Global Nephropathic Cystinosis Treatment Market
시장 규모 (USD 10억)
연평균 성장률 :
% 
 USD
1.39 Billion
USD
2.73 Billion
2024
2032
USD
1.39 Billion
USD
2.73 Billion
2024
2032
| 2025 –2032 | |
| USD 1.39 Billion | |
| USD 2.73 Billion | |
|
|
|
|
글로벌 신병성 시스틴증 치료 시장 세분화, 치료(시스틴 감소 치료, 증상 치료, 신장 이식), 투여 경로(경구 및 기타), 최종 사용자(병원, 진료소 및 기타), 유통 채널(병원 약국, 소매 약국 및 온라인 약국) - 2032년까지의 산업 동향 및 전망
신병성 시스틴증 치료 시장 규모
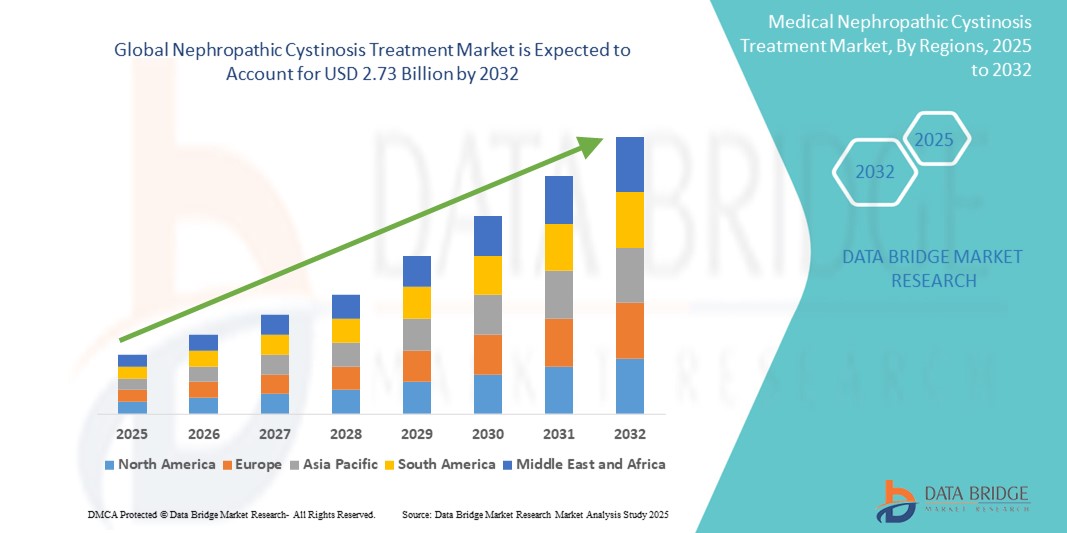
- 글로벌 신병성 시스틴증 치료 시장 규모는 2024년에 13억 9천만 달러 로 평가되었으며, 예측 기간 동안 8.81%의 CAGR 로 2032년까지 27억 3천만 달러에 도달할 것으로 예상됩니다 .
- 시장 성장은 주로 인지도 향상, 조기 진단 및 희귀 약물 개발의 발전에 의해 주도되고 있으며, 이로 인해 이 희귀 유전 질환의 관리가 크게 개선되었습니다.
- 또한, 시스티노신 유전자 돌연변이를 표적으로 삼는 정부 지원 사업과 연구 활동의 증가는 치료 혁신과 접근성을 강화하고 있습니다. 이러한 공동의 노력은 더욱 효과적인 치료 옵션의 개발과 이용을 촉진하여 시장 확대에 크게 기여하고 있습니다.
신병성 시스틴증 치료 시장 분석
- 이 희귀한 상염색체 열성 리소좀 저장 장애를 관리하는 것을 목표로 하는 신병성 시스틴증 치료법은 표적 시스틴 고갈 치료법을 통해 질병 진행을 지연시키고, 신장 기능을 보존하고, 삶의 질을 개선할 수 있는 능력으로 인해 희귀 질환 치료에 점점 더 필수적이 되고 있습니다.
- 시스티노시스 치료에 대한 수요 증가는 주로 조기 진단 개선, 의료 서비스 제공자의 인식 증가, 희귀 유전 질환 에 대한 희귀 의약품 접근성 증가에 따른 것입니다.
- 북미는 2024년 42.2%의 가장 큰 매출 점유율로 신병성 시스틴증 치료 시장을 장악했으며, 이는 첨단 의료 인프라, 상당한 R&D 투자, 희귀 질환에 대한 유리한 규제 경로, 특히 미국 내 주요 제약 혁신 기업의 존재에 힘입은 것입니다.
- 아시아 태평양 지역은 의료 인프라 개선, 희귀 질환 진단 및 치료법 접근성 증가, 환자 지원 프로그램 확대로 인해 예측 기간 동안 신병성 시스틴증 치료 시장에서 가장 빠르게 성장하는 지역이 될 것으로 예상됩니다.
- 시스틴 감소 치료 부문은 2024년 68%의 시장 점유율로 신병성 시스틴증 치료 시장을 장악했으며, 세포 내 시스틴 수치를 낮추고 말기 신장 질환으로의 진행을 지연시키는 효과에 힘입어 성장했습니다.
보고서 범위 및 신병성 시스틴증 치료 시장 세분화
|
속성 |
신병성 시스틴증 치료 주요 시장 통찰력 |
|
다루는 세그먼트 |
|
|
포함 국가 |
북아메리카
유럽
아시아 태평양
중동 및 아프리카
남아메리카
|
|
주요 시장 참여자 |
|
|
시장 기회 |
|
|
부가가치 데이터 정보 세트 |
Data Bridge Market Research에서 큐레이팅한 시장 보고서에는 시장 가치, 성장률, 세분화, 지리적 적용 범위, 주요 기업 등 시장 시나리오에 대한 통찰력 외에도 심층적인 전문가 분석, 가격 분석, 브랜드 점유율 분석, 소비자 설문 조사, 인구 통계 분석, 공급망 분석, 가치 사슬 분석, 원자재/소모품 개요, 공급업체 선택 기준, PESTLE 분석, Porter 분석 및 규제 프레임워크가 포함되어 있습니다. |
신병성 시스틴증 치료 시장 동향
지연 방출 요법 및 개인 맞춤 치료 접근법의 발전
- 전 세계 신병증성 시스틴증 치료 시장에서 중요하고 빠르게 성장하는 추세는 지연 방출 시스테아민 제형의 도입 증가와 환자 개개인의 필요에 맞춘 맞춤형 의료 접근법의 등장입니다. 이러한 혁신은 치료 순응도를 크게 향상시키고, 투약 빈도를 줄이며, 환자의 삶의 질을 향상시키고 있습니다.
- 예를 들어, Procysbi(지연 방출 시스테아민 비타르트레이트)는 6시간마다 투여해야 하는 Cystagon과 같은 기존 제형에 비해 하루 두 번 투여할 수 있어 특히 소아 환자의 치료 부담을 덜어줍니다.
- 유전자 검사와 조기 진단 기술의 발전 또한 환자 맞춤형 치료 계획 개발을 촉진하고 있습니다. 유전자 치료와 mRNA 기반 기술 연구는 아직 초기 단계이지만, 질병의 근본적인 유전적 원인(CTNS 유전자 돌연변이)을 해결하여 잠재적인 장기적인 해결책을 제시하는 것을 목표로 합니다.
- 또한, 준수 모니터링 앱 및 원격 환자 지원 서비스와 같은 디지털 건강 도구의 통합으로 환자와 의료 서비스 제공자가 질병을 효과적으로 관리할 수 있는 능력이 더욱 강화되고 있습니다.
- Horizon Therapeutics와 같은 제약 회사는 약물 내약성과 장기적 결과를 향상시키기 위해 환자 지원 프로그램과 차세대 치료법에 투자하고 있습니다.
- 더욱 개인화되고 편리하며 진보된 치료 전략을 향한 이러한 추세는 신병성 시스틴증 환경을 재편하고 질병 관리를 개선하며 부문 전반에 걸쳐 혁신을 촉진하고 있습니다.
신병성 시스틴증 치료 시장 동향
운전사
“인식 향상, 조기 진단 및 희귀의약품 개발”
- 향상된 진단 능력과 정부 주도 이니셔티브에 힘입어 희귀 질환 인식에 대한 전 세계적 관심이 높아지고 있으며 이는 신병성 시스틴증 치료 시장 성장을 촉진하는 주요 요인입니다.
- 예를 들어, 조기 유전자 검사 및 신생아 검사 프로그램을 통해 시스틴증의 시기적절한 탐지가 개선되어 더 빠른 치료적 개입과 더 나은 환자 결과가 가능해졌습니다.
- 미국 FDA 및 EMA와 같은 규제 기관의 희귀의약품 지정은 제약 회사에 시장 독점권, 세액 공제 및 가속 승인 등의 인센티브를 제공하여 Procysbi® 및 Cystagon®과 같은 표적 치료제 개발을 장려합니다.
- 또한 환자 옹호 단체와 희귀 질환 재단은 글로벌 협력 및 교육 캠페인을 통해 인식 제고, 연구 자금 지원, 치료 접근성 확대에 중요한 역할을 해왔습니다.
- 정책 지원, 조기 진단 및 제약 혁신의 결합으로 효과적인 치료법에 대한 접근성이 크게 향상되고 신병성 시스틴증에 대한 글로벌 치료 환경이 확대되고 있습니다.
제지/도전
“제한된 환자 수와 높은 치료 비용”
- 세계 신병성 시스틴증 치료 시장의 주요 제약은 환자 인구가 적고 지리적으로 분산되어 있어 광범위한 R&D 투자에 대한 상업적 인센티브가 제한되고 치료 접근성에 문제가 발생한다는 점입니다.
- 시스틴증은 10만~20만 명의 출생아 중 약 1명에게 영향을 미치는 극히 드문 질환으로, 특히 개발도상국에서 진단이 부족하고 의료에 대한 인식이 제한적입니다.
- 게다가 평생 치료의 높은 비용(예: Procysbi의 연간 가격이 25만 달러를 초과)은 환자와 지불자에게 부담을 주는 문제를 야기합니다. 특히 강력한 보험 적용 범위나 공공 의료 지원이 부족한 지역에서는 더욱 그렇습니다.
- 평생 치료 준수는 부작용, 복잡한 요법 및 만성 희귀 질환 관리의 정신사회적 부담에 의해 영향을 받습니다.
- 이러한 장애물을 극복하려면 환자 등록 확대, 저렴한 가격으로 의료 서비스를 제공하는 가격 책정 전략, 자원이 부족한 환경에서의 진단 접근성 확대, 비용 효율적이고 내약성이 좋은 치료법의 지속적인 개발 등 다양한 이해관계자의 노력이 필요합니다.
신병성 시스틴증 치료 시장 범위
시장은 치료, 투여 경로, 최종 사용자, 유통 채널을 기준으로 세분화됩니다.
- 치료별
신병증성 시스틴증 치료 시장은 치료 방식에 따라 시스틴 제거 요법, 대증 요법, 그리고 신장 이식 수술로 구분됩니다. 시스틴 제거 요법은 2024년 68%의 매출 점유율로 시장을 장악했으며, 세포 내 시스틴 축적을 감소시키는 1차 치료제이자 가장 효과적인 치료법으로서의 역할을 주도했습니다. 프로시스비®(Procysbi®)와 시스타곤®(Cystagon®)과 같은 약물은 시장에서 확고한 입지를 굳건히 하고 있으며, 질병 진행을 지연시키고 장기적인 신장 예후를 개선하는 데 필수적인 역할을 하고 있습니다. 시스틴 제거 요법의 일관된 임상 효능은 전 세계 시스틴증 치료 프로토콜에서 핵심적인 역할을 뒷받침합니다.
증상 치료 분야는 갑상선 기능 저하증, 광선공포증, 근육 위축과 같은 이차 합병증 관리에 대한 관심이 증가함에 따라 2025년부터 2032년까지 가장 빠른 연평균 성장률을 기록할 것으로 예상됩니다. 치료 전략이 더욱 포괄적으로 발전함에 따라 지지 요법에 대한 수요 또한 증가할 것으로 예상됩니다.
- 관리 경로별
투여 경로를 기준으로 신병증성 시스틴증 치료 시장은 경구 투여와 기타 투여로 구분됩니다. 2024년 경구 투여 부문은 72.3%의 가장 큰 매출 점유율을 기록했는데, 이는 속효성 및 서방성 시스테아민을 포함한 대부분의 표준 치료제가 경구 투여되기 때문입니다. 투여의 용이성과 비침습적 투여 경로에 대한 환자의 선호도는 시스틴증 치료에서 경구 치료의 우위를 더욱 강화합니다.
기타 부문은 유전자 및 세포 기반 전달 방법을 탐구하는 새로운 치료법과 임상 시험이 계속 개발됨에 따라 2025년부터 2032년까지 가장 빠른 CAGR을 기록할 것으로 예상됩니다.
- 최종 사용자에 의해
신병증성 시스틴증 치료 시장은 최종 사용자 기준으로 병원, 의원, 기타 의료기관으로 구분됩니다. 병원 부문은 2024년 시장 점유율 54.8%로 가장 큰 비중을 차지했는데, 이는 전문 진료, 첨단 진단 도구, 그리고 희귀 유전 질환 관리에 필요한 다학제 의료진의 가용성 덕분입니다. 병원은 진행성 질환의 치료 시작, 모니터링, 그리고 신장 이식의 주요 기관입니다.
클리닉 부문은 2025년부터 2032년까지 가장 빠른 연평균 성장률(CAGR)을 기록할 것으로 예상되며, 특히 외래 희귀질환 프로그램이 확대되고 있는 도시 및 준도시 지역에서 더욱 그렇습니다. 희귀질환 관리에 대한 인식이 높아지고 분산화됨에 따라 더 많은 환자가 3차 병원 밖에서도 일관된 치료를 받도록 유도하고 있습니다.
- 유통 채널별
신병증성 시스틴증 치료 시장은 유통 채널을 기준으로 병원 약국, 소매 약국, 그리고 온라인 약국으로 구분됩니다. 병원 약국 부문은 2024년 45.6%의 점유율로 시장을 주도했으며, 이는 희귀의약품과 고가 치료제의 직접 조제 및 감독 하에 이루어지는 관리에 힘입은 것입니다. 이러한 약국들은 규제된 치료법에 대한 접근성을 높이고 장기 치료 프로토콜을 준수하는 데 필수적입니다.
온라인 약국 시장은 디지털 건강 플랫폼의 수용 증가, 만성 약물 가정 배송의 편의성 증대, 그리고 외딴 지역의 접근성 확대로 인해 2025년부터 2032년까지 가장 빠른 연평균 성장률(CAGR)을 기록할 것으로 예상됩니다. 이러한 추세는 장기적인 시스틴증 관리가 필요한 환자들의 치료 연속성을 크게 향상시킬 것으로 예상됩니다.
신병성 시스틴증 치료 시장 지역 분석
- 북미는 2024년 42.2%의 가장 큰 매출 점유율로 신병성 시스틴증 치료 시장을 장악했으며, 이는 첨단 의료 인프라, 상당한 R&D 투자, 희귀 질환에 대한 유리한 규제 경로, 특히 미국 내 주요 제약 혁신 기업의 존재에 힘입은 것입니다.
- 이 지역의 환자와 의료 제공자는 희귀 질환에 대한 장기간의 임상 경험을 통해 개발된 시스테아민 기반 치료법, 포괄적인 보험 적용 범위, 확립된 치료 프로토콜에 대한 광범위한 접근성의 혜택을 받습니다.
- 이러한 리더십 위치는 FDA와 같은 규제 기관의 적극적인 지원, 제약 회사의 상당한 R&D 투자, 의료 기관과 환자 옹호 단체 간의 협력 노력으로 더욱 강화되어 북미가 시스틴증 치료 분야에서 혁신과 접근성을 위한 핵심 허브가 되었습니다.
미국 신병성 시스틴증 치료 시장 통찰력
미국 신병증성 시스틴증 치료 시장은 2024년 북미에서 84%의 매출 점유율을 기록하며 가장 큰 매출 점유율을 기록했습니다. 이는 광범위한 신생아 선별 검사 프로그램, 확립된 희귀 질환 프로토콜, 그리고 Procysbi® 및 Cystagon®과 같은 FDA 승인 치료제의 조기 접근성에 힘입은 것입니다. 미국의 탄탄한 의료 인프라와 희귀의약품에 대한 높은 R&D 투자는 시장 성장에 크게 기여하고 있습니다. 또한, 환자 권익 옹호 단체의 강력한 지원, 유리한 보험급여 정책, 그리고 임상 연구에 대한 적극적인 참여는 미국이 시스틴증 치료 접근성 및 혁신을 선도하는 국가로서 입지를 강화하고 있습니다.
유럽 신병성 시스틴증 치료 시장 통찰력
유럽 신병증성 시스틴증 치료 시장은 희귀 질환에 대한 인식 제고, 공동 연구 사업, 그리고 유럽 의약품청(EMA) 승인 희귀 의약품 접근성 향상에 힘입어 예측 기간 동안 상당한 연평균 성장률(CAGR)을 기록할 것으로 예상됩니다. 환자 등록 시스템과 중앙 집중식 치료 체계의 확대는 조기 진단 및 장기 치료를 향상시킵니다. 또한, 이 지역은 소아 희귀 질환 프로그램에 대한 투자도 증가하고 있어 서유럽과 동유럽 모두에서 시스틴증 치료제 접근성 확대에 기여하고 있습니다.
영국 신병성 시스틴증 치료 시장 통찰력
영국 신병증성 시스틴증 치료 시장은 예측 기간 동안 주목할 만한 연평균 성장률(CAGR)로 성장할 것으로 예상되며, 이는 강력한 국가 의료 시스템, 유전자 검사 접근성, 그리고 희귀 질환 정책 시행에 대한 강조에 힘입은 것입니다. 조기 진단 및 평생 치료 보장에 대한 국민보건서비스(NHS)의 지원과 연구 기관 및 글로벌 제약 회사 간의 파트너십 확대는 시장 내 치료 접근성과 혁신을 강화하고 있습니다.
독일 신병성 시스틴증 치료 시장 통찰력
독일 신병증성 시스틴증 치료 시장은 예측 기간 동안 상당한 연평균 성장률(CAGR)로 성장할 것으로 예상되며, 이는 희귀 질환 연구에 대한 탄탄한 투자, 정밀 치료법의 조기 도입, 그리고 지원적인 보험급여 체계에 힘입은 것입니다. 독일은 생명공학 혁신과 통합 치료 경로에 중점을 두고 있으며, 특히 소아 신장내과 센터에서 첨단 시스틴증 치료법 도입을 가속화하고 있습니다. 정부 지원 의료 사업과 데이터 기반 환자 관리 플랫폼 또한 시장 확대에 기여하고 있습니다.
아시아 태평양 신병성 시스틴증 치료 시장 통찰력
아시아 태평양 신병증성 시스틴증 치료 시장은 2025년부터 2032년까지 예측 기간 동안 23.5%라는 가장 빠른 연평균 성장률(CAGR)로 성장할 것으로 예상됩니다. 이는 의료 서비스 접근성 향상, 희귀 질환에 대한 인식 제고, 그리고 중국, 일본, 인도 등 여러 국가에서 새롭게 도입되는 유전자 검사 프로그램에 힘입은 것입니다. 희귀 질환 기반 시설에 대한 투자 증가와 제약 회사와의 협력 확대는 이 지역 전역에 시스틴증 치료제 도입을 뒷받침하고 있습니다. 또한, 신생아 선별 검사 및 진단 역량 강화를 장려하는 사업들은 조기 개입의 격차를 해소하는 데 기여하고 있습니다.
일본 신병성 시스틴증 치료 시장 통찰력
일본의 신병증성 시스틴증 치료 시장은 선진 의료 시스템, 유전자 연구 전문성, 그리고 정부의 강력한 희귀 질환 관리 지원으로 인해 성장세가 가속화되고 있습니다. 조기 진단율 증가와 환자 중심 치료에 대한 집중은 기존 및 연구 중인 시스틴증 치료법의 도입을 촉진하고 있습니다. 시스틴증 치료를 더 광범위한 국가 희귀 질환 정책에 통합함으로써 시장의 장기적인 성장과 혁신을 유지할 것으로 예상됩니다.
인도 신병성 시스틴증 치료 시장 통찰력
인도 신병증성 시스틴증 치료 시장은 2024년 아시아 태평양 지역에서 가장 큰 시장 점유율을 차지했는데, 이는 인도의 의료 인프라 개선, 홍보 캠페인을 통한 인식 제고, 그리고 유전자 검사 접근성 향상에 기인합니다. 농촌 지역에서는 치료 접근성이 제한적인 반면, 도시 지역에서는 희귀 질환 진단 및 수입 시스틴증 치료제에 대한 수요가 증가하고 있습니다. 국가 희귀 질환 정책에 따른 정부 사업과 민관 협력 확대를 통해 치료 접근성과 인식이 확대될 것으로 예상됩니다.
신병성 시스틴증 치료 시장 점유율
신병성 시스틴증 치료 산업은 주로 다음을 포함한 잘 정립된 회사들이 주도하고 있습니다.
- Horizon Therapeutics plc(아일랜드)
- 레코다티 희귀질환(미국)
- Leadiant Biosciences, Inc. (미국)
- Chiesi Farmaceutici SpA(이탈리아)
- Orphan Europe SARL(프랑스)
- 테바 제약 산업 유한회사(이스라엘)
- 노바티스 AG(스위스)
- 화이자(미국)
- 사노피(프랑스)
- 아미커스 테라퓨틱스(Amicus Therapeutics, Inc.)(미국)
- 다케다제약 주식회사(일본)
- 알렉시온 파마슈티컬스(미국)
- 바이오마린 파마슈티컬 주식회사(미국)
- Reata Pharmaceuticals, Inc.(미국)
- Ultragenyx Pharmaceutical Inc. (미국)
- Zydus Lifesciences Limited(인도)
- Sun Pharmaceutical Industries Ltd.(인도)
- Apotex Inc.(캐나다)
- 랩터 파마슈티컬스(미국)
글로벌 신병성 시스틴증 치료 시장의 최근 동향은 무엇인가?
- 2024년 5월, 프로시스비(Procysbi) 제조사인 호라이즌 테라퓨틱스(Horizon Therapeutics plc)는 라틴 아메리카와 아시아 태평양 일부 지역을 포함하여 글로벌 접근성 프로그램을 확대했습니다. 이 전략적 조치는 지역 의료 서비스 제공자 및 환자 권익 옹호 단체와 협력하여 소외된 시스틴증 환자의 치료 접근성을 개선하는 것을 목표로 합니다. 이 이니셔티브는 전 세계 희귀 질환 환자들에게 생명을 구하는 치료법에 대한 공평한 접근성을 제공하려는 호라이즌의 노력을 강조하며, 글로벌 희귀 의약품 시장에서 호라이즌의 입지를 더욱 강화합니다.
- 2024년 3월, Leadiant Biosciences는 Cystagon의 장기적인 안전성과 효능을 평가하기 위해 여러 유럽 국가에서 시판 후 감시 연구(PMS) 시작을 발표했습니다. 이 연구는 향후 치료 프로토콜을 개발하고 규제 체계를 뒷받침할 귀중한 실제 임상 데이터를 제공할 것으로 기대됩니다. 이는 희귀 질환 관리에서 데이터 기반 의사 결정의 중요성이 커지고 있으며, 시스틴증 환자의 임상 결과 개선을 위한 Leadiant Biosciences의 지속적인 투자를 반영하는 것입니다.
- 2024년 2월, 유니버시티 칼리지 런던(UCL) 연구진은 유럽 바이오테크 기업들과 협력하여 신병증성 시스틴증을 유발하는 CTNS 유전자 돌연변이를 표적으로 하는 새로운 유전자 치료법을 연구하는 임상시험을 시작했습니다. 이 획기적인 임상시험은 환자의 정상적인 리소좀 기능 회복을 목표로 하는 잠재적인 치료 접근법으로 나아가는 중요한 발걸음을 의미합니다. 이는 증상 관리를 넘어 질병의 근본 원인을 해결하는 차세대 치료법에 대한 관심이 높아지고 있음을 보여줍니다.
- 2024년 1월, 시스틴증 연구 재단(Cystinosis Research Foundation)은 mRNA 기반 치료법, 줄기세포 이식, 질병 조절제 등 혁신적인 치료법 개발에 주력하는 국제 연구팀에 총 300만 달러 이상의 새로운 연구비를 지원했습니다. 이러한 연구 지원 사업은 시스틴증에 대한 혁신적인 해결책 개발을 가속화하고 희귀 질환 분야의 발전을 촉진하는 비영리 단체의 중요한 역할을 보여주는 것을 목표로 합니다.
- 2023년 12월, 레코다티 희귀질환(Recordati Rare Diseases)은 유럽과 북미 지역에서 시스틴증 환자 지원 프로그램을 확대하여 간호사 교육, 가정 배달 서비스, 다국어 헬프라인 등의 서비스를 제공한다고 발표했습니다. 이러한 확대는 만성 희귀 질환 환자의 치료 순응도, 삶의 질, 그리고 치료 결과를 개선하는 포괄적인 치료 모델에 대한 강조가 점차 커지고 있음을 보여줍니다.
SKU-
세계 최초의 시장 정보 클라우드 보고서에 온라인으로 접속하세요
- 대화형 데이터 분석 대시보드
- 높은 성장 잠재력 기회를 위한 회사 분석 대시보드
- 사용자 정의 및 질의를 위한 리서치 분석가 액세스
- 대화형 대시보드를 통한 경쟁자 분석
- 최신 뉴스, 업데이트 및 추세 분석
- 포괄적인 경쟁자 추적을 위한 벤치마크 분석의 힘 활용
연구 방법론
데이터 수집 및 기준 연도 분석은 대규모 샘플 크기의 데이터 수집 모듈을 사용하여 수행됩니다. 이 단계에는 다양한 소스와 전략을 통해 시장 정보 또는 관련 데이터를 얻는 것이 포함됩니다. 여기에는 과거에 수집한 모든 데이터를 미리 검토하고 계획하는 것이 포함됩니다. 또한 다양한 정보 소스에서 발견되는 정보 불일치를 검토하는 것도 포함됩니다. 시장 데이터는 시장 통계 및 일관된 모델을 사용하여 분석하고 추정합니다. 또한 시장 점유율 분석 및 주요 추세 분석은 시장 보고서의 주요 성공 요인입니다. 자세한 내용은 분석가에게 전화를 요청하거나 문의 사항을 드롭하세요.
DBMR 연구팀에서 사용하는 주요 연구 방법론은 데이터 마이닝, 시장에 대한 데이터 변수의 영향 분석 및 주요(산업 전문가) 검증을 포함하는 데이터 삼각 측량입니다. 데이터 모델에는 공급업체 포지셔닝 그리드, 시장 타임라인 분석, 시장 개요 및 가이드, 회사 포지셔닝 그리드, 특허 분석, 가격 분석, 회사 시장 점유율 분석, 측정 기준, 글로벌 대 지역 및 공급업체 점유율 분석이 포함됩니다. 연구 방법론에 대해 자세히 알아보려면 문의를 통해 업계 전문가에게 문의하세요.
사용자 정의 가능
Data Bridge Market Research는 고급 형성 연구 분야의 선두 주자입니다. 저희는 기존 및 신규 고객에게 목표에 맞는 데이터와 분석을 제공하는 데 자부심을 느낍니다. 보고서는 추가 국가에 대한 시장 이해(국가 목록 요청), 임상 시험 결과 데이터, 문헌 검토, 재생 시장 및 제품 기반 분석을 포함하도록 사용자 정의할 수 있습니다. 기술 기반 분석에서 시장 포트폴리오 전략에 이르기까지 타겟 경쟁업체의 시장 분석을 분석할 수 있습니다. 귀하가 원하는 형식과 데이터 스타일로 필요한 만큼 많은 경쟁자를 추가할 수 있습니다. 저희 분석가 팀은 또한 원시 엑셀 파일 피벗 테이블(팩트북)로 데이터를 제공하거나 보고서에서 사용 가능한 데이터 세트에서 프레젠테이션을 만드는 데 도움을 줄 수 있습니다.