Global Neuromyelitis Optica Treatment Market
시장 규모 (USD 10억)
연평균 성장률 :
% 
 USD
297.72 Million
USD
503.94 Million
2024
2032
USD
297.72 Million
USD
503.94 Million
2024
2032
| 2025 –2032 | |
| USD 297.72 Million | |
| USD 503.94 Million | |
|
|
|
|
글로벌 시신경척수염 치료 시장 세분화, 유형별(아쿠아포린-4 항체를 사용한 시신경척수염 스펙트럼 장애 및 아쿠아포린-4 항체를 사용하지 않은 시신경척수염 스펙트럼 장애), 치료 유형(약물, 혈장 교환 요법, 면역글로불린 요법), 약물(C5 단백질 억제제, 경구 코르티코스테로이드, 비스테로이드 면역억제제 등), 투여 경로(경구 및 주사), 최종 사용자(병원, 재택 치료, 전문 클리닉 등) - 2032년까지의 산업 동향 및 예측
시신경척수염 치료제 시장 분석
시신경척수염 스펙트럼 장애 유병률 증가와 치료 옵션의 발전으로 시신경척수염 치료 시장이 주목을 받고 있습니다. 시신경척수염 스펙트럼 장애는 시신경과 척수에 심한 염증을 유발하여 시력 상실과 장애를 유발하는 희귀 자가면역 질환입니다. 최근 시장 개발은 염증 과정에 관여하는 주요 단백질을 억제하는 단일클론 항체를 포함하여 이 질환의 근본적인 병태생리를 구체적으로 다루는 표적 치료에 중점을 두고 있습니다 . 의료 전문가와 환자들 사이에서 시신경척수염 스펙트럼 장애에 대한 인식이 높아짐에 따라 효과적인 치료 옵션에 대한 수요가 증가하고 있습니다. 또한, 진행 중인 임상 시험 과 연구를 통해 잠재적인 치료법의 파이프라인이 확장되고 있으며, 이는 환자 치료 결과를 향상시킬 것으로 예상됩니다. 이 질환에 대한 이해가 발전함에 따라 시장은 상당한 성장을 예상하고 있으며, 제약 회사와 의료 서비스 제공업체에게 환자의 요구에 맞는 혁신적인 솔루션을 개발할 수 있는 기회를 제공합니다.
시신경척수염 치료 시장 규모
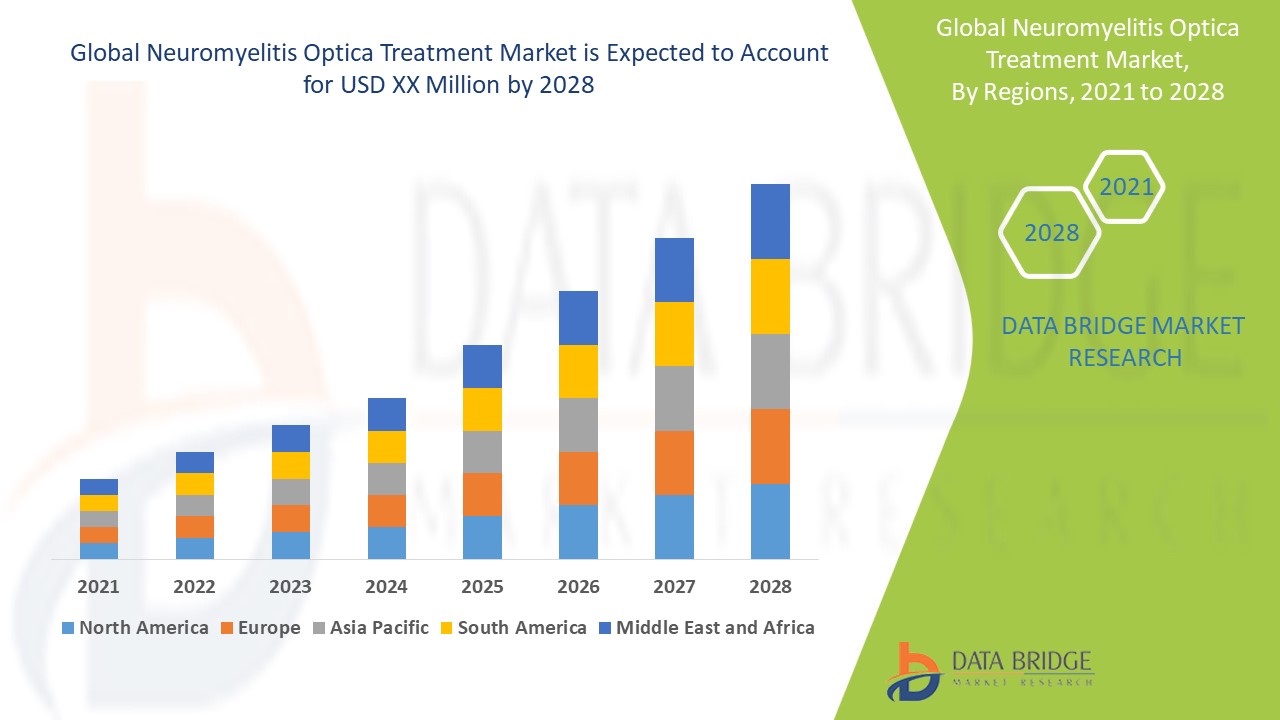
전 세계 시신경척수염 치료 시장 규모는 2024년에 2억 9,772만 달러로 평가되었으며, 2032년까지 5억 394만 달러에 이를 것으로 예상되며, 2025년부터 2032년까지의 예측 기간 동안 연평균 성장률은 6.80%입니다. Data Bridge Market Research에서 큐레이팅한 시장 보고서에는 시장 가치, 성장률, 세분화, 지리적 범위, 주요 기업 등 시장 시나리오에 대한 통찰력 외에도 심층 전문가 분석, 환자 역학, 파이프라인 분석, 가격 분석 및 규제 프레임워크가 포함됩니다.
시신경척수염 치료 시장 동향
“ 표적 치료제 개발”
시신경척수염 치료 시장은 치료 접근법의 혁신과 연구 활동의 증가에 힘입어 빠르게 발전하고 있습니다. 주목할 만한 추세는 시신경척수염 스펙트럼 장애와 관련된 염증 과정을 특이적으로 억제하도록 설계된 표적 치료제, 특히 단일클론 항체의 개발입니다. 이러한 발전은 치료의 효능과 안전성을 향상시켜 환자 치료 결과를 개선할 수 있는 희망을 제공합니다. 또한, 정밀 의학의 통합이 확대되어 개별 환자 프로필에 기반한 개인 맞춤형 치료 계획이 가능해지고 있습니다. 이 질환에 대한 인식이 높아지고 더 많은 임상 시험이 시작됨에 따라, 이 시장은 상당한 성장을 보일 것으로 예상되며, 이는 이 심각한 질환으로 고통받는 환자들을 위한 치료의 질을 향상시키려는 노력을 반영합니다.
보고서 범위 및 시신경척수염 치료 시장 세분화
|
속성 |
시신경척수염 치료 주요 시장 통찰력 |
|
다루는 세그먼트 |
|
|
포함 국가 |
미국, 캐나다 및 멕시코(북미), 독일, 프랑스, 영국, 네덜란드, 스위스, 벨기에, 러시아, 이탈리아, 스페인, 터키, 유럽의 기타 유럽 국가, 중국, 일본, 인도, 한국, 싱가포르, 말레이시아, 호주, 태국, 인도네시아, 필리핀, 아시아 태평양(APAC)의 기타 아시아 태평양 국가(APAC), 사우디 아라비아, UAE, 남아프리카 공화국, 이집트, 이스라엘, 중동 및 아프리카(MEA)의 일부인 기타 중동 및 아프리카(MEA), 브라질, 아르헨티나 및 남미의 일부인 기타 남미 |
|
주요 시장 참여자 |
F. Hoffmann-La Roche Ltd(스위스), AstraZeneca(영국), Teva Pharmaceutical Industries Ltd.(이스라엘), Alexion Pharmaceuticals, Inc(미국), Viela Bio(미국), Anvil Biosciences(미국), Opexa Therapeutics, Inc(미국), Arrien Pharmaceuticals, LLC(미국), TG Therapeutics, Inc(미국), Bionure(스페인) |
|
시장 기회 |
|
|
부가가치 데이터 정보 세트 |
Data Bridge Market Research에서 큐레이팅한 시장 보고서에는 시장 가치, 성장률, 세분화, 지리적 범위, 주요 업체 등 시장 시나리오에 대한 통찰력 외에도 심층적인 전문가 분석, 환자 역학, 파이프라인 분석, 가격 분석, 규제 프레임워크가 포함됩니다. |
시신경척수염 치료제 시장 정의
시신경척수염 치료는 시신경과 척수에 염증을 유발하는 자가면역 질환인 시신경척수염 스펙트럼 장애를 관리하기 위한 의학적 개입을 포함합니다. 치료에는 일반적으로 면역억제제, 단일클론항체 , 코르티코스테로이드, 혈장 교환 요법이 포함되며, 염증 감소, 증상 완화, 재발 방지, 그리고 신경 기능 보존을 통해 환자의 삶의 질을 향상시키는 데 중점을 둡니다.
시신경척수염 치료 시장 동향
운전자
- 시신경척수염의 증가
시신경척수염 스펙트럼 장애의 유병률 증가는 효과적인 치료 옵션에 대한 수요를 크게 증가시켜 시장 성장을 촉진하고 있습니다. 이 희귀 자가면역 질환에 대한 인식이 높아짐에 따라, 더 많은 환자들이 진단을 받고 증상 관리와 삶의 질 향상을 위한 의료적 개입을 모색하고 있습니다. 이러한 유병률 증가는 표적 치료제와 혁신적인 치료 솔루션의 개발 및 이용을 필요로 합니다. 의료 서비스 제공자들은 이 질환의 시의적절하고 효과적인 관리의 중요성을 점점 더 인식하고 있으며, 이는 치료 옵션에 대한 시장 확대로 이어지고 있습니다. 결과적으로 제약 회사들은 증가하는 환자들의 요구를 충족하기 위해 연구 개발에 투자하고 있으며, 이는 시장 성장을 더욱 촉진하고 있습니다.
- 증가하는 연구 및 임상 시험
진행 중인 임상 시험과 연구 계획은 시신경척수염 스펙트럼 장애의 잠재적 치료법 파이프라인을 확장하는 데 매우 중요하며, 이를 통해 치료 시장에 대한 관심을 높이고 있습니다. 이러한 노력은 시신경척수염 스펙트럼 장애 특유의 어려움을 해결하는 혁신적이고 표적화된 치료법 개발에 집중되어 있으며, 이는 환자의 치료 결과를 크게 향상시킬 수 있습니다. 임상 시험을 통해 새로운 치료법이 등장함에 따라, 이러한 치료법들은 기존 치료법에 대한 유망한 대안을 제시하여 치료 전략의 전반적인 효능과 안전성을 향상시킵니다. 또한, 연구 투자 증가는 시신경척수염에 대한 이해를 증진시키고 새로운 치료법 개발을 촉진하며 시장 성장을 더욱 촉진하는 역동적인 환경을 조성하려는 제약 회사와 연구자들의 헌신을 보여줍니다.
기회
- 원격진료의 부상
원격진료의 부상은 시신경척수염 스펙트럼 장애 환자의 전문의 접근성을 높이고 원격 모니터링을 용이하게 하는 중요한 기회를 제공합니다. 원격진료 플랫폼을 활용하면 환자는 집에서 편안하게 신경과 전문의 및 기타 의료 전문가와 쉽게 상담할 수 있어 이동 및 일정 예약과 관련된 장벽을 해소할 수 있습니다. 이러한 접근성 향상은 시의적절한 중재와 정기적인 추적 관찰을 가능하게 하며, 이는 질환을 효과적으로 관리하는 데 필수적입니다. 또한, 원격 모니터링 기술을 통해 의료 서비스 제공자는 환자의 건강 데이터를 실시간으로 추적하여 개인 맞춤형 치료 조정 및 증상의 선제적 관리를 용이하게 할 수 있습니다. 결과적으로, 시신경척수염 치료에 원격진료를 통합하면 환자 치료 결과가 개선되고 혁신적인 의료 솔루션에 대한 시장 잠재력이 확대됩니다.
- 새롭고 혁신적인 치료법 개발
시신경척수염 스펙트럼 장애의 기저 기전을 특이적으로 표적으로 하는 단일클론 항체 및 소분자 치료제 와 같은 새롭고 혁신적인 치료법 개발에 대한 상당한 기회가 있습니다 . 이러한 첨단 치료법은 질병의 근본 원인을 해결하여 더욱 효과적인 치료 옵션을 제공하고, 잠재적으로 환자 치료 결과를 크게 개선할 수 있습니다. 시신경척수염의 병태생리에 대한 이해가 높아짐에 따라 질병 활성도를 감소시키고 재발을 예방할 수 있는 표적 중재의 길이 열리고 있습니다. 또한, 연구를 통해 새로운 치료 경로가 발견됨에 따라 제약 회사들은 이러한 유망한 치료법 개발에 투자하여 시장 점유율을 확대하고 변화하는 환자 요구에 부응하도록 장려되고 있습니다. 이러한 혁신은 치료 옵션을 개선하고 시신경척수염 치료에 대한 더욱 개인화된 접근 방식을 구축하는 데 기여합니다.
제약/도전
- 경쟁적인 치료 옵션
자가면역 질환에 대한 대체 치료법의 존재는 시신경척수염 스펙트럼 장애를 표적으로 하는 치료법에 상당한 어려움을 야기합니다. 다른 자가면역 질환에 대한 기존 치료법이 시신경척수염 치료법을 압도하여, 새롭고 잠재적으로 더 효과적인 치료법이 시장 점유율을 확보하는 데 어려움을 겪을 수 있습니다. 의사들은 입증된 효능을 가진 익숙한 치료법을 우선시하는 경우가 많아, 시신경척수염에 대한 새로운 치료법 도입을 주저하게 만듭니다. 이러한 경쟁은 시신경척수염 치료법의 이점과 고유한 작용 기전에 대한 인지도를 저하시켜 궁극적으로 환자의 접근성을 떨어뜨리고 제약 회사의 연구 개발 투자 유인을 감소시킵니다. 결과적으로, 효과적인 치료 옵션의 필요성에도 불구하고 시장은 잠재력을 최대한 발휘하는 데 어려움을 겪습니다.
- 높은 치료 비용
단일클론 항체와 같은 혁신적인 치료법과 관련된 상당한 비용은 시신경척수염 치료 시장에 상당한 제약을 가하고 있습니다. 이러한 첨단 치료법은 종종 고가로 책정되어, 특히 의료 예산이 부족한 지역에서 환자의 접근성을 제한할 수 있습니다. 많은 의료 서비스 제공자는 환자의 비용 부담이나 보험 적용 범위 부족에 대한 우려로 이러한 고가 치료법을 처방하기를 꺼릴 수 있습니다. 이러한 재정적 장벽은 치료 순응도에 영향을 미치고 의료 접근성의 불균형을 초래하여 궁극적으로 시신경척수염 스펙트럼 장애 치료 전략의 전반적인 효과를 저해합니다. 결과적으로, 혁신적인 치료법과 관련된 높은 비용은 시장 성장과 환자 치료에 있어 여전히 중요한 과제로 남아 있습니다.
이 시장 보고서는 최근 동향, 무역 규제, 수출입 분석, 생산 분석, 가치 사슬 최적화, 시장 점유율, 국내 및 현지 시장 참여자의 영향, 신규 매출 창출 기회 분석, 시장 규제 변화, 전략적 시장 성장 분석, 시장 규모, 카테고리별 시장 성장, 응용 분야별 틈새 시장 및 시장 점유율, 제품 승인, 제품 출시, 지리적 확장, 시장 기술 혁신 등에 대한 세부 정보를 제공합니다. 시장에 대한 더 자세한 정보를 원하시면 Data Bridge Market Research에 문의하여 분석 브리핑을 요청하십시오. 저희 팀은 시장 성장을 위한 정보에 기반한 시장 결정을 내릴 수 있도록 도와드리겠습니다.
시신경척수염 치료 시장 범위
시장은 치료 유형, 약물, 투여 경로 및 최종 사용자를 기준으로 세분화됩니다. 이러한 세그먼트의 성장은 산업 내 저조한 성장 세그먼트를 분석하고, 사용자에게 핵심 시장 응용 분야를 파악하기 위한 전략적 의사 결정에 도움이 되는 귀중한 시장 개요와 시장 통찰력을 제공하는 데 도움이 될 것입니다.
유형
- 아쿠아포린-4 항체를 이용한 시신경척수염 스펙트럼 장애
- 아쿠아포린-4 항체가 없는 시신경 척수염 스펙트럼 장애
치료 유형
- 약물
- 혈장 교환 요법
- 면역글로불린 치료
약제
- C5 단백질 억제제
- 경구 코르티코스테로이드
- 비스테로이드 면역억제제
- 기타
투여 경로
- 경구
- 주사 가능
최종 사용자
- 병원
- 홈케어
- 전문 클리닉
- 기타
시신경척수염 치료 시장 지역 분석
위에 언급된 바와 같이 국가, 치료 유형, 약물, 투여 경로 및 최종 사용자별로 시장을 분석하고 시장 규모에 대한 통찰력과 추세를 제공합니다.
시장 보고서에 포함된 국가는 북미의 미국, 캐나다, 멕시코, 유럽의 독일, 프랑스, 영국, 네덜란드, 스위스, 벨기에, 러시아, 이탈리아, 스페인, 터키, 유럽의 기타 유럽 국가, 중국, 일본, 인도, 한국, 싱가포르, 말레이시아, 호주, 태국, 인도네시아, 필리핀, 아시아 태평양(APAC)의 기타 아시아 태평양(APAC), 사우디 아라비아, UAE, 남아프리카 공화국, 이집트, 이스라엘, 중동 및 아프리카(MEA)의 일부인 기타 중동 및 아프리카(MEA), 브라질, 아르헨티나, 남미의 일부인 기타 남미입니다.
북미 지역은 시신경척수염 치료 시장을 선도하고 있으며, 이는 주로 조기 진단 및 치료 옵션의 중요성에 대한 인식이 높아진 데 기인합니다. 의료 전문가와 환자 모두의 이러한 인지도 향상은 신제품 출시 및 치료 접근법 발전에 유리한 환경을 조성했습니다. 결과적으로, 북미 지역은 시신경척수염 스펙트럼 장애의 효과적인 치료법 개발 및 접근성 향상에 있어 핵심적인 역할을 지속적으로 수행하고 있습니다.
아시아 태평양 지역은 시신경척수염 스펙트럼 장애 환자 증가로 인해 2025년부터 2032년까지 상당한 성장을 경험할 것으로 예상됩니다. 이러한 환자 증가는 효과적인 치료 옵션과 의료 서비스에 대한 수요 증가를 촉진하고 있습니다. 의료 서비스에 대한 인식과 접근성이 향상됨에 따라, 아시아 태평양 지역은 시신경척수염 치료 분야의 주요 시장으로 부상할 것으로 예상됩니다.
보고서의 국가별 섹션은 개별 시장 영향 요인과 국내 시장 규제 변화도 제공하며, 이는 현재 및 미래 시장 동향에 영향을 미칩니다. 다운스트림 및 업스트림 가치 사슬 분석, 기술 동향, 포터의 5가지 힘 분석, 사례 연구 등의 데이터 포인트는 개별 국가의 시장 시나리오를 예측하는 데 활용됩니다. 또한, 글로벌 브랜드의 존재 및 가용성, 그리고 국내 및 국내 브랜드와의 경쟁이 심화되거나 부족해짐에 따라 직면하는 과제, 국내 관세 및 무역 경로의 영향 등을 고려하여 국가별 데이터를 예측 분석합니다.
시신경척수염 치료제 시장 점유율
시장 경쟁 구도는 경쟁사별 세부 정보를 제공합니다. 여기에는 회사 개요, 회사 재무 상태, 창출된 매출, 시장 잠재력, 연구 개발 투자, 신규 시장 진출, 글로벌 입지, 생산 시설 및 시설, 생산 능력, 회사의 강점과 약점, 제품 출시, 제품 종류 및 범위, 응용 분야별 우위 등이 포함됩니다. 위에 제공된 데이터는 해당 회사의 시장 집중도와 관련된 데이터입니다.
시신경척수염 치료제 시장에서 활동하는 선두주자는 다음과 같습니다.
- F. Hoffmann-La Roche Ltd(스위스)
- 아스트라제네카(영국)
- Teva Pharmaceutical Industries Ltd.(이스라엘)
- 알렉시온 파마슈티컬스(미국)
- 비엘라 바이오(미국)
- 앤빌 바이오사이언스(미국)
- 오펙사 테라퓨틱스(Opexa Therapeutics, Inc)(미국)
- Arrien Pharmaceuticals, LLC(미국)
- TG Therapeutics, Inc(미국)
- 바이오누레(스페인)
시신경척수염 치료 시장의 최신 동향
- 2024년 3월, 아스트라제네카의 울토미리스(Ravulizumab-cwvz)는 미국 식품의약국(FDA)으로부터 시신경척수염 스펙트럼 장애(NESD) 치료제로 승인을 받았습니다. 시신경척수염 스펙트럼 장애는 미국 성인 약 6,000명에게 영향을 미치는 희귀 자가면역 질환입니다. 이번 승인은 이 질환을 앓고 있는 환자들을 위한 치료 옵션에 있어 중요한 진전을 의미합니다. 아스트라제네카는 NMOSD에 대한 표적 치료를 제공함으로써, 의료 서비스가 부족한 이 환자들의 치료 결과와 삶의 질을 개선하고자 합니다.
- 2024년 3월, 아스트라제네카의 알렉시온은 울토미리스의 네 번째 적응증 승인을 발표하며, 희귀 자가면역 질환인 시신경척수염 스펙트럼 장애 치료에 사용될 수 있게 되었다고 발표했습니다. 이 획기적인 성과는 다양한 중증 자가면역 질환 치료에 있어 울토미리스의 다재다능함을 보여줍니다. 아스트라제네카는 이 새로운 적응증을 통해 이 까다로운 질환으로 고통받는 환자들의 치료 옵션을 강화하고 더 나은 건강 결과를 얻을 가능성을 높이는 것을 목표로 합니다.
- 2023년 10월, 암젠은 시신경척수염 스펙트럼 장애(NMOSD)와 관련된 염증성 바이오마커의 존재에 대한 귀중한 통찰력을 제공하는 3상 N-MOmentum 임상 시험의 새로운 결과를 발표했습니다. 이러한 결과는 질병 관련 발작을 최소화하는 데 있어 업리즈나(이네빌리주맙)의 "지속적인 효과"를 더욱 강화합니다. 이 연구는 업리즈나가 효과적인 치료 옵션으로서 잠재력을 발휘할 수 있음을 강조하며, NMOSD와 관련된 근본적인 염증 과정을 표적으로 삼아 질환을 관리하고 환자 치료 결과를 개선하는 데 있어 업리즈나의 역할을 강조합니다.
SKU-
세계 최초의 시장 정보 클라우드 보고서에 온라인으로 접속하세요
- 대화형 데이터 분석 대시보드
- 높은 성장 잠재력 기회를 위한 회사 분석 대시보드
- 사용자 정의 및 질의를 위한 리서치 분석가 액세스
- 대화형 대시보드를 통한 경쟁자 분석
- 최신 뉴스, 업데이트 및 추세 분석
- 포괄적인 경쟁자 추적을 위한 벤치마크 분석의 힘 활용
목차
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF GLOBAL NEUROMYELITIS OPTICA TREATMENT MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 KEY TAKEAWAYS
2.2 ARRIVING AT THE GLOBAL NEUROMYELITIS OPTICA TREATMENT MARKET SIZE
2.2.1 VENDOR POSITIONING GRID
2.2.2 TECHNOLOGY LIFE LINE CURVE
2.2.3 TRIPOD DATA VALIDATION MODEL
2.2.4 MARKET GUIDE
2.2.5 MULTIVARIATE MODELLING
2.2.6 TOP TO BOTTOM ANALYSIS
2.2.7 CHALLENGE MATRIX
2.2.8 APPLICATION COVERAGE GRID
2.2.9 STANDARDS OF MEASUREMENT
2.2.10 VENDOR SHARE ANALYSIS
2.2.11 EPIDEMIOLOGY BASED MODEL
2.2.12 DATA POINTS FROM KEY PRIMARY INTERVIEWS
2.2.13 DATA POINTS FROM KEY SECONDARY DATABASES
2.3 GLOBAL NEUROMYELITIS OPTICA TREATMENT MARKET: RESEARCH SNAPSHOT
2.4 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL ANALYSIS
4.2 PORTER’S FIVE FORCES MODEL
5 INDUSTRY INSIGHTS
5.1 PATENT ANALYSIS
5.1.1 PATENT LANDSCAPE
5.1.2 USPTO NUMBER
5.1.3 PATENT EXPIRY
5.1.4 EPIO NUMBER
5.1.5 PATENT STRENGTH AND QUALITY
5.1.6 PATENT CLAIMS
5.1.7 PATENT CITATIONS
5.1.8 PATENT LITIGATION AND LICENSING
5.1.9 FILE OF PATENT
5.1.10 PATENT RECEIVED CONTRIES
5.1.11 TECHNOLOGY BACKGROUND
5.2 DRUG TREATMENT RATE BY MATURED MARKETS
5.3 DEMOGRAPHIC TRENDS: IMPACTS ON ALL INCIDENCE RATES
5.4 PATIENT FLOW DIAGRAM
5.5 KEY PRICING STRATEGIES
5.6 KEY PATIENT ENROLLMENT STRATEGIES
5.7 INTERVIEWS WITH SPECIALIST
5.8 OTHER KOL SNAPSHOTS
6 EPIDEMIOLOGY
6.1 INCIDENCE OF ALL BY GENDER
6.2 TREATMENT RATE
6.3 MORTALITY RATE
6.4 DRUG ADHERENCE AND THERAPY SWITCH MODEL
6.5 PATIENT TREATMENT SUCCESS RATES
7 MERGERS AND ACQUISITION
7.1 LICENSING
7.2 COMMERCIALIZATION AGREEMENTS
8 REGULATORY FRAMEWORK
8.1 REGULATORY APPROVAL PROCESS
8.2 GEOGRAPHIES’ EASE OF REGULATORY APPROVAL
8.3 REGULATORY APPROVAL PATHWAYS
8.4 LICENSING AND REGISTRATION
8.5 POST-MARKETING SURVEILLANCE
8.6 GOOD MANUFACTURING PRACTICES (GMPS) GUIDELINES
9 PIPELINE ANALYSIS
9.1 CLINICAL TRIALS AND PHASE ANALYSIS
9.2 DRUG THERAPY PIPELINE
9.3 PHASE III CANDIDATES
9.4 PHASE II CANDIDATES
9.5 PHASE I CANDIDATES
9.6 OTHERS (PRE-CLINICAL AND RESEARCH)
TABLE 1 GLOBAL CLINICAL TRIAL MARKET FOR NEUROMYELITIS OPTICA TREATMENT MARKET
Company Name Therapeutic Area
XX XX
XX XX
XX XX
XX XX
XX XX
XX XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
TABLE 2 DISTRIBUTION OF PRODUCTS AND PROJECTS BY PHASE NEUROMYELITIS OPTICA TREATMENT MARKET
Phase Number of Projects
Preclinical/Research Projects XX
Clinical Development XX
Phase I XX
Phase II XX
Phase III XX
U.S. Filed/Approved But Not Yest Marketed XX
Total XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
TABLE 3 DISTRIBUTION OF PROJECTS BY THERAPEUTIC AREA AND PHASE NEUROMYELITIS OPTICA TREATMENT MARKET
Therapeutic Area Preclinical/ Research Project
XX XX
XX XX
XX XX
XX XX
XX XX
Total Projects XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
TABLE 4 DISTRIBUTION OF PROJECTS BY SCIENTIFIC APPROACH AND PHASE NEUROMYELITIS OPTICA TREATMENT MARKET
Technology Preclinical/ Research Project
XX XX
XX XX
XX XX
XX XX
XX XX
Total Projects XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
FIGURE 1 TOP ENTITIES BASED ON R&D GLANCE FOR NEUROMYELITIS OPTICA TREATMENT MARKET
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
10 MARKETED DRUG ANALYSIS
10.1 DRUG
10.1.1 BRAND NAME
10.1.2 GENERICS NAME
10.2 THERAPEUTIC INDIACTION
10.3 PHARACOLOGICAL CLASS OD THE DRUG
10.4 DRUG PRIMARY INDICATION
10.5 MARKET STATUS
10.6 MEDICATION TYPE
10.7 DRUG DOSAGES FORM
10.8 DOSAGES AVAILABILITY
10.9 PACKAGING TYPE
10.1 DRUG ROUTE OF ADMINISTRATION
10.11 DOSING FREQUENCY
10.12 DRUG INSIGHT
10.13 AN OVERVIEW OF THE DRUG DEVELOPMENT ACTIVITIES SUCH AS REGULATORY MILSTONE, SAFETY DATA AND EFFICACY DATA, MARKET EXCLUSIVITY DATA.
10.13.1 FORECAST MARKET OUTLOOK
10.13.2 CROSS COMPETITION
10.13.3 THERAPEUTIC PORTFOLIO
10.13.4 CURRENT DEVELOPMENT SCENARIO
11 MARKET ACCESS
11.1 10-YEAR MARKET FORECAST
11.2 CLINICAL TRIAL RECENT UPDATES
11.3 ANNUAL NEW FDA APPROVED DRUGS
11.4 DRUGS MANUFACTURER AND DEALS
11.5 MAJOR DRUG UPTAKE
11.6 CURRENT TREATMENT PRACTICES
11.7 IMPACT OF UPCOMING THERAPY
12 R & D ANALYSIS
12.1 COMPARATIVE ANALYSIS
12.2 DRUG DEVELOPMENTAL LANDSCAPE
12.3 IN-DEPTH INSIGHTS ON REGULATORY MILESTONES
12.4 THERAPEUTIC ASSESSMENT
12.5 ASSET-BASED COLLABORATIONS AND PARTNERSHIPS
13 MARKET OVERVIEW
13.1 DRIVERS
13.2 RESTRAINTS
13.3 OPPORTUNITIES
13.4 CHALLENGES
14 GLOBAL NEUROMYELITIS OPTICA TREATMENT MARKET, BY TYPE
14.1 OVERVIEW
14.2 WITH AQUAPORIN-4 ANTIBODIES
14.3 WITHOUT AQUAPORIN-4 ANTIBODIES
15 GLOBAL NEUROMYELITIS OPTICA TREATMENT MARKET, BY DISEASE TYPE
15.1 OVERVIEW
15.2 RELAPSING FORM
15.3 MONOPHASIC FORM
16 GLOBAL NEUROMYELITIS OPTICA TREATMENT MARKET, BY TREATMENT TYPE
16.1 OVERVIEW
16.2 MEDICATION
16.2.1 APPROVED/MARKETED TREATMENT
16.2.1.1. IMMUNOSUPPRESSANT
16.2.1.1.1. AZATHIOPRINE
16.2.1.1.2. MYCOPHENOLATE
16.2.1.1.3. RITUXIMAB
16.2.1.1.4. CORTICOSTEROID
16.2.1.1.4.1 METHYLPREDNISOLONE
16.2.1.1.4.2 PREDNISONE
16.2.1.1.4.3 OTHERS
16.2.1.1.5. OTHERS
16.2.1.2. MONOCLONAL ANTIBODY
16.2.1.2.1. ECULIZUMAB
16.2.1.2.2. INEBILIZUMAB
16.2.1.2.3. SATRALIZUMAB-MWGE
16.2.1.2.4. OTHERS
16.2.1.3. ANTI-EPILEPTIC MEDICATIONS
16.2.1.3.1. GABAPENTIN
16.2.1.3.2. CARBAMAZEPINE
16.2.1.3.3. OTHERS
16.2.1.4. ANTI-SPASMODICS
16.2.1.4.1. BACLOFEN
16.2.1.4.2. TIZANIDINE
16.2.1.4.3. OTHERS
16.2.1.5. ANTI-DEPRESSANTS
16.2.1.5.1. AMITRIPTYLINE
16.2.1.5.2. DULOXETINE
16.2.1.5.3. OTHERS
16.2.1.6. ANALGESICS
16.2.1.6.1. TRAMADOL
16.2.1.6.2. OPIATES
16.2.1.6.3. OTHERS
16.2.1.7. OTHERS
16.2.2 PIPELINE MEDICATION
16.2.2.1. ANTI-FCRN BATOCLIMAB
16.2.2.2. ABX-1431
16.2.2.3. TG-1101
16.2.2.4. NDC-1308
16.2.2.5. OTHERS
16.3 PLASMAPHERESIS
16.3.1 FILTERS
16.3.1.1. PLASMA EXCHANGE
16.3.1.2. DOUBLE FILTRATION PLASMAPHERESIS
16.3.1.3. IMMUNOADSORPTION
16.3.1.4. OTHERS
16.3.2 MACHINES
16.3.2.1. BY TYPE
16.3.2.1.1. THERAPEUTIC APHAERESISI MACHINE
16.3.2.1.2. PLASMAPHERESIS MACHINE
16.3.2.1.3. CENTRIFUGES
16.3.2.1.4. OTHERS
16.3.2.2. BY MODALITY
16.3.2.2.1. TROLLEY MOUNTED
16.3.2.2.2. BENCH TOP
16.3.2.2.3. OTHERS
16.3.3 REPLACEMENT FLUIDS
16.3.3.1. ALBUMIN
16.3.3.2. ELECTROLYTE
16.3.3.3. HYDROXYETHYL STARCH
16.3.3.4. FFP
16.3.3.5. PURIFIED PROTEIN PRODUCTS
16.3.3.6. OTHERS
16.3.4 DISPOSABLES
16.3.4.1. SINGLE USE TUBING SETS
16.3.4.2. INJECTION NEEDLE
16.3.4.3. SPARE FILTERS
16.3.4.4. SOLUTIONS
16.3.4.5. OTHERS
16.3.5 OTHERS
16.4 STEM CELL THERAPY
16.4.1 HEMATOPOIETIC STEM CELL TRANSPLANTATION (HSCT)
16.4.1.1. AUTOLOGOUS
16.4.1.2. ALLOGENEIC
16.4.2 MESENCHYMAL STEM CELLS
16.5 OTHERS
17 GLOBAL NEUROMYELITIS OPTICA TREATMENT MARKET, BY DRUG TYPE
17.1 OVERVIEW
17.2 BRANDED
17.2.1 SOLIRIS
17.2.2 UPLIZNA
17.2.3 ENSPRYNG
17.2.4 OTHERS
17.3 GENERICS
18 GLOBAL NEUROMYELITIS OPTICA TREATMENT MARKET, BY ROUTE OF ADMINISTRATION
18.1 OVERVIEW
18.2 ORAL
18.2.1 TABLETS
18.2.2 SUSPENSION
18.2.3 OTHERS
18.3 PARENTERAL
18.3.1 INTRAVENOUS
18.3.2 SUBCUTANEOUS
18.3.3 OTHERS
18.4 OTHERS
19 GLOBAL NEUROMYELITIS OPTICA TREATMENT MARKET, BY POPULATION TYPE
19.1 OVERVIEW
19.2 MALE
19.2.1 PEDIATRIC
19.2.2 ADULTS
19.2.3 GERIATRIC
19.3 FEMALE
19.3.1 PEDIATRIC
19.3.2 ADULTS
19.3.3 GERIATRIC
20 GLOBAL NEUROMYELITIS OPTICA TREATMENT MARKET, BY AGE GROUP
20.1 OVERVIEW
20.2 PEDIATRIC
20.3 ADULTS
20.4 GERIATRIC
21 GLOBAL NEUROMYELITIS OPTICA TREATMENT MARKET, BY END USER
21.1 OVERVIEW
21.2 HOSPITALS
21.2.1 BY TYPE
21.2.1.1. PUBLIC
21.2.1.2. PRIVATE
21.2.2 BY LEVEL
21.2.2.1. TIER 1
21.2.2.2. TIER 2
21.2.2.3. TIER 3
21.3 SPECIALTY CLINICS
21.4 HOME HEALTHCARE
21.5 ACADEMIC AND RESEARCH INSTITUTES
21.6 OTHERS
22 GLOBAL NEUROMYELITIS OPTICA TREATMENT MARKET, BY DISTRIBUTION CHANNEL
22.1 OVERVIEW
22.2 DIRECT TENDER
22.3 RETAIL SALES
22.3.1 OFFLINE SALES
22.3.1.1. HOSPITAL PHARMACIES
22.3.1.2. RETAIL PHARMACIES
22.3.1.3. OTHERS
22.3.2 ONLINE SALES
22.3.2.1. E-STORES
22.3.2.2. COMPANY WEBSITE
22.3.2.3. OTHERS
22.4 OTHERS
23 GLOBAL NEUROMYELITIS OPTICA TREATMENT MARKET, BY GEOGRAPHY
23.1 GLOBAL NEUROMYELITIS OPTICA TREATMENT MARKET, (ALL SEGMENTATION PROVIDED ABOVE IS REPRESENTED IN THIS CHAPTER BY COUNTRY)
23.1.1 NORTH AMERICA
23.1.1.1. U.S.
23.1.1.2. CANADA
23.1.1.3. MEXICO
23.1.2 EUROPE
23.1.2.1. GERMANY
23.1.2.2. FRANCE
23.1.2.3. U.K.
23.1.2.4. HUNGARY
23.1.2.5. LITHUANIA
23.1.2.6. AUSTRIA
23.1.2.7. IRELAND
23.1.2.8. NORWAY
23.1.2.9. POLAND
23.1.2.10. ITALY
23.1.2.11. SPAIN
23.1.2.12. RUSSIA
23.1.2.13. TURKEY
23.1.2.14. BELGIUM
23.1.2.15. NETHERLANDS
23.1.2.16. SWITZERLAND
23.1.2.17. REST OF EUROPE
23.1.3 ASIA-PACIFIC
23.1.3.1. JAPAN
23.1.3.2. CHINA
23.1.3.3. SOUTH KOREA
23.1.3.4. INDIA
23.1.3.5. AUSTRALIA
23.1.3.6. SINGAPORE
23.1.3.7. THAILAND
23.1.3.8. MALAYSIA
23.1.3.9. INDONESIA
23.1.3.10. PHILIPPINES
23.1.3.11. VIETNAM
23.1.3.12. REST OF ASIA-PACIFIC
23.1.4 SOUTH AMERICA
23.1.4.1. BRAZIL
23.1.4.2. ARGENTINA
23.1.4.3. PERU
23.1.4.4. REST OF SOUTH AMERICA
23.1.5 MIDDLE EAST AND AFRICA
23.1.5.1. SOUTH AFRICA
23.1.5.2. SAUDI ARABIA
23.1.5.3. UAE
23.1.5.4. EGYPT
23.1.5.5. KUWAIT
23.1.5.6. ISRAEL
23.1.5.7. REST OF MIDDLE EAST AND AFRICA
23.1.6 KEY PRIMARY INSIGHTS: BY MAJOR COUNTRIES
24 GLOBAL NEUROMYELITIS OPTICA TREATMENT MARKET, COMPANY LANDSCAPE
24.1 COMPANY SHARE ANALYSIS: GLOBAL
24.2 COMPANY SHARE ANALYSIS: NORTH AMERICA
24.3 COMPANY SHARE ANALYSIS: EUROPE
24.4 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
24.5 MERGERS & ACQUISITIONS
24.6 NEW PRODUCT DEVELOPMENT & APPROVALS
24.7 EXPANSIONS
24.8 REGULATORY CHANGES
24.9 PARTNERSHIP AND OTHER STRATEGIC DEVELOPMENTS
25 GLOBAL NEUROMYELITIS OPTICA TREATMENT MARKET, SWOT AND DBMR ANALYSIS
26 GLOBAL NEUROMYELITIS OPTICA TREATMENT MARKET, COMPANY PROFILE
26.1 PHARMA MANUFACTURES
26.1.1 CORESTEMCHEMON INC.
26.1.1.1. COMPANY OVERVIEW
26.1.1.2. REVENUE ANALYSIS
26.1.1.3. GEOGRAPHIC PRESENCE
26.1.1.4. PRODUCT PORTFOLIO
26.1.1.5. RECENT DEVELOPMENTS
26.1.2 ASTRAZENECA
26.1.2.1. COMPANY OVERVIEW
26.1.2.2. REVENUE ANALYSIS
26.1.2.3. GEOGRAPHIC PRESENCE
26.1.2.4. PRODUCT PORTFOLIO
26.1.2.5. RECENT DEVELOPMENTS
26.1.3 HARBOUR BIOMED
26.1.3.1. COMPANY OVERVIEW
26.1.3.2. REVENUE ANALYSIS
26.1.3.3. GEOGRAPHIC PRESENCE
26.1.3.4. PRODUCT PORTFOLIO
26.1.3.5. RECENT DEVELOPMENTS
26.1.4 TG THERAPEUTICS
26.1.4.1. COMPANY OVERVIEW
26.1.4.2. REVENUE ANALYSIS
26.1.4.3. GEOGRAPHIC PRESENCE
26.1.4.4. PRODUCT PORTFOLIO
26.1.4.5. RECENT DEVELOPMENTS
26.1.5 ENDECE
26.1.5.1. COMPANY OVERVIEW
26.1.5.2. REVENUE ANALYSIS
26.1.5.3. GEOGRAPHIC PRESENCE
26.1.5.4. PRODUCT PORTFOLIO
26.1.5.5. RECENT DEVELOPMENTS
26.1.6 ALEXION PHARMACEUTICALS
26.1.6.1. COMPANY OVERVIEW
26.1.6.2. REVENUE ANALYSIS
26.1.6.3. GEOGRAPHIC PRESENCE
26.1.6.4. PRODUCT PORTFOLIO
26.1.6.5. RECENT DEVELOPMENTS
26.1.7 HORIZON THERAPEUTICS PLC
26.1.7.1. COMPANY OVERVIEW
26.1.7.2. REVENUE ANALYSIS
26.1.7.3. GEOGRAPHIC PRESENCE
26.1.7.4. PRODUCT PORTFOLIO
26.1.7.5. RECENT DEVELOPMENTS
26.1.8 F. HOFFMANN-LA ROCHE LTD
26.1.8.1. COMPANY OVERVIEW
26.1.8.2. REVENUE ANALYSIS
26.1.8.3. GEOGRAPHIC PRESENCE
26.1.8.4. PRODUCT PORTFOLIO
26.1.8.5. RECENT DEVELOPMENTS
26.1.9 PFIZER INC.
26.1.9.1. COMPANY OVERVIEW
26.1.9.2. REVENUE ANALYSIS
26.1.9.3. GEOGRAPHIC PRESENCE
26.1.9.4. PRODUCT PORTFOLIO
26.1.9.5. RECENT DEVELOPMENTS
26.1.10 AMNEAL PHARMACEUTICALS LLC.
26.1.10.1. COMPANY OVERVIEW
26.1.10.2. REVENUE ANALYSIS
26.1.10.3. GEOGRAPHIC PRESENCE
26.1.10.4. PRODUCT PORTFOLIO
26.1.10.5. RECENT DEVELOPMENTS
26.1.11 ZYDUS GROUP
26.1.11.1. COMPANY OVERVIEW
26.1.11.2. REVENUE ANALYSIS
26.1.11.3. GEOGRAPHIC PRESENCE
26.1.11.4. PRODUCT PORTFOLIO
26.1.11.5. RECENT DEVELOPMENTS
26.1.12 JUBILANT CADISTA PHARMACEUTICALS INC.
26.1.12.1. COMPANY OVERVIEW
26.1.12.2. REVENUE ANALYSIS
26.1.12.3. GEOGRAPHIC PRESENCE
26.1.12.4. PRODUCT PORTFOLIO
26.1.12.5. RECENT DEVELOPMENTS
26.1.13 HIKMA PHARMACEUTICALS PLC
26.1.13.1. COMPANY OVERVIEW
26.1.13.2. REVENUE ANALYSIS
26.1.13.3. GEOGRAPHIC PRESENCE
26.1.13.4. PRODUCT PORTFOLIO
26.1.13.5. RECENT DEVELOPMENTS
26.1.14 VIATRIS INC.
26.1.14.1. COMPANY OVERVIEW
26.1.14.2. REVENUE ANALYSIS
26.1.14.3. GEOGRAPHIC PRESENCE
26.1.14.4. PRODUCT PORTFOLIO
26.1.14.5. RECENT DEVELOPMENTS
26.1.15 SEBELA PHARMACEUTICALS
26.1.15.1. COMPANY OVERVIEW
26.1.15.2. REVENUE ANALYSIS
26.1.15.3. GEOGRAPHIC PRESENCE
26.1.15.4. PRODUCT PORTFOLIO
26.1.15.5. RECENT DEVELOPMENTS
26.1.16 NOVARTIS AG
26.1.16.1. COMPANY OVERVIEW
26.1.16.2. REVENUE ANALYSIS
26.1.16.3. GEOGRAPHIC PRESENCE
26.1.16.4. PRODUCT PORTFOLIO
26.1.16.5. RECENT DEVELOPMENTS
26.1.17 ACCORD-UK LTD
26.1.17.1. COMPANY OVERVIEW
26.1.17.2. REVENUE ANALYSIS
26.1.17.3. GEOGRAPHIC PRESENCE
26.1.17.4. PRODUCT PORTFOLIO
26.1.17.5. RECENT DEVELOPMENTS
26.1.18 TEVA PHARMACEUTICALS USA, INC.
26.1.18.1. COMPANY OVERVIEW
26.1.18.2. REVENUE ANALYSIS
26.1.18.3. GEOGRAPHIC PRESENCE
26.1.18.4. PRODUCT PORTFOLIO
26.1.18.5. RECENT DEVELOPMENTS
26.1.19 ALKEM LABS
26.1.19.1. COMPANY OVERVIEW
26.1.19.2. REVENUE ANALYSIS
26.1.19.3. GEOGRAPHIC PRESENCE
26.1.19.4. PRODUCT PORTFOLIO
26.1.19.5. RECENT DEVELOPMENTS
26.1.20 STRIDES PHARMA INC.
26.1.20.1. COMPANY OVERVIEW
26.1.20.2. REVENUE ANALYSIS
26.1.20.3. GEOGRAPHIC PRESENCE
26.1.20.4. PRODUCT PORTFOLIO
26.1.20.5. RECENT DEVELOPMENTS
26.1.21 CONCORD BIOTECH
26.1.21.1. COMPANY OVERVIEW
26.1.21.2. REVENUE ANALYSIS
26.1.21.3. GEOGRAPHIC PRESENCE
26.1.21.4. PRODUCT PORTFOLIO
26.1.21.5. RECENT DEVELOPMENTS
26.2 MEDICAL DEVICE MANUFACTURE
26.2.1 ASAHI KESEI CORPORATION
26.2.1.1. COMPANY OVERVIEW
26.2.1.2. REVENUE ANALYSIS
26.2.1.3. GEOGRAPHIC PRESENCE
26.2.1.4. PRODUCT PORTFOLIO
26.2.1.5. RECENT DEVELOPMENTS
26.2.2 HAEMONETICS CORPORATION
26.2.2.1. COMPANY OVERVIEW
26.2.2.2. REVENUE ANALYSIS
26.2.2.3. GEOGRAPHIC PRESENCE
26.2.2.4. PRODUCT PORTFOLIO
26.2.2.5. RECENT DEVELOPMENTS
26.2.3 MILTENYI BIOTEC AND/OR ITS AFFILIATES
26.2.3.1. COMPANY OVERVIEW
26.2.3.2. REVENUE ANALYSIS
26.2.3.3. GEOGRAPHIC PRESENCE
26.2.3.4. PRODUCT PORTFOLIO
26.2.3.5. RECENT DEVELOPMENTS
26.2.4 GRIFOLS, S.A.
26.2.4.1. COMPANY OVERVIEW
26.2.4.2. REVENUE ANALYSIS
26.2.4.3. GEOGRAPHIC PRESENCE
26.2.4.4. PRODUCT PORTFOLIO
26.2.4.5. RECENT DEVELOPMENTS
26.2.5 OCTAPHARMA AG
26.2.5.1. COMPANY OVERVIEW
26.2.5.2. REVENUE ANALYSIS
26.2.5.3. GEOGRAPHIC PRESENCE
26.2.5.4. PRODUCT PORTFOLIO
26.2.5.5. RECENT DEVELOPMENTS
26.2.6 3M (SOLVENTUM)
26.2.6.1. COMPANY OVERVIEW
26.2.6.2. REVENUE ANALYSIS
26.2.6.3. GEOGRAPHIC PRESENCE
26.2.6.4. PRODUCT PORTFOLIO
26.2.6.5. RECENT DEVELOPMENTS
26.2.7 B. BRAUN SE
26.2.7.1. COMPANY OVERVIEW
26.2.7.2. REVENUE ANALYSIS
26.2.7.3. GEOGRAPHIC PRESENCE
26.2.7.4. PRODUCT PORTFOLIO
26.2.7.5. RECENT DEVELOPMENTS
26.2.8 FRESENIUS SE & CO. KGAA
26.2.8.1. COMPANY OVERVIEW
26.2.8.2. REVENUE ANALYSIS
26.2.8.3. GEOGRAPHIC PRESENCE
26.2.8.4. PRODUCT PORTFOLIO
26.2.8.5. RECENT DEVELOPMENTS
26.2.9 INFOMED SA
26.2.9.1. COMPANY OVERVIEW
26.2.9.2. REVENUE ANALYSIS
26.2.9.3. GEOGRAPHIC PRESENCE
26.2.9.4. PRODUCT PORTFOLIO
26.2.9.5. RECENT DEVELOPMENTS
26.2.10 SB-KAWASUMI LABORATORIES, INC.
26.2.10.1. COMPANY OVERVIEW
26.2.10.2. REVENUE ANALYSIS
26.2.10.3. GEOGRAPHIC PRESENCE
26.2.10.4. PRODUCT PORTFOLIO
26.2.10.5. RECENT DEVELOPMENTS
26.2.11 TERUMO BCT, INC.
26.2.11.1. COMPANY OVERVIEW
26.2.11.2. REVENUE ANALYSIS
26.2.11.3. GEOGRAPHIC PRESENCE
26.2.11.4. PRODUCT PORTFOLIO
26.2.11.5. RECENT DEVELOPMENTS
26.2.12 BIOBASE BIOTECH (JINAN) CO., LTD
26.2.12.1. COMPANY OVERVIEW
26.2.12.2. REVENUE ANALYSIS
26.2.12.3. GEOGRAPHIC PRESENCE
26.2.12.4. PRODUCT PORTFOLIO
26.2.12.5. RECENT DEVELOPMENTS
26.2.13 BAXTER
26.2.13.1. COMPANY OVERVIEW
26.2.13.2. REVENUE ANALYSIS
26.2.13.3. GEOGRAPHIC PRESENCE
26.2.13.4. PRODUCT PORTFOLIO
26.2.13.5. RECENT DEVELOPMENTS
26.2.14 KANEKA CORPORATION
26.2.14.1. COMPANY OVERVIEW
26.2.14.2. REVENUE ANALYSIS
26.2.14.3. GEOGRAPHIC PRESENCE
26.2.14.4. PRODUCT PORTFOLIO
26.2.14.5. RECENT DEVELOPMENTS
26.2.15 SHANGHAI DAHUA MEDICAL APPARATUS CO., LTD
26.2.15.1. COMPANY OVERVIEW
26.2.15.2. REVENUE ANALYSIS
26.2.15.3. GEOGRAPHIC PRESENCE
26.2.15.4. PRODUCT PORTFOLIO
26.2.15.5. RECENT DEVELOPMENTS
26.2.16 ZHENGYUAN TECHNOLOGY CO., LTD.
26.2.16.1. COMPANY OVERVIEW
26.2.16.2. REVENUE ANALYSIS
26.2.16.3. GEOGRAPHIC PRESENCE
26.2.16.4. PRODUCT PORTFOLIO
26.2.16.5. RECENT DEVELOPMENTS
NOTE: THE COMPANIES PROFILED IS NOT EXHAUSTIVE LIST AND IS AS PER OUR PREVIOUS CLIENT REQUIREMENT. WE PROFILE MORE THAN 100 COMPANIES IN OUR STUDY AND HENCE THE LIST OF COMPANIES CAN BE MODIFIED OR REPLACED ON REQUEST
27 RELATED REPORTS
28 CONCLUSION
29 QUESTIONNAIRE
30 ABOUT DATA BRIDGE MARKET RESEARCH

연구 방법론
데이터 수집 및 기준 연도 분석은 대규모 샘플 크기의 데이터 수집 모듈을 사용하여 수행됩니다. 이 단계에는 다양한 소스와 전략을 통해 시장 정보 또는 관련 데이터를 얻는 것이 포함됩니다. 여기에는 과거에 수집한 모든 데이터를 미리 검토하고 계획하는 것이 포함됩니다. 또한 다양한 정보 소스에서 발견되는 정보 불일치를 검토하는 것도 포함됩니다. 시장 데이터는 시장 통계 및 일관된 모델을 사용하여 분석하고 추정합니다. 또한 시장 점유율 분석 및 주요 추세 분석은 시장 보고서의 주요 성공 요인입니다. 자세한 내용은 분석가에게 전화를 요청하거나 문의 사항을 드롭하세요.
DBMR 연구팀에서 사용하는 주요 연구 방법론은 데이터 마이닝, 시장에 대한 데이터 변수의 영향 분석 및 주요(산업 전문가) 검증을 포함하는 데이터 삼각 측량입니다. 데이터 모델에는 공급업체 포지셔닝 그리드, 시장 타임라인 분석, 시장 개요 및 가이드, 회사 포지셔닝 그리드, 특허 분석, 가격 분석, 회사 시장 점유율 분석, 측정 기준, 글로벌 대 지역 및 공급업체 점유율 분석이 포함됩니다. 연구 방법론에 대해 자세히 알아보려면 문의를 통해 업계 전문가에게 문의하세요.
사용자 정의 가능
Data Bridge Market Research는 고급 형성 연구 분야의 선두 주자입니다. 저희는 기존 및 신규 고객에게 목표에 맞는 데이터와 분석을 제공하는 데 자부심을 느낍니다. 보고서는 추가 국가에 대한 시장 이해(국가 목록 요청), 임상 시험 결과 데이터, 문헌 검토, 재생 시장 및 제품 기반 분석을 포함하도록 사용자 정의할 수 있습니다. 기술 기반 분석에서 시장 포트폴리오 전략에 이르기까지 타겟 경쟁업체의 시장 분석을 분석할 수 있습니다. 귀하가 원하는 형식과 데이터 스타일로 필요한 만큼 많은 경쟁자를 추가할 수 있습니다. 저희 분석가 팀은 또한 원시 엑셀 파일 피벗 테이블(팩트북)로 데이터를 제공하거나 보고서에서 사용 가능한 데이터 세트에서 프레젠테이션을 만드는 데 도움을 줄 수 있습니다.