.19.2 REVENUE ANALYSIS
16.19.3 PRODUCT PORTFOLIO
16.19.4 RECENT DEVELOPMENTS
16.2 QIAGEN
16.20.1 COMPANY SNAPSHOT
16.20.2 REVENUE ANALYSIS
16.20.3 PRODUCT PORTFOLIO
16.20.4 RECENT DEVELOPMENTS
17 QUESTIONNAIRE
18 RELATED REPORTS
표 목록
TABLE 1 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 2 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET, BY TYPE, 2021-2030 (UNITS)
TABLE 3 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET, BY TYPE, 2021-2030 (ASP)
TABLE 4 MIDDLE EAST AND AFRICA PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 5 MIDDLE EAST AND AFRICA PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (UNITS)
TABLE 6 MIDDLE EAST AND AFRICA PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (ASP)
TABLE 7 MIDDLE EAST AND AFRICA NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2021-2030 (USD MILLION)
TABLE 8 MIDDLE EAST AND AFRICA NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2021-2030 (UNITS)
TABLE 9 MIDDLE EAST AND AFRICA NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2021-2030 (ASP)
TABLE 10 MIDDLE EAST AND AFRICA NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY CONDITION, 2021-2030 (USD MILLION)
TABLE 11 MIDDLE EAST AND AFRICA NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2021-2030 (USD MILLION)
TABLE 12 MIDDLE EAST AND AFRICA NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2021-2030 (UNITS)
TABLE 13 MIDDLE EAST AND AFRICA NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2021-2030 (ASP)
TABLE 14 MIDDLE EAST AND AFRICA NEW BORN SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 15 MIDDLE EAST AND AFRICA CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 16 MIDDLE EAST AND AFRICA CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 17 MIDDLE EAST AND AFRICA CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 18 MIDDLE EAST AND AFRICA CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 19 MIDDLE EAST AND AFRICA CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (UNITS)
TABLE 20 MIDDLE EAST AND AFRICA CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 21 MIDDLE EAST AND AFRICA EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 22 MIDDLE EAST AND AFRICA EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (UNITS)
TABLE 23 MIDDLE EAST AND AFRICA CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 24 MIDDLE EAST AND AFRICA CARRIER TESTING IN GENETIC TESTING MARKET, BY MEDICAL CONDITION 2021-2030 (USD MILLION)
TABLE 25 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 26 MIDDLE EAST AND AFRICA POLYMERASE CHAIN REACTION IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 27 MIDDLE EAST AND AFRICA DNA SEQUENCING (NGS-BASED TESTING) IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 28 MIDDLE EAST AND AFRICA MICROARRAYS IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 29 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET , BY DISEASES, 2021-2030 (USD MILLION)
TABLE 30 MIDDLE EAST AND AFRICA CANCER IN GENETIC TESTING MARKET, BY DISEASES, 2021-2030 (USD MILLION)
TABLE 31 MIDDLE EAST AND AFRICA RARE GENETIC DISORDER IN GENETIC TESTING MARKET, BY DISEASES, 2021-2030 (USD MILLION)
TABLE 32 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 33 IDDLE EAST AND AFRICA GENETIC TESTING MARKET, BY COUNTRY 2021-2030 (USD MILLION)
TABLE 34 SOUTH AFRICA GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 35 SOUTH AFRICA GENETIC TESTING MARKET, BY TYPE, 2021-2030 (UNITS)
TABLE 36 SOUTH AFRICA GENETIC TESTING MARKET, BY TYPE, 2021-2030 (ASP)
TABLE 37 SOUTH AFRICA PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 38 SOUTH AFRICA PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (UNITS)
TABLE 39 SOUTH AFRICA PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (ASP)
TABLE 40 SOUTH AFRICA NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2021-2030 (USD MILLION)
TABLE 41 SOUTH AFRICA NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2021-2030 (UNITS)
TABLE 42 SOUTH AFRICA NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2021-2030 (ASP)
TABLE 43 SOUTH AFRICA NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY CONDITION, 2021-2030 (USD MILLION)
TABLE 44 SOUTH AFRICA NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2021-2030 (USD MILLION)
TABLE 45 SOUTH AFRICA NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2021-2030 (UNITS)
TABLE 46 SOUTH AFRICA NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2021-2030 (ASP)
TABLE 47 SOUTH AFRICA NEW-BORN SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 48 SOUTH AFRICA CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 49 SOUTH AFRICA CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 50 SOUTH AFRICA CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2021-2030 (ASP)
TABLE 51 SOUTH AFRICA CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 52 SOUTH AFRICA CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (UNITS)
TABLE 53 SOUTH AFRICA CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (ASP)
TABLE 54 SOUTH AFRICA EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 55 SOUTH AFRICA EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (UNITS)
TABLE 56 SOUTH AFRICA EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (ASP)
TABLE 57 SOUTH AFRICA CARRIER TESTING IN GENETIC TESTING MARKET, BY MEDICAL CONDITION, 2021-2030 (USD MILLION)
TABLE 58 SOUTH AFRICA GENETIC TESTING MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 59 SOUTH AFRICA POLYMERASE CHAIN REACTION IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 60 SOUTH AFRICA DNA SEQUENCING (NGS-BASED TESTING) IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 61 SOUTH AFRICA MICROARRAYS IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 62 SOUTH AFRICA GENETIC TESTING MARKET, BY DISEASES, 2021-2030 (USD MILLION)
TABLE 63 SOUTH AFRICA RARE GENETIC DISORDER IN GENETIC TESTING MARKET, BY DISEASES, 2021-2030 (USD MILLION)
TABLE 64 SOUTH AFRICA CANCER IN GENETIC TESTING MARKET, BY DISEASES, 2021-2030 (USD MILLION)
TABLE 65 SOUTH AFRICA GENETIC TESTING MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 66 SAUDI ARABIA GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 67 SAUDI ARABIA GENETIC TESTING MARKET, BY TYPE, 2021-2030 (UNITS)
TABLE 68 SAUDI ARABIA GENETIC TESTING MARKET, BY TYPE, 2021-2030 (ASP)
TABLE 69 SAUDI ARABIA PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 70 SAUDI ARABIA PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (UNITS)
TABLE 71 SAUDI ARABIA PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (ASP)
TABLE 72 SAUDI ARABIA NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2021-2030 (USD MILLION)
TABLE 73 SAUDI ARABIA NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2021-2030 (UNITS)
TABLE 74 SAUDI ARABIA NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2021-2030 (ASP)
TABLE 75 SAUDI ARABIA NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY CONDITION, 2021-2030 (USD MILLION)
TABLE 76 SAUDI ARABIA NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2021-2030 (USD MILLION)
TABLE 77 SAUDI ARABIA NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2021-2030 (UNITS)
TABLE 78 SAUDI ARABIA NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2021-2030 (ASP)
TABLE 79 SAUDI ARABIA NEW-BORN SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 80 SAUDI ARABIA CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 81 SAUDI ARABIA CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 82 SAUDI ARABIA CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2021-2030 (ASP)
TABLE 83 SAUDI ARABIA CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 84 SAUDI ARABIA CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (UNITS)
TABLE 85 SAUDI ARABIA CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (ASP)
TABLE 86 SAUDI ARABIA EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 87 SAUDI ARABIA EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (UNITS)
TABLE 88 SAUDI ARABIA EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (ASP)
TABLE 89 SAUDI ARABIA CARRIER TESTING IN GENETIC TESTING MARKET, BY MEDICAL CONDITION, 2021-2030 (USD MILLION)
TABLE 90 SAUDI ARABIA GENETIC TESTING MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 91 SAUDI ARABIA POLYMERASE CHAIN REACTION IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 92 SAUDI ARABIA DNA SEQUENCING (NGS-BASED TESTING) IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 93 SAUDI ARABIA MICROARRAYS IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 94 SAUDI ARABIA GENETIC TESTING MARKET, BY DISEASES, 2021-2030 (USD MILLION)
TABLE 95 SAUDI ARABIA RARE GENETIC DISORDER IN GENETIC TESTING MARKET, BY DISEASES, 2021-2030 (USD MILLION)
TABLE 96 SAUDI ARABIA CANCER IN GENETIC TESTING MARKET, BY DISEASES, 2021-2030 (USD MILLION)
TABLE 97 SAUDI ARABIA GENETIC TESTING MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 98 U.A.E. GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 99 U.A.E. GENETIC TESTING MARKET, BY TYPE, 2021-2030 (UNITS)
TABLE 100 U.A.E. GENETIC TESTING MARKET, BY TYPE, 2021-2030 (ASP)
TABLE 101 U.A.E. PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 102 U.A.E. PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (UNITS)
TABLE 103 U.A.E. PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (ASP)
TABLE 104 U.A.E. NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2021-2030 (USD MILLION)
TABLE 105 U.A.E. NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2021-2030 (UNITS)
TABLE 106 U.A.E. NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2021-2030 (ASP)
TABLE 107 U.A.E. NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY CONDITION, 2021-2030 (USD MILLION)
TABLE 108 U.A.E. NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2021-2030 (USD MILLION)
TABLE 109 U.A.E. NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2021-2030 (UNITS)
TABLE 110 U.A.E. NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2021-2030 (ASP)
TABLE 111 U.A.E. NEW-BORN SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 112 U.A.E. CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 113 U.A.E. CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 114 U.A.E. CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2021-2030 (ASP)
TABLE 115 U.A.E. CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 116 U.A.E. CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (UNITS)
TABLE 117 U.A.E. CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (ASP)
TABLE 118 U.A.E. EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 119 U.A.E. EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (UNITS)
TABLE 120 U.A.E. EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (ASP)
TABLE 121 U.A.E. CARRIER TESTING IN GENETIC TESTING MARKET, BY MEDICAL CONDITION, 2021-2030 (USD MILLION)
TABLE 122 U.A.E. GENETIC TESTING MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 123 U.A.E. POLYMERASE CHAIN REACTION IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 124 U.A.E. DNA SEQUENCING (NGS-BASED TESTING) IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 125 U.A.E. MICROARRAYS IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 126 U.A.E. GENETIC TESTING MARKET, BY DISEASES, 2021-2030 (USD MILLION)
TABLE 127 U.A.E. RARE GENETIC DISORDER IN GENETIC TESTING MARKET, BY DISEASES, 2021-2030 (USD MILLION)
TABLE 128 U.A.E. CANCER IN GENETIC TESTING MARKET, BY DISEASES, 2021-2030 (USD MILLION)
TABLE 129 U.A.E. GENETIC TESTING MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 130 ISRAEL GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 131 ISRAEL GENETIC TESTING MARKET, BY TYPE, 2021-2030 (UNITS)
TABLE 132 ISRAEL GENETIC TESTING MARKET, BY TYPE, 2021-2030 (ASP)
TABLE 133 ISRAEL PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 134 ISRAEL PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (UNITS)
TABLE 135 ISRAEL PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (ASP)
TABLE 136 ISRAEL NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2021-2030 (USD MILLION)
TABLE 137 ISRAEL NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2021-2030 (UNITS)
TABLE 138 ISRAEL NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2021-2030 (ASP)
TABLE 139 ISRAEL NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY CONDITION, 2021-2030 (USD MILLION)
TABLE 140 ISRAEL NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2021-2030 (USD MILLION)
TABLE 141 ISRAEL NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2021-2030 (UNITS)
TABLE 142 ISRAEL NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2021-2030 (ASP)
TABLE 143 ISRAEL NEW-BORN SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 144 ISRAEL CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 145 ISRAEL CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 146 ISRAEL CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2021-2030 (ASP)
TABLE 147 ISRAEL CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 148 ISRAEL CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (UNITS)
TABLE 149 ISRAEL CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (ASP)
TABLE 150 ISRAEL EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 151 ISRAEL EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (UNITS)
TABLE 152 ISRAEL EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (ASP)
TABLE 153 ISRAEL CARRIER TESTING IN GENETIC TESTING MARKET, BY MEDICAL CONDITION, 2021-2030 (USD MILLION)
TABLE 154 ISRAEL GENETIC TESTING MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 155 ISRAEL POLYMERASE CHAIN REACTION IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 156 ISRAEL DNA SEQUENCING (NGS-BASED TESTING) IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 157 ISRAEL MICROARRAYS IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 158 ISRAEL GENETIC TESTING MARKET, BY DISEASES, 2021-2030 (USD MILLION)
TABLE 159 ISRAEL RARE GENETIC DISORDER IN GENETIC TESTING MARKET, BY DISEASES, 2021-2030 (USD MILLION)
TABLE 160 ISRAEL CANCER IN GENETIC TESTING MARKET, BY DISEASES, 2021-2030 (USD MILLION)
TABLE 161 ISRAEL GENETIC TESTING MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 162 EGYPT GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 163 EGYPT GENETIC TESTING MARKET, BY TYPE, 2021-2030 (UNITS)
TABLE 164 EGYPT GENETIC TESTING MARKET, BY TYPE, 2021-2030 (ASP)
TABLE 165 EGYPT PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 166 EGYPT PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (UNITS)
TABLE 167 EGYPT PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (ASP)
TABLE 168 EGYPT NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2021-2030 (USD MILLION)
TABLE 169 EGYPT NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2021-2030 (UNITS)
TABLE 170 EGYPT NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2021-2030 (ASP)
TABLE 171 EGYPT NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY CONDITION, 2021-2030 (USD MILLION)
TABLE 172 EGYPT NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2021-2030 (USD MILLION)
TABLE 173 EGYPT NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2021-2030 (UNITS)
TABLE 174 EGYPT NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2021-2030 (ASP)
TABLE 175 EGYPT NEW-BORN SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 176 EGYPT CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 177 EGYPT CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 178 EGYPT CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 179 EGYPT CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (UNITS)
TABLE 180 EGYPT CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (ASP)
TABLE 181 EGYPT EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 182 EGYPT EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (UNITS)
TABLE 183 EGYPT EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (ASP)
TABLE 184 EGYPT CARRIER TESTING IN GENETIC TESTING MARKET, BY MEDICAL CONDITION, 2021-2030 (USD MILLION)
TABLE 185 EGYPT GENETIC TESTING MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 186 EGYPT POLYMERASE CHAIN REACTION IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 187 EGYPT DNA SEQUENCING (NGS-BASED TESTING) IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 188 EGYPT MICROARRAYS IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 189 EGYPT GENETIC TESTING MARKET, BY DISEASES, 2021-2030 (USD MILLION)
TABLE 190 EGYPT RARE GENETIC DISORDER IN GENETIC TESTING MARKET, BY DISEASES, 2021-2030 (USD MILLION)
TABLE 191 EGYPT CANCER IN GENETIC TESTING MARKET, BY DISEASES, 2021-2030 (USD MILLION)
TABLE 192 EGYPT GENETIC TESTING MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 193 REST OF MIDDLE EAST AND AFRICA GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
그림 목록
FIGURE 1 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: SEGMENTATION
FIGURE 2 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET:DATA TRIANGULATION
FIGURE 3 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: DROC ANALYSIS
FIGURE 4 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: GLOBAL VS REGIONAL MARKET ANALYSIS
FIGURE 5 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET :COMPANY RESEARCH ANALYSIS
FIGURE 6 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET : INTERVIEW DEMOGRAPHICS
FIGURE 7 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET : DBMR MARKET POSITION GRID
FIGURE 8 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET : MARKET APPLICATION COVERAGE GRID
FIGURE 9 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: SEGMENTATION
FIGURE 11 THE INCREASING PREVALENCE OF GENETIC DISEASES AND RISING HEALTHCARE EXPENDITURE ARE EXPECTED TO DRIVE THE MIDDLE EAST AND AFRICA GENETIC TESTING MARKET GROWTH IN THE FORECAST PERIOD OF 2023 TO 2030
FIGURE 12 DIGANOSTIC TESTING SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE MIDDLE EAST AND AFRICA GENETIC TESTING MARKET IN 2023 & 2030
FIGURE 13 EPIDEMIOLOGY
FIGURE 14 INCIDENCE OF ALL GENDER-SOUTH AFRICA AND SAUDI ARABIA
FIGURE 15 INCIDENCE OF ALL GENDER-UAE AND EGYPT
FIGURE 16 INCIDENCE OF ALL GENDER-ISRAEL
FIGURE 17 MORTALITY RATE- SOUTH AFRICA AND SAUDI ARABIA
FIGURE 18 MORTALITY RATE-UAE AND EGYPT
FIGURE 19 MORTALITY RATE-ISRAEL
FIGURE 20 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF THE MIDDLE EAST AND AFRICA GENETIC TESTING MARKET
FIGURE 21 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: BY TYPE, 2022
FIGURE 22 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: BY TYPE, 2023-2030 (USD MILLION)
FIGURE 23 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: BY TYPE, CAGR (2023-2030)
FIGURE 24 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: BY TYPE, LIFELINE CURVE
FIGURE 25 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: BY TECHNOLOGY, 2022
FIGURE 26 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: BY TECHNOLOGY, 2023-2030 (USD MILLION)
FIGURE 27 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: BY TECHNOLOGY, CAGR (2023-2030)
FIGURE 28 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: BY TECHNOLOGY, LIFELINE CURVE
FIGURE 29 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: BY DISEASES, 2022
FIGURE 30 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: BY DISEASES, 2023-2030 (USD MILLION)
FIGURE 31 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: BY DISEASES, CAGR (2023-2030)
FIGURE 32 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: BY DISEASES, LIFELINE CURVE
FIGURE 33 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: BY END USER, 2022
FIGURE 34 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: BY END USER, 2023-2030 (USD MILLION)
FIGURE 35 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: BY END USER, CAGR (2023-2030)
FIGURE 36 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: BY END USER, LIFELINE CURVE
FIGURE 37 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: SNAPSHOT (2022)
FIGURE 38 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: BY COUNTRY (2022)
FIGURE 39 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: BY COUNTRY (2023 & 2030)
FIGURE 40 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: BY COUNTRY (2022 & 2030)
FIGURE 41 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: BY TYPE (2023-2030)
FIGURE 42 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: COMPANY SHARE 2022 (%)

연구 방법론
데이터 수집 및 기준 연도 분석은 대규모 샘플 크기의 데이터 수집 모듈을 사용하여 수행됩니다. 이 단계에는 다양한 소스와 전략을 통해 시장 정보 또는 관련 데이터를 얻는 것이 포함됩니다. 여기에는 과거에 수집한 모든 데이터를 미리 검토하고 계획하는 것이 포함됩니다. 또한 다양한 정보 소스에서 발견되는 정보 불일치를 검토하는 것도 포함됩니다. 시장 데이터는 시장 통계 및 일관된 모델을 사용하여 분석하고 추정합니다. 또한 시장 점유율 분석 및 주요 추세 분석은 시장 보고서의 주요 성공 요인입니다. 자세한 내용은 분석가에게 전화를 요청하거나 문의 사항을 드롭하세요.
DBMR 연구팀에서 사용하는 주요 연구 방법론은 데이터 마이닝, 시장에 대한 데이터 변수의 영향 분석 및 주요(산업 전문가) 검증을 포함하는 데이터 삼각 측량입니다. 데이터 모델에는 공급업체 포지셔닝 그리드, 시장 타임라인 분석, 시장 개요 및 가이드, 회사 포지셔닝 그리드, 특허 분석, 가격 분석, 회사 시장 점유율 분석, 측정 기준, 글로벌 대 지역 및 공급업체 점유율 분석이 포함됩니다. 연구 방법론에 대해 자세히 알아보려면 문의를 통해 업계 전문가에게 문의하세요.
사용자 정의 가능
Data Bridge Market Research는 고급 형성 연구 분야의 선두 주자입니다. 저희는 기존 및 신규 고객에게 목표에 맞는 데이터와 분석을 제공하는 데 자부심을 느낍니다. 보고서는 추가 국가에 대한 시장 이해(국가 목록 요청), 임상 시험 결과 데이터, 문헌 검토, 재생 시장 및 제품 기반 분석을 포함하도록 사용자 정의할 수 있습니다. 기술 기반 분석에서 시장 포트폴리오 전략에 이르기까지 타겟 경쟁업체의 시장 분석을 분석할 수 있습니다. 귀하가 원하는 형식과 데이터 스타일로 필요한 만큼 많은 경쟁자를 추가할 수 있습니다. 저희 분석가 팀은 또한 원시 엑셀 파일 피벗 테이블(팩트북)로 데이터를 제공하거나 보고서에서 사용 가능한 데이터 세트에서 프레젠테이션을 만드는 데 도움을 줄 수 있습니다.













