글로벌 듀센 근이영양실조 치료 시장, 치료 유형(분자 기반 치료, 스테로이드 치료 및 기타), 치료(엑손 스키핑 접근법, 돌연변이 억제 및 디스트로핀 표적 치료), 투여 경로(경구, 비경구 및 기타), 최종 사용자(병원, 전문 클리닉, 재택 치료 및 기타), 유통 채널(병원 약국, 소매 약국 및 온라인 약국) - 업계 동향 및 2030년까지의 예측.
듀센 근이영양증 치료 시장 분석 및 규모
시장 성장을 견인할 것으로 예상되는 주요 요인은 DMD 치료에 대한 인식 증가와 DMD 장애에 대한 새로운 치료법의 도입입니다. 또한, DMD 장애의 유병률과 영향 증가는 시장 성장을 견인할 것으로 예상되는 또 다른 주요 요인입니다. 임상 시험 수의 증가는 최근 추세이며 시장 성장을 견인할 것으로 예상됩니다. 그러나 첨단 기술로 인해 DMD 치료의 가용성이 제한적이고 DMD 장애의 높은 치료 비용이 시장 성장을 억제할 것으로 예상됩니다.
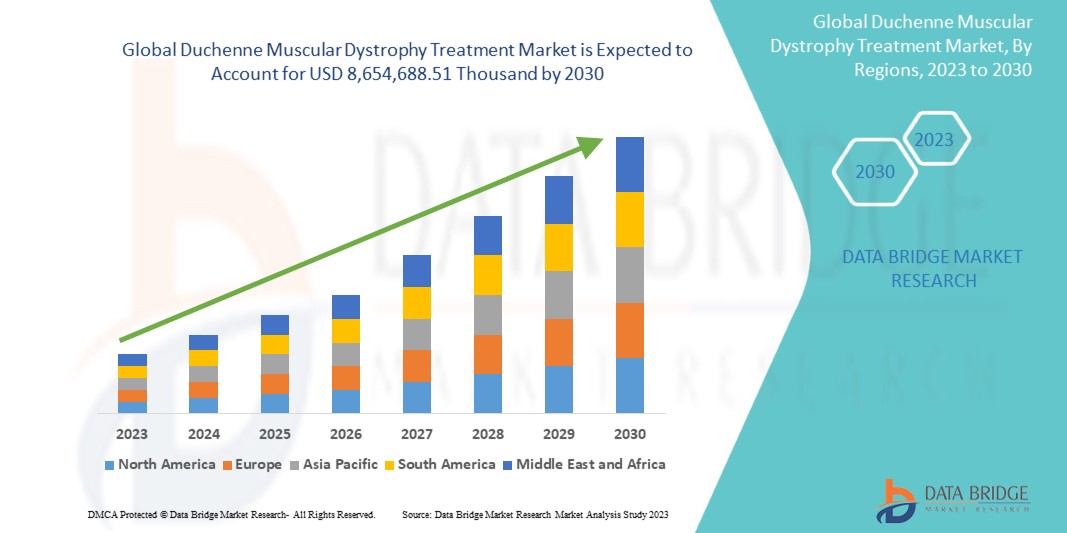
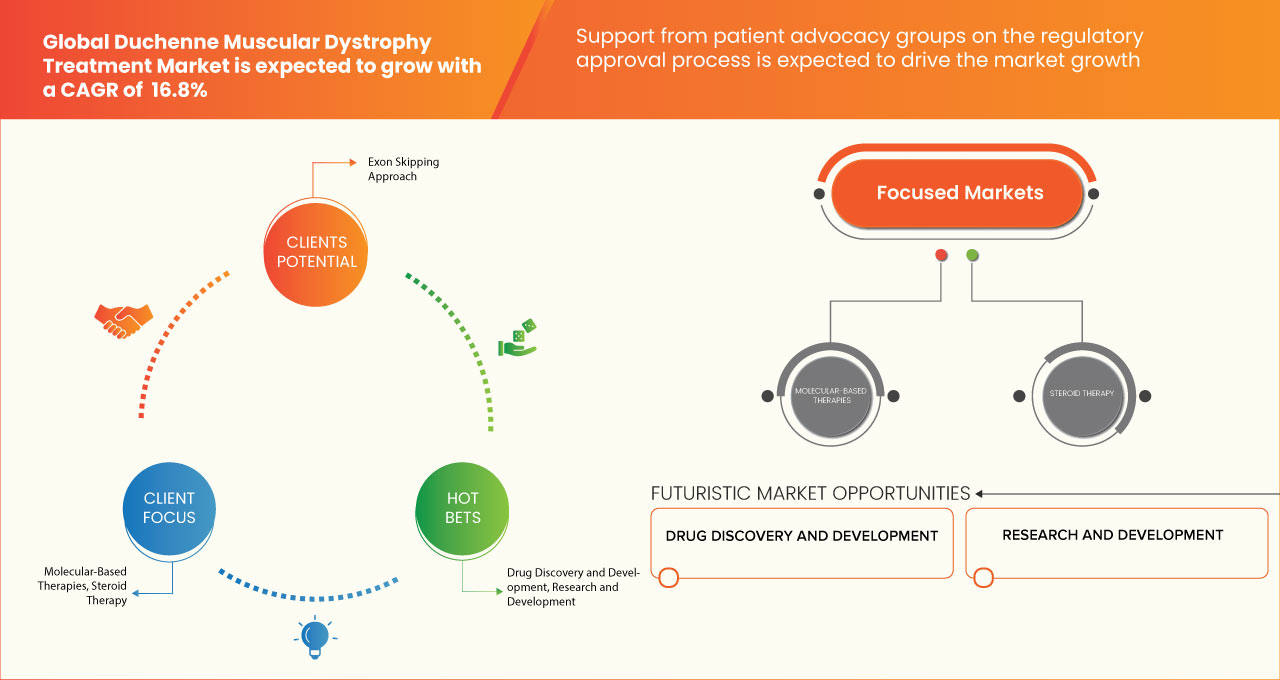
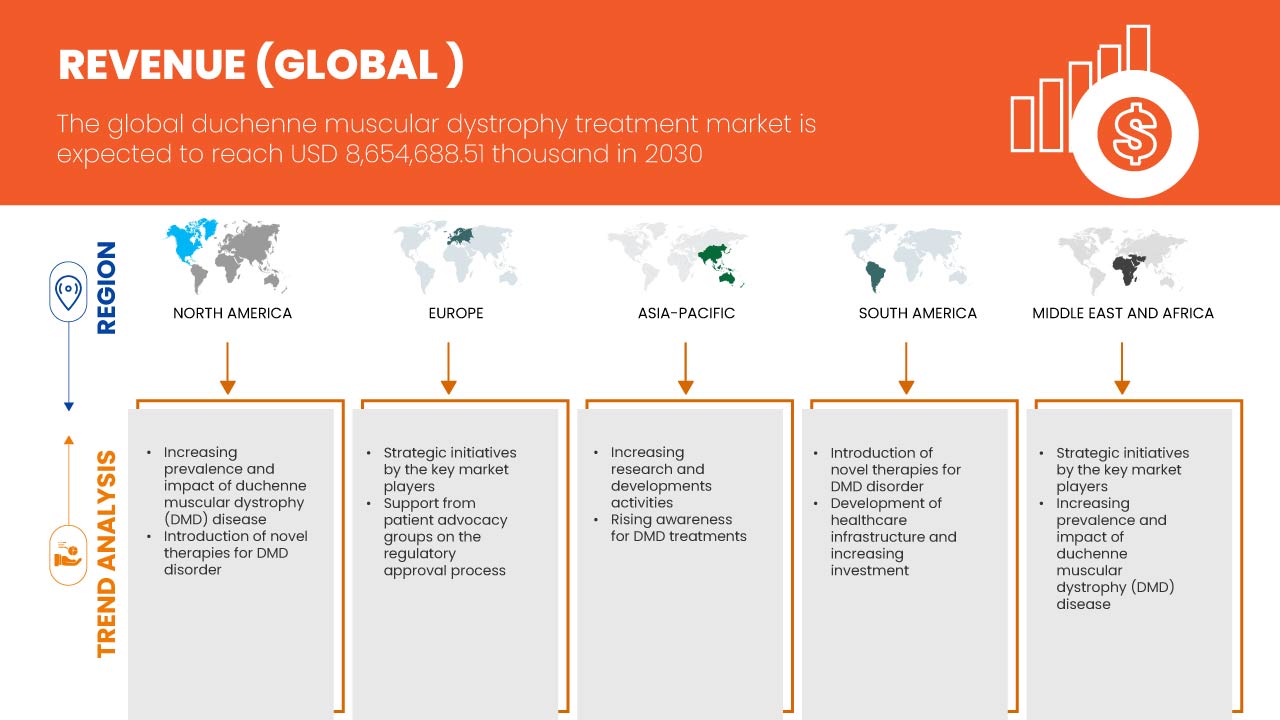
Data Bridge Market Research는 글로벌 듀센 근위축증 치료 시장이 2030년까지 8,654,688.51천 달러에 도달할 것으로 예상되며, 2023-2030년 예측 기간 동안 CAGR은 16.8%가 될 것으로 분석했습니다. 이 시장 보고서는 또한 가격 분석과 기술 발전에 대한 심층적인 내용을 다룹니다.
|
보고서 메트릭 |
세부 |
|
예측 기간 |
2023년부터 2030년까지 |
|
기준 연도 |
2022 |
|
역사적 연도 |
2021 (2015-2020까지 사용자 정의 가능) |
|
양적 단위 |
수익 (USD 천) |
|
다루는 세그먼트 |
치료 유형(분자 기반 치료, 스테로이드 치료 및 기타), 치료(엑손 스킵핑 접근법, 돌연변이 억제 및 디스트로핀 표적 치료), 투여 경로(경구, 비경구 및 기타), 최종 사용자(병원, 전문 클리닉, 홈 케어 및 기타), 유통 채널(병원 약국, 소매 약국 및 온라인 약국) |
|
적용 국가 |
미국, 캐나다, 멕시코, 독일, 프랑스, 영국, 이탈리아, 스페인, 러시아, 터키, 벨기에, 네덜란드, 스위스, 유럽 기타 지역, 일본, 중국, 한국, 인도, 호주, 싱가포르, 태국, 말레이시아, 인도네시아, 필리핀, 아시아 태평양 기타 지역, 브라질, 아르헨티나, 남미 기타 지역, 남아프리카, 사우디 아라비아, UAE, 이집트, 이스라엘, 중동 및 아프리카 기타 지역 |
|
시장 참여자 포함 |
Sarepta Therapeutics, Inc., GSK plc., Capricor Therapeutics, Inc., Dyne Therapeutics, Solid Biosciences Inc., BioMarin, Stealth BioTherapeutics Inc., Avidity Biosciences, ReveraGen BioPharma, Inc. PTC Therapeutics., NS Pharma, Inc, ITALFARMACO SpA, FibroGen, Inc, SANTHERA PHARMACEUTICALS, Pfizer Inc., F. Hoffmann-La Roche Ltd, Akashi RX, TAIHO PHARMACEUTICAL CO., LTD 등 |
시장 정의
듀센 근이영양증(DMD)은 점진적인 근육 퇴화와 약화를 특징으로 하는 희귀 유전적 질환입니다. DMD의 주요 치료에는 프레드니손이나 데플라자코트와 같은 코르티코스테로이드를 사용하는 것이 포함됩니다. 이러한 약물은 염증을 줄이고 근육 퇴화를 지연시켜 궁극적으로 걷는 능력을 연장하고 근육 기능을 보존하는 데 도움이 됩니다. 코르티코스테로이드 치료는 DMD 환자의 근력, 호흡 기능 및 전반적인 삶의 질을 개선하는 것으로 나타났습니다.
글로벌 듀센 근이영양증 치료 시장 역학
이 섹션에서는 시장 동인, 이점, 제약 및 과제를 이해하는 것을 다룹니다. 이 모든 내용은 아래에서 자세히 설명합니다.
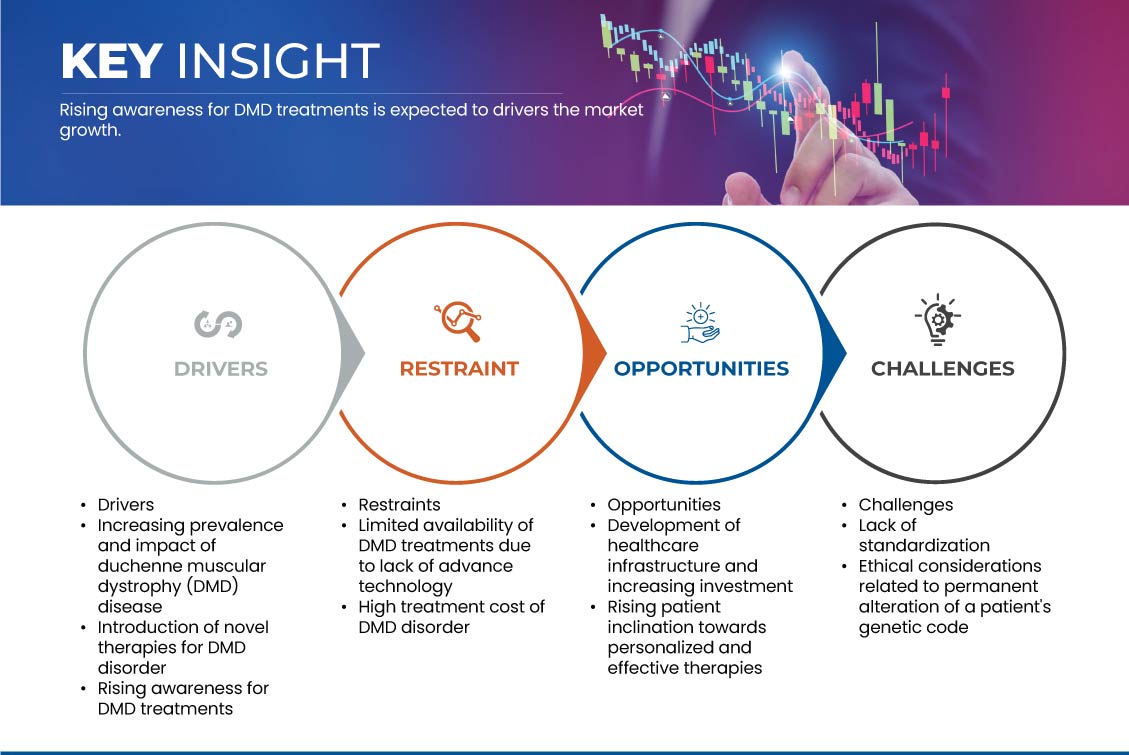
운전자
- 듀센 근이영양증(DMD) 질병 의 유병률 및 영향 증가
듀센 근이영양증(DMD)은 점진적인 근육 약화와 기능 상실을 특징으로 하는 희귀하고 쇠약해지는 유전적 질환입니다. 주로 남성에게 영향을 미치며 증상은 일반적으로 유아기에 나타납니다. DMD 진단을 받는 개인 수도 증가하고 있으며, 이러한 유병률 증가로 인해 세계 인구가 계속 증가함에 따라 효과적인 치료법과 요법에 대한 필요성이 생겨났습니다.
DMD의 유병률이 증가함에 따라 환자와 의료 전문가의 옹호 활동도 증가했습니다. 이러한 그룹은 DMD에 대한 인식 제고, 연구 자금 지원 옹호, 잠재적 치료법의 빠른 승인에 중요한 역할을 합니다. DMD 커뮤니티가 증가하고 있으므로 이 의료 분야에서의 기술 발전과 투자도 증가하고 있습니다.
- DMD 장애 에 대한 새로운 치료법 소개
혁신적인 치료 접근 방식의 등장은 DMD 치료의 증가로 이어지고 시장 확장에 크게 기여합니다. 전통적인 치료 옵션은 주로 증상 관리와 지지 치료에 초점을 맞췄습니다. 그러나 새로운 치료법의 도입은 DMD의 원인이 되는 근본적인 유전자 돌연변이를 해결하는 것을 목표로 하기 때문에 패러다임의 전환을 나타냅니다.
게다가, 엑손 스키핑 약물은 DMD에 대한 또 다른 유망한 치료법으로 부상했습니다. 이러한 약물은 디스트로핀 유전자의 특정 엑손을 "스킵"하도록 설계되어 절단되었지만 부분적으로 기능하는 디스트로핀 단백질을 생산할 수 있습니다. 이러한 약물은 질병의 진행을 상당히 늦추고 DMD 환자의 삶의 질을 향상시킬 수 있습니다. 이러한 치료법의 개발 및 승인은 DMD 환자에게 새로운 기대감을 제공했으며 시장 성장을 더욱 촉진할 것으로 예상됩니다.
기회
- 의료 인프라 개발 및 투자 증가
DMD 환자는 잘 갖춰진 병원과 진단 시설을 포함한 더 강력한 의료 인프라를 통해 더 일찍 진단받을 수 있습니다. 조기 진단은 시기적절한 개입과 치료 시작을 가능하게 하여 질병 진행을 늦추고 환자 결과를 개선할 수 있습니다. 선진 의료 시스템에는 종종 DMD와 같은 희귀 질환에 전념하는 전문 센터와 진료소가 있습니다. 이러한 센터는 전문 의료 전문가, 물리 치료 및 지원 서비스에 대한 접근을 포함한 포괄적인 치료를 제공하여 DMD 환자의 삶의 질을 향상시킬 수 있습니다.
개발된 의료 시스템은 종종 물리 치료 및 작업 치료 , 보조 장치, 상담과 같은 광범위한 지원 서비스를 제공하며 , 이는 DMD 환자의 삶의 질을 크게 향상시킬 수 있습니다. 정부, 개인 투자자, 자선 단체는 잘 확립된 의료 인프라가 있는 경우 DMD와 같은 희귀 질환에 대한 약물 개발에 투자할 가능성이 더 높습니다. 이러한 투자는 연구, 임상 시험 및 혁신적인 치료법 개발을 지원할 수 있습니다.
제지/ 도전
- 첨단 기술 부족으로 DMD 치료의 가용성 제한
DMD에 대한 치료 옵션은 제한적이어서 환자와 그 가족은 질병 진행을 늦추거나 전반적인 웰빙을 개선할 방법이 거의 없습니다.
기술의 발전은 종종 유전자 치료 나 개인화된 의료 접근 방식과 같은 DMD에 대한 고도로 전문화되고 혁신적인 치료법의 개발로 이어진다 . 이러한 최첨단 치료법은 개발, 제조 및 투여가 복잡하다. 특히 유전자 치료법의 개발에는 복잡한 공정과 특수 제조 기술이 필요하다. 이러한 치료법은 DMD의 근본 원인을 해결하기 위해 결함이 있는 유전자를 수정하거나 교체해야 하므로 치료 비용이 많이 든다. 또한 유전자 치료법을 생산할 수 있는 제조 시설의 수가 제한되어 가용성이 제한적이다.
고급 DMD 치료의 복잡성으로 인해 제조 및 투여가 어려워지고, 가용성이 제한되는 데 더욱 기여합니다. 생산에는 특수 시설과 전문 지식이 필요할 수 있으며, 이러한 치료를 투여하려면 특정 지역에서는 부족할 수 있는 전문 의료 전문가가 필요할 수 있습니다.
최근 개발 사항
- 2023년 3월, Dyne Therapeutics는 엑손 51 스키핑에 적합한 듀센 근이영양증(DMD) 돌연변이에 대한 조사 치료제인 DYNE-251이 미국 FDA 희귀의약품 및 희귀 소아 질환 지정을 받았다고 발표했습니다. DYNE-251은 1/2상 DELIVER 임상 시험에서 평가되고 있습니다. 이를 통해 조직은 제품 범주와 전체 매출을 늘리는 데 도움이 됩니다.
- 2022년 8월, FibroGen, Inc.는 배경 전신 코르티코스테로이드를 사용하는 외래 DMD 환자를 치료하기 위한 pamrevlumab의 3상 LELANTOS-2 시험의 최상위 데이터를 발표했습니다. 이를 통해 회사는 파이프라인 포트폴리오를 강화할 수 있었습니다.
글로벌 듀센 근이영양증 치료 시장 범위
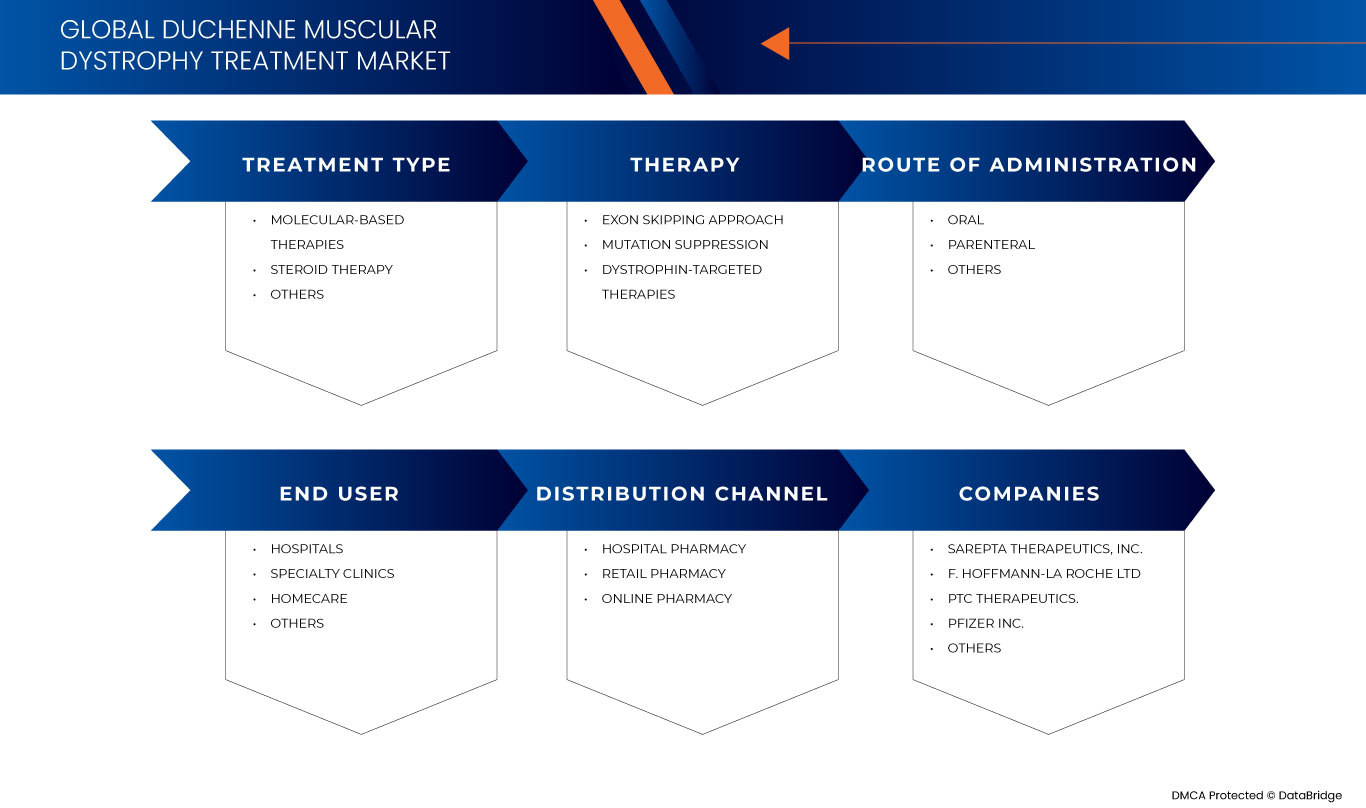
글로벌 듀센 근이영양증 치료 시장은 치료 유형, 요법, 투여 경로, 최종 사용자 및 유통 채널을 기준으로 5개의 주요 세그먼트로 세분화됩니다. 이러한 세그먼트 간의 성장은 산업의 빈약한 성장 세그먼트를 분석하고 사용자에게 핵심 시장 응용 프로그램을 식별하기 위한 전략적 결정을 내리는 데 도움이 되는 귀중한 시장 개요와 시장 통찰력을 제공하는 데 도움이 됩니다.
치료 유형
- 분자 기반 치료법
- 스테로이드 치료
- 기타
치료 유형을 기준으로 시장은 분자 기반 치료법, 스테로이드 치료법 및 기타 치료법으로 구분됩니다.
요법
- 엑손 스킵핑 접근법
- 돌연변이 억제
- 디스트로핀 표적 치료
치료법을 기준으로 시장은 엑손 스키핑 접근법, 돌연변이 억제, 디스트로핀 표적 치료법으로 구분됩니다.
투여 경로
- 경구
- 비경구적
- 기타
투여 경로를 기준으로 시장은 경구, 비경구 및 기타로 구분됩니다.
최종 사용자
- 병원
- 홈 헬스케어
- 전문 클리닉
- 기타
최종 사용자를 기준으로 시장은 병원, 가정 건강 관리, 전문 클리닉 및 기타로 구분됩니다.
유통 채널
- 병원 약국
- 온라인 약국
- 소매 약국
유통 채널을 기준으로 시장은 병원 약국, 온라인 약국, 소매 약국으로 구분됩니다 .
글로벌 듀센 근이영양증 치료 시장 지역 분석/통찰력
글로벌 듀센 근이영양증 치료 시장은 치료 유형, 요법, 투여 경로, 최종 사용자 및 유통 채널을 기준으로 5개의 주요 부문으로 구분됩니다.
이 글로벌 듀센 근이영양증 치료 시장 보고서에서 다루는 국가는 미국, 캐나다, 멕시코, 독일, 프랑스, 영국, 이탈리아, 스페인, 러시아, 터키, 벨기에, 네덜란드, 스위스, 유럽의 다른 지역, 일본, 중국, 한국, 인도, 호주, 싱가포르, 태국, 말레이시아, 인도네시아, 필리핀, 아시아 태평양의 다른 지역, 브라질, 아르헨티나, 남미의 다른 지역, 남아프리카, 사우디 아라비아, UAE, 이집트, 이스라엘, 그리고 중동 및 아프리카의 다른 지역입니다.
미국은 DMD에 대한 인식과 스크리닝이 증가함에 따라 북미에서 우위를 점할 것으로 예상됩니다. 독일은 근위축증의 유병률 증가와 새로운 기술 발전의 침투로 인해 유럽에서 우위를 점할 것으로 예상됩니다. 중국은 주요 시장 참여자의 전략적 이니셔티브가 크게 확대됨에 따라 아시아 태평양에서 우위를 점할 것으로 예상됩니다.
보고서의 국가 섹션은 또한 현재 및 미래 시장 추세에 영향을 미치는 개별 시장 영향 요인과 시장 규제의 변화를 제공합니다. 하류 및 상류 가치 사슬 분석, 기술 추세, 포터의 5가지 힘 분석, 사례 연구와 같은 데이터 포인트는 개별 국가의 시장 시나리오를 예측하는 데 사용되는 몇 가지 포인터입니다. 또한 글로벌 브랜드의 존재 및 가용성과 지역 및 국내 브랜드와의 대규모 또는 희소한 경쟁으로 인해 직면한 과제, 국내 관세의 영향 및 무역 경로가 국가 데이터의 예측 분석을 제공하는 동안 고려됩니다.
경쟁 환경 및 글로벌 듀센 근이영양증 치료 시장 점유율 분석
글로벌 듀센 근위축증 치료 시장 경쟁 구도는 경쟁자에 대한 세부 정보를 제공합니다. 포함된 세부 정보는 회사 개요, 회사 재무, 창출된 수익, 시장 잠재력, 새로운 시장 이니셔티브, 글로벌 입지, 생산 현장 및 시설, 생산 용량, 회사의 강점과 약점, 제품 출시, 제품 폭과 범위, 응용 분야 우세입니다. 제공된 위의 데이터 포인트는 시장과 관련된 회사의 초점에만 관련이 있습니다.
글로벌 듀센 근이영양증 치료 시장에서 활동하는 주요 기업으로는 Sarepta Therapeutics, Inc., GSK plc., Capricor Therapeutics, Inc., Dyne Therapeutics, Solid Biosciences Inc., BioMarin, Stealth BioTherapeutics Inc., Avidity Biosciences, ReveraGen BioPharma, Inc., PTC Therapeutics., NS Pharma, Inc, ITALFARMACO SpA, FibroGen, Inc, SANTHERA PHARMACEUTICALS, Pfizer Inc., F. Hoffmann-La Roche Ltd, Akashi RX, TAIHO PHARMACEUTICAL CO., LTD 등이 있습니다.
SKU-
세계 최초의 시장 정보 클라우드 보고서에 온라인으로 접속하세요
- 대화형 데이터 분석 대시보드
- 높은 성장 잠재력 기회를 위한 회사 분석 대시보드
- 사용자 정의 및 질의를 위한 리서치 분석가 액세스
- 대화형 대시보드를 통한 경쟁자 분석
- 최신 뉴스, 업데이트 및 추세 분석
- 포괄적인 경쟁자 추적을 위한 벤치마크 분석의 힘 활용
목차
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF THE GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATIONS
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 DBMR TRIPOD DATA VALIDATION MODEL
2.5 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.6 MULTIVARIATE MODELLING
2.7 TREATMENT TYPE SEGMENT LIFELINE CURVE
2.8 MARKET END USER COVERAGE GRID
2.9 DBMR MARKET POSITION GRID
2.1 VENDOR SHARE ANALYSIS
2.11 SECONDARY SOURCES
2.12 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL'S MODEL
4.2 PORTER'S FIVE FORCES MODEL
4.3 PRICING ANALYSIS
5 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: REGULATIONS
5.1 REGULATIONS IN U.S.
5.2 REGULATTIONS IN EUROPE
5.3 REGULATTIONS IN AUSTRALIA
5.4 REGULATIONS IN SOUTH AFRICA
5.5 REGULATIONS IN BRAZIL
6 MARKET OVERVIEW
6.1 DRIVERS
6.1.1 INCREASING PREVALENCE AND IMPACT OF DUCHENNE MUSCULAR DYSTROPHY (DMD) DISEASE
6.1.2 INTRODUCTION OF NOVEL THERAPIES FOR DMD DISORDER
6.1.3 RISING AWARENESS FOR DMD TREATMENTS
6.1.4 INCREASE IN THE NUMBER OF CLINICAL TRIALS IS A RECENT TREND
6.2 RESTRAINTS
6.2.1 LIMITED AVAILABILITY OF DMD TREATMENTS DUE TO LACK OF ADVANCE TECHNOLOGY
6.2.2 HIGH TREATMENT COST OF DMD DISORDER
6.3 OPPORTUNITIES
6.3.1 DEVELOPMENT OF HEALTHCARE INFRASTRUCTURE AND INCREASING INVESTMENT
6.3.2 RISING PATIENT INCLINATION TOWARDS PERSONALIZED AND EFFECTIVE THERAPIES
6.3.3 STRATEGIC INITIATIVES BY THE KEY MARKET PLAYERS
6.3.4 SUPPORT FROM PATIENT ADVOCACY GROUPS ON THE REGULATORY APPROVAL PROCESS
6.4 CHALLENGES
6.4.1 LACK OF STANDARDIZATION IN DMD DIAGNOSIS
6.4.2 ETHICAL CONSIDERATIONS RELATED TO PERMANENT ALTERATION OF A PATIENT'S GENETIC CODE
7 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE
7.1 OVERVIEW
7.2 MOLECULAR-BASED THERAPIES
7.2.1 ANTISENSE OLIGONUCLEOTIDE THERAPY
7.2.1.1 EXONDYS 51
7.2.1.2 AMONDYS 45
7.2.1.3 VYONDYS 53
7.3 NONSENSE MUTATION
7.3.1.1 Translarna
7.4 STEROID THERAPY
7.4.1 PREDNISONE
7.4.2 DEFLAZACORT
7.5 OTHERS
8 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY
8.1 OVERVIEW
8.2 EXON SKIPPING APPROACH
8.2.1 MULTI-EXON SKIPPING APPROACH
8.2.2 SINGLE-EXON SKIPPING APPROACH
8.3 MUTATION SUPPRESSION
8.4 DYSTROPHIN-TARGETED THERAPIES
8.4.1 GENE THERAPIES
8.4.2 CELL THERAPIES
8.4.2.1 Gene Editing
8.4.2.2 Gene Addition
9 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION
9.1 OVERVIEW
9.2 PARENTERAL
9.3 ORAL
9.4 OTHERS
10 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER
10.1 OVERVIEW
10.2 HOSPITALS
10.3 SPECIALTY CLINICS
10.4 HOMECARE
10.5 OTHERS
11 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL
11.1 OVERVIEW
11.2 HOSPITAL PHARMACY
11.3 RETAIL PHARMACY
11.4 ONLINE PHARMACY
12 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY REGION
12.1 OVERVIEW
12.2 NORTH AMERICA
12.2.1 U.S.
12.2.2 CANADA
12.2.3 MEXICO
12.3 EUROPE
12.3.1 GERMANY
12.3.2 FRANCE
12.3.3 U.K.
12.3.4 ITALY
12.3.5 SPAIN
12.3.6 RUSSIA
12.3.7 TURKEY
12.3.8 BELGIUM
12.3.9 NETHERLANDS
12.3.10 SWITZERLAND
12.3.11 REST OF EUROPE
12.4 ASIA-PACIFIC
12.4.1 CHINA
12.4.2 JAPAN
12.4.3 INDIA
12.4.4 SOUTH KOREA
12.4.5 AUSTRALIA
12.4.6 SINGAPORE
12.4.7 THAILAND
12.4.8 MALAYSIA
12.4.9 INDONESIA
12.4.10 PHILIPPINES
12.4.11 REST OF ASIA-PACIFIC
12.5 SOUTH AMERICA
12.5.1 BRAZIL
12.5.2 ARGENTINA
12.5.3 REST OF SOUTH AMERICA
12.6 MIDDLE EAST AND AFRICA
12.6.1 SOUTH AFRICA
12.6.2 SAUDI ARABIA
12.6.3 U.A.E
12.6.4 EGYPT
12.6.5 ISRAEL
12.6.6 REST OF MIDDLE EAST AND AFRICA
13 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, COMPANY LANDSCAPE
13.1 COMPANY SHARE ANALYSIS: GLOBAL
14 NORTH AMERICA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, COMPANY LANDSCAPE
14.1 COMPANY SHARE ANALYSIS: NORTH AMERICA
15 EUROPE DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, COMPANY LANDSCAPE
15.1 COMPANY SHARE ANALYSIS: EUROPE
16 ASIA-PACIFIC DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, COMPANY LANDSCAPE
16.1 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
17 SWOT ANALYSIS
18 COMPANY PROFILES
18.1 SAREPTA THERAPEUTICS, INC.
18.1.1 COMPANY SNAPSHOT
18.1.1 REVENUE ANALYSIS
18.1.2 COMPANY SHARE ANALYSIS
18.1.3 PRODUCT PORTFOLIO
18.1.4 PIPELINE PORTFOLIO
18.1.5 RECENT DEVELOPMENTS
18.2 F. HOFFMANN-LA ROCHE LTD
18.2.1 COMPANY SNAPSHOT
18.2.2 REVENUE ANALYSIS
18.2.3 COMPANY SHARE ANALYSIS
18.2.4 PRODUCT PORTFOLIO
18.2.5 PIPELINE PORTFOLIO
18.2.6 RECENT DEVELOPMENTS
18.3 PTC THERAPEUTICS.
18.3.1 COMPANY SNAPSHOT
18.3.2 REVENUE ANALYSIS
18.3.3 COMPANY SHARE ANALYSIS
18.3.4 PRODUCT PORTFOLIO
18.3.5 RECENT DEVELOPMENT
18.4 PFIZER INC.
18.4.1 COMPANY SNAPSHOT
18.4.2 REVENUE ANALYSIS
18.4.3 COMPANY SHARE ANALYSIS
18.4.4 PIPELINE PORTFOLIO
18.4.5 PRODUCT PORTFOLIO
18.4.6 RECENT DEVELOPMENT
18.5 AKASHI RX
18.5.1 COMPANY SNAPSHOT
18.5.2 PIPELINE PORTFOLIO
18.5.3 RECENT DEVELOPMENTS
18.6 AVIDITY BIOSCIENCES
18.6.1 COMPANY SNAPSHOT
18.6.2 REVENUE ANALYSIS
18.6.3 PIPELINE PORTFOLIO
18.6.4 RECENT DEVELOPMENTS
18.7 BIOMARIN
18.7.1 COMPANY SNAPSHOT
18.7.2 REVENUE ANALYSIS
18.7.3 PIPELINE PORTFOLIO
18.7.4 RECENT DEVELOPMENTS
18.8 CAPRICOR THERAPEUTICS, INC.
18.8.1 COMPANY SNAPSHOT
18.8.2 PIPELINE PORTFOLIO
18.8.3 RECENT DEVELOPMENTS
18.9 DYNE THERAPEUTICS
18.9.1 COMPANY SNAPSHOT
18.9.2 PIPELINE PORTFOLIO
18.9.3 RECENT DEVELOPMENTS
18.1 FIBROGEN, INC.
18.10.1 COMPANY SNAPSHOT
18.10.2 REVENUE ANALYSIS
18.10.3 PIPELINE PORTFOLIO
18.10.4 RECENT DEVELOPMENT
18.11 ITALFARMACO S.P.A.
18.11.1 COMPANY SNAPSHOT
18.11.2 PIPELINE PORTFOLIO
18.11.3 RECENT DEVELOPMENT
18.12 NS PHARMA, INC.
18.12.1 COMPANY SNAPSHOT
18.12.2 PIPELINE PORTFOLIO
18.12.3 RECENT DEVELOPMENTS
18.13 REVERAGEN BIOPHARMA, INC.
18.13.1 COMPANY SNAPSHOT
18.13.2 PIPELINE PORTFOLIO
18.13.3 RECENT DEVELOPMENT
18.14 SANTHERA PHARMACEUTICALS
18.14.1 COMPANY SNAPSHOT
18.14.2 REVENUE ANALYSIS
18.14.3 PIPELINE PORTFOLIO
18.14.4 RECENT DEVELOPMENT
18.15 TAIHO PHARMACEUTICAL CO., LTD.
18.15.1 COMPANY SNAPSHOT
18.15.2 PIPELINE PORTFOLIO
18.15.3 RECENT DEVELOPMENTS
18.16 SOLID BIOSCIENCES INC.
18.16.1 COMPANY SNAPSHOT
18.16.2 PIPELINE PORTFOLIO
18.16.3 RECENT DEVELOPMENT
18.17 STEALTH BIOTHERAPEUTICS INC
18.17.1 COMPANY SNAPSHOT
18.17.2 PIPELINE PORTFOLIO
18.17.3 RECENT DEVELOPMENT
19 QUESTIONNAIRE
20 RELATED REPORTS
표 목록
TABLE 1 LIST OF PRICES FOR APPROVED DRUGS OF THE GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET
TABLE 2 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE , 2021-2030 (USD THOUSAND)
TABLE 3 GLOBAL MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY REGION, 2021-2030 (USD THOUSAND)
TABLE 4 GLOBAL MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 5 GLOBAL ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 6 GLOBAL NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 7 GLOBAL STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY REGION, 2021-2030 (USD THOUSAND)
TABLE 8 GLOBAL STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 9 GLOBAL OTHERS IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY REGION, 2021-2030 (USD THOUSAND)
TABLE 10 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 11 GLOBAL EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY REGION, 2021-2030 (USD THOUSAND)
TABLE 12 GLOBAL EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 13 GLOBAL MUTATION SUPPRESSION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY REGION, 2021-2030 (USD THOUSAND)
TABLE 14 GLOBAL DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY REGION, 2021-2030 (USD THOUSAND)
TABLE 15 GLOBAL DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 16 GLOBAL CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 17 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION , 2021-2030 (USD THOUSAND)
TABLE 18 GLOBAL PARENTERAL IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY REGION, 2021-2030 (USD THOUSAND)
TABLE 19 GLOBAL ORAL IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY REGION, 2021-2030 (USD THOUSAND)
TABLE 20 GLOBAL OTHERS IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY REGION, 2021-2030 (USD THOUSAND)
TABLE 21 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USERS, 2021-2030 (USD THOUSAND)
TABLE 22 GLOBAL HOSPITALS IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY REGION, 2021-2030 (USD THOUSAND)
TABLE 23 GLOBAL SPECIALITY CLINICS IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY REGION, 2021-2030 (USD THOUSAND)
TABLE 24 GLOBAL HOMECARE IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY REGION, 2021-2030 (USD THOUSAND)
TABLE 25 GLOBAL OTHERS IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY REGION, 2021-2030 (USD THOUSAND)
TABLE 26 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 27 GLOBAL HOSPITAL PHARMACY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY REGION, 2021-2030 (USD THOUSAND)
TABLE 28 GLOBAL RETAIL PHARMACY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY REGION, 2021-2030 (USD THOUSAND)
TABLE 29 GLOBAL ONLINE PHARMACY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY REGION, 2021-2030 (USD THOUSAND)
TABLE 30 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY REGION, 2021-2030 (USD THOUSAND)
TABLE 31 NORTH AMERICA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY COUNTRY, 2021-2030 (USD THOUSAND) COUNTRY
TABLE 32 NORTH AMERICA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 33 NORTH AMERICA MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 34 NORTH AMERICA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 35 NORTH AMERICA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 36 NORTH AMERICA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 37 NORTH AMERICA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 38 NORTH AMERICA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 39 NORTH AMERICA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 40 NORTH AMERICA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 41 NORTH AMERICA EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 42 NORTH AMERICA DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 43 NORTH AMERICA CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 44 NORTH AMERICA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 45 NORTH AMERICA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 46 NORTH AMERICA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 47 U.S. DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 48 U.S. MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 49 U.S. ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 50 U.S. ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 51 U.S. ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 52 U.S. NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 53 U.S. NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 54 U.S. NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 55 U.S. STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 56 U.S. STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 57 U.S. STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 58 U.S.DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 59 U.S. EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 60 U.S. DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 61 U.S. CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 62 U.S. DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 63 U.S. DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 64 U.S. DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 65 CANADA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 66 CANADA MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 67 CANADA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 68 CANADA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 69 CANADA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 70 CANADA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 71 CANADA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 72 CANADA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 73 CANADA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 74 CANADA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 75 CANADA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 76 CANADA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 77 CANADA EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 78 CANADA DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 79 CANADA CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 80 CANADA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 81 CANADA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 82 CANADA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 83 MEXICO DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 84 MEXICO MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 85 MEXICO ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 86 MEXICO ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 87 MEXICO ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 88 MEXICO NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 89 MEXICO NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 90 MEXICO NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 91 MEXICO STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 92 MEXICO STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 93 MEXICO STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 94 MEXICO DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 95 MEXICO EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 96 MEXICO DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 97 MEXICO CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 98 MEXICO DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 99 MEXICO DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 100 MEXICO DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 101 EUROPE DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY COUNTRY, 2021-2030 (USD THOUSAND)
TABLE 102 EUROPE DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 103 EUROPE MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 104 EUROPE ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 105 EUROPE ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 106 EUROPE NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 107 EUROPE NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 108 EUROPE STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 109 EUROPE STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 110 EUROPE DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 111 EUROPE EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 112 EUROPE DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 113 EUROPE CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 114 EUROPE DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 115 EUROPE DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 116 EUROPE DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 117 GERMANY DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 118 GERMANY MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 119 GERMANY ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 120 GERMANY ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 121 GERMANY ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 122 GERMANY NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 123 GERMANY NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 124 GERMANY NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 125 GERMANY STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 126 GERMANY STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 127 GERMANY STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 128 GERMANY DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 129 GERMANY EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 130 GERMANY DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 131 GERMANY CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 132 GERMANY DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 133 GERMANY DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 134 GERMANY DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 135 FRANCE DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 136 FRANCE MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 137 FRANCE ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 138 FRANCE ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 139 FRANCE ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 140 FRANCE NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 141 FRANCE NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 142 FRANCE NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 143 FRANCE STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 144 FRANCE STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 145 FRANCE STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 146 FRANCE DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 147 FRANCE EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 148 FRANCE DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 149 FRANCE CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 150 FRANCE DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 151 FRANCE DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 152 FRANCE DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 153 U.K. DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 154 U.K. MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 155 U.K. ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 156 U.K. ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 157 U.K. ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 158 U.K. NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 159 U.K. NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 160 U.K. NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 161 U.K. STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 162 U.K. STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 163 U.K. STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 164 U.K. DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 165 U.K. EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 166 U.K. DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 167 U.K. CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 168 U.K. DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 169 U.K. DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 170 U.K. DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 171 ITALY DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 172 ITALY MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 173 ITALY ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 174 ITALY ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 175 ITALY ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 176 ITALY NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 177 ITALY NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 178 ITALY NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 179 ITALY STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 180 ITALY STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 181 ITALY STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 182 ITALYDUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 183 ITALY EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 184 ITALY DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 185 ITALY CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 186 ITALY DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 187 ITALY DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 188 ITALY DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 189 SPAIN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 190 SPAIN MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 191 SPAIN ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 192 SPAIN ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 193 SPAIN ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 194 SPAIN NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 195 SPAIN NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 196 SPAIN NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 197 SPAIN STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 198 SPAIN STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 199 SPAIN STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 200 SPAIN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 201 SPAIN EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 202 SPAIN DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 203 SPAIN CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 204 SPAIN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 205 SPAIN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 206 SPAIN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 207 RUSSIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 208 RUSSIA MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 209 RUSSIA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 210 RUSSIA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 211 RUSSIA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 212 RUSSIA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 213 RUSSIA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 214 RUSSIA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 215 RUSSIA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 216 RUSSIA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 217 RUSSIA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 218 RUSSIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 219 RUSSIA EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 220 RUSSIA DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 221 RUSSIA CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 222 RUSSIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 223 RUSSIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 224 RUSSIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 225 TURKEY DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 226 TURKEY MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 227 TURKEY ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 228 TURKEY ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 229 TURKEY ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 230 TURKEY NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 231 TURKEY NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 232 TURKEY NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 233 TURKEY STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 234 TURKEY STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 235 TURKEY STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 236 TURKEY DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 237 TURKEY EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 238 TURKEY DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 239 TURKEY CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 240 TURKEY DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 241 TURKEY DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 242 TURKEY DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 243 BELGIUM DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 244 BELGIUM MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 245 BELGIUM ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 246 BELGIUM ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 247 BELGIUM ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 248 BELGIUM NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 249 BELGIUM NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 250 BELGIUM NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 251 BELGIUM STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 252 BELGIUM STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 253 BELGIUM STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 254 BELGIUM DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 255 BELGIUM EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 256 BELGIUM DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 257 BELGIUM CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 258 BELGIUM DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 259 BELGIUM DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 260 BELGIUM DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 261 NETHERLANDS DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 262 NETHERLANDS MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 263 NETHERLANDS ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 264 NETHERLANDS ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 265 NETHERLANDS ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 266 NETHERLANDS NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 267 NETHERLANDS NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 268 NETHERLANDS NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 269 NETHERLANDS STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 270 NETHERLANDS STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 271 NETHERLANDS STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 272 NETHERLANDS DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 273 NETHERLANDS EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 274 NETHERLANDS DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 275 NETHERLANDS CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 276 NETHERLANDS DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 277 NETHERLANDS DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 278 NETHERLANDS DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 279 SWITZERLAND DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 280 SWITZERLAND MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 281 SWITZERLAND ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 282 SWITZERLAND ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 283 SWITZERLAND ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 284 SWITZERLAND NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 285 SWITZERLAND NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 286 SWITZERLAND NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 287 SWITZERLAND STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 288 SWITZERLAND STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 289 SWITZERLAND STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 290 SWITZERLANDDUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 291 SWITZERLAND EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 292 SWITZERLAND DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 293 SWITZERLAND CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 294 SWITZERLAND DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 295 SWITZERLAND DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 296 SWITZERLAND DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 297 REST OF EUROPE DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 298 ASIA-PACIFIC DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY COUNTRY, 2021-2030 (USD THOUSAND)
TABLE 299 ASIA-PACIFIC DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 300 ASIA-PACIFIC MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 301 ASIA-PACIFIC ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 302 ASIA-PACIFIC ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 303 ASIA-PACIFIC NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 304 ASIA-PACIFIC NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 305 ASIA-PACIFIC STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 306 ASIA-PACIFIC STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 307 ASIA-PACIFIC DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 308 ASIA-PACIFIC EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 309 ASIA-PACIFIC DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 310 ASIA-PACIFIC CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 311 ASIA-PACIFIC DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 312 ASIA-PACIFIC DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 313 ASIA-PACIFIC DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 314 CHINA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 315 CHINA MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 316 CHINA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 317 CHINA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 318 CHINA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 319 CHINA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 320 CHINA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 321 CHINA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 322 CHINA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 323 CHINA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 324 CHINA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 325 CHINA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 326 CHINA EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 327 CHINA DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 328 CHINA CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 329 CHINA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 330 CHINA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 331 CHINA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 332 JAPAN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 333 JAPAN MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 334 JAPAN ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 335 JAPAN ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 336 JAPAN ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 337 JAPAN NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 338 JAPAN NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 339 JAPAN NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 340 JAPAN STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 341 JAPAN STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 342 JAPAN STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 343 JAPAN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 344 JAPAN EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 345 JAPAN DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 346 JAPAN CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 347 JAPAN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 348 JAPAN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 349 JAPAN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 350 INDIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 351 INDIA MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 352 INDIA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 353 INDIA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 354 INDIA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 355 INDIA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 356 INDIA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 357 INDIA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 358 INDIA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 359 INDIA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 360 INDIA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 361 INDIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 362 INDIA EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 363 INDIA CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 364 INDIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 365 INDIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 366 INDIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 367 SOUTH KOREA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 368 SOUTH KOREA MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 369 SOUTH KOREA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 370 SOUTH KOREA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 371 SOUTH KOREA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 372 SOUTH KOREA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 373 SOUTH KOREA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 374 SOUTH KOREA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 375 SOUTH KOREA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 376 SOUTH KOREA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 377 SOUTH KOREA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 378 SOUTH KOREADUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 379 SOUTH KOREA EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 380 SOUTH KOREA DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 381 SOUTH KOREA CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 382 SOUTH KOREA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 383 SOUTH KOREA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 384 SOUTH KOREA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 385 AUSTRALIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 386 AUSTRALIA MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 387 AUSTRALIA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 388 AUSTRALIA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 389 AUSTRALIA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 390 AUSTRALIA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 391 AUSTRALIA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 392 AUSTRALIA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 393 AUSTRALIA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 394 AUSTRALIA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 395 AUSTRALIA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 396 AUSTRALIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 397 AUSTRALIA EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 398 AUSTRALIA DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 399 AUSTRALIA CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 400 AUSTRALIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 401 AUSTRALIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 402 AUSTRALIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 403 SINGAPORE DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 404 SINGAPORE MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 405 SINGAPORE ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 406 SINGAPORE ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 407 SINGAPORE ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 408 SINGAPORE NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 409 SINGAPORE NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 410 SINGAPORE NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 411 SINGAPORE STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 412 SINGAPORE STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 413 SINGAPORE STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 414 SINGAPORE DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 415 SINGAPORE EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 416 SINGAPORE DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 417 SINGAPORE CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 418 SINGAPORE DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 419 SINGAPORE DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 420 SINGAPORE DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 421 THAILAND DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 422 THAILAND MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 423 THAILAND ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 424 THAILAND ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 425 THAILAND ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 426 THAILAND NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 427 THAILAND NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 428 THAILAND NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 429 THAILAND STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 430 THAILAND STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 431 THAILAND STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 432 THAILAND DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 433 THAILAND EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 434 THAILAND DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 435 THAILAND CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 436 THAILAND DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 437 THAILAND DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 438 THAILAND DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 439 MALAYSIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 440 MALAYSIA MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 441 MALAYSIA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 442 MALAYSIA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 443 MALAYSIA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 444 MALAYSIA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 445 MALAYSIA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 446 MALAYSIA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 447 MALAYSIA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 448 MALAYSIA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 449 MALAYSIA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 450 MALAYSIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 451 MALAYSIA EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 452 MALAYSIA DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 453 MALAYSIA CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 454 MALAYSIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 455 MALAYSIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 456 MALAYSIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 457 INDONESIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 458 INDONESIA MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 459 INDONESIA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 460 INDONESIA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 461 INDONESIA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 462 INDONESIA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 463 INDONESIA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 464 INDONESIA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 465 INDONESIA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 466 INDONESIA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 467 INDONESIA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 468 INDONESIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 469 INDONESIA EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 470 INDONESIA DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 471 INDONESIA CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 472 INDONESIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 473 INDONESIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 474 INDONESIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 475 PHILIPPINES DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 476 PHILIPPINES MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 477 PHILIPPINES ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 478 PHILIPPINES ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 479 PHILIPPINES ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 480 PHILIPPINES NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 481 PHILIPPINES NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 482 PHILIPPINES NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 483 PHILIPPINES STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 484 PHILIPPINES STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 485 PHILIPPINES STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 486 PHILIPPINESDUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 487 PHILIPPINES EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 488 PHILIPPINES DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 489 PHILIPPINES CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 490 PHILIPPINES DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 491 PHILIPPINES DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 492 PHILIPPINES DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 493 REST OF ASIA-PACIFIC DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 494 SOUTH AMERICA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY COUNTRY, 2021-2030 (USD THOUSAND)
TABLE 495 SOUTH AMERICA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 496 SOUTH AMERICA MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 497 SOUTH AMERICA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 498 SOUTH AMERICA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 499 SOUTH AMERICA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 500 SOUTH AMERICA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 501 SOUTH AMERICA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 502 SOUTH AMERICA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 503 SOUTH AMERICA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 504 SOUTH AMERICA EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 505 SOUTH AMERICA DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 506 SOUTH AMERICA CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 507 SOUTH AMERICA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 508 SOUTH AMERICA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 509 SOUTH AMERICA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 510 BRAZIL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 511 BRAZIL MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 512 BRAZIL ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 513 BRAZIL ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 514 BRAZIL ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 515 BRAZIL NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 516 BRAZIL NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 517 BRAZIL NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 518 BRAZIL STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 519 BRAZIL STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 520 BRAZIL STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 521 BRAZIL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 522 BRAZIL EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 523 BRAZIL DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 524 BRAZIL CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 525 BRAZIL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 526 BRAZIL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 527 BRAZIL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 528 ARGENTINA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 529 ARGENTINA MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 530 ARGENTINA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 531 ARGENTINA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 532 ARGENTINA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 533 ARGENTINA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 534 ARGENTINA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 535 ARGENTINA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 536 ARGENTINA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 537 ARGENTINA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 538 ARGENTINA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 539 ARGENTINA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 540 ARGENTINA EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 541 ARGENTINA DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 542 ARGENTINA CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 543 ARGENTINA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 544 ARGENTINA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 545 ARGENTINA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 546 REST OF SOUTH AMERICA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 547 MIDDLE EAST AND AFRICA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY COUNTRY, 2021-2030 (USD THOUSAND)
TABLE 548 MIDDLE EAST AND AFRICA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 549 MIDDLE EAST AND AFRICA MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 550 MIDDLE EAST AND AFRICA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 551 MIDDLE EAST AND AFRICA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 552 MIDDLE EAST AND AFRICA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 553 MIDDLE EAST AND AFRICA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 554 MIDDLE EAST AND AFRICA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 555 MIDDLE EAST AND AFRICA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 556 MIDDLE EAST AND AFRICA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 557 MIDDLE EAST AND AFRICA EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 558 MIDDLE EAST AND AFRICA DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 559 MIDDLE EAST AND AFRICA CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 560 MIDDLE EAST AND AFRICA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 561 MIDDLE EAST AND AFRICA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 562 MIDDLE EAST AND AFRICA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 563 SOUTH AFRICA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 564 SOUTH AFRICA MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 565 SOUTH AFRICA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 566 SOUTH AFRICA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 567 SOUTH AFRICA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 568 SOUTH AFRICA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 569 SOUTH AFRICA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 570 SOUTH AFRICA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 571 SOUTH AFRICA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 572 SOUTH AFRICA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 573 SOUTH AFRICA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 574 SOUTH AFRICA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 575 SOUTH AFRICA EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 576 SOUTH AFRICA DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 577 SOUTH AFRICA CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 578 SOUTH AFRICA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 579 SOUTH AFRICA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 580 SOUTH AFRICA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 581 SAUDI ARABIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 582 SAUDI ARABIA MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 583 SAUDI ARABIA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 584 SAUDI ARABIA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 585 SAUDI ARABIA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 586 SAUDI ARABIA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 587 SAUDI ARABIA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 588 SAUDI ARABIA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 589 SAUDI ARABIA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 590 SAUDI ARABIA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 591 SAUDI ARABIA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 592 SAUDI ARABIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 593 SAUDI ARABIA EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 594 SAUDI ARABIA DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 595 SAUDI ARABIA CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 596 SAUDI ARABIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 597 SAUDI ARABIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 598 SAUDI ARABIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 599 U.A.E DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 600 U.A.E MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 601 U.A.E ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 602 U.A.E ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 603 U.A.E ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 604 U.A.E NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 605 U.A.E NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 606 U.A.E NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 607 U.A.E STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 608 U.A.E STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 609 U.A.E STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 610 U.A.E DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 611 U.A.E EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 612 U.A.E DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 613 U.A.E CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 614 U.A.E DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 615 U.A.E DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 616 U.A.E DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 617 EGYPT DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 618 EGYPT MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 619 EGYPT ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 620 EGYPT ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 621 EGYPT ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 622 EGYPT NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 623 EGYPT NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 624 EGYPT NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 625 EGYPT STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 626 EGYPT STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 627 EGYPT STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 628 EGYPT DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 629 EGYPT EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 630 EGYPT DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 631 EGYPT CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 632 EGYPT DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 633 EGYPT DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 634 EGYPT DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 635 ISRAEL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 636 ISRAEL MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 637 ISRAEL ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 638 ISRAEL ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 639 ISRAEL ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 640 ISRAEL NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 641 ISRAEL NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 642 ISRAEL NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 643 ISRAEL STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 644 ISRAEL STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 645 ISRAEL STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 646 ISRAEL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 647 ISRAEL EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 648 ISRAEL DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 649 ISRAEL CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 650 ISRAEL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 651 ISRAEL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 652 ISRAEL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 653 REST OF MIDDLE EAST AND AFRICA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
그림 목록
FIGURE 1 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: SEGMENTATION
FIGURE 2 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: DATA TRIANGULATION
FIGURE 3 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: DROC ANALYSIS
FIGURE 4 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: GLOBAL VS REGIONAL MARKET ANALYSIS
FIGURE 5 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: MARKET END USER COVERAGE GRID
FIGURE 8 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: DBMR MARKET POSITION GRID
FIGURE 9 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: SEGMENTATION
FIGURE 11 THE RISING PREVALENCE AND IMPACT OF DUCHENNE MUSCULAR DYSTROPHY (DMD) AND INTRODUCTION OF NOVEL THERAPIES FOR DMD DISORDER ARE EXPECTED TO DRIVE THE GROWTH OF THE GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET FROM 2023 TO 2030
FIGURE 12 MOLECULAR-BASED THERAPIES SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET IN 2023 AND 2030
FIGURE 13 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF THE GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET
FIGURE 14 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: BY TREATMENT TYPE , 2022
FIGURE 15 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: BY TREATMENT TYPE, 2023-2030 (USD THOUSAND)
FIGURE 16 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: BY TREATMENT TYPE , CAGR (2023-2030)
FIGURE 17 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: BY TREATMENT TYPE, LIFELINE CURVE
FIGURE 18 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: BY THERAPY, 2022
FIGURE 19 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: BY THERAPY, 2023-2030 (USD THOUSAND)
FIGURE 20 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: BY THERAPY, CAGR (2023-2030)
FIGURE 21 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: BY THERAPY, LIFELINE CURVE
FIGURE 22 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: BY ROUTE OF ADMINISTRATION, 2022
FIGURE 23 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: BY ROUTE OF ADMINISTRATION, 2023-2030 (USD THOUSAND)
FIGURE 24 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: BY ROUTE OF ADMINISTRATION, CAGR (2023-2030)
FIGURE 25 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: BY ROUTE OF ADMINISTRATION, LIFELINE CURVE
FIGURE 26 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: BY END USER, 2022
FIGURE 27 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: BY END USER, 2023-2030 (USD THOUSAND)
FIGURE 28 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: BY END USER, CAGR (2023-2030)
FIGURE 29 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: BY END USER, LIFELINE CURVE
FIGURE 30 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: BY DISTRIBUTION CHANNEL, 2022
FIGURE 31 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: BY DISTRIBUTION CHANNEL, 2023-2030 (USD THOUSAND)
FIGURE 32 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: BY DISTRIBUTION CHANNEL, CAGR (2023-2030)
FIGURE 33 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 34 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET SNAPSHOT
FIGURE 35 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: COMPANY SHARE 2022 (%)
FIGURE 36 NORTH AMERICA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: COMPANY SHARE 2022 (%)
FIGURE 37 EUROPE DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: COMPANY SHARE 2022 (%)
FIGURE 38 ASIA-PACIFIC DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: COMPANY SHARE 2022 (%)

연구 방법론
데이터 수집 및 기준 연도 분석은 대규모 샘플 크기의 데이터 수집 모듈을 사용하여 수행됩니다. 이 단계에는 다양한 소스와 전략을 통해 시장 정보 또는 관련 데이터를 얻는 것이 포함됩니다. 여기에는 과거에 수집한 모든 데이터를 미리 검토하고 계획하는 것이 포함됩니다. 또한 다양한 정보 소스에서 발견되는 정보 불일치를 검토하는 것도 포함됩니다. 시장 데이터는 시장 통계 및 일관된 모델을 사용하여 분석하고 추정합니다. 또한 시장 점유율 분석 및 주요 추세 분석은 시장 보고서의 주요 성공 요인입니다. 자세한 내용은 분석가에게 전화를 요청하거나 문의 사항을 드롭하세요.
DBMR 연구팀에서 사용하는 주요 연구 방법론은 데이터 마이닝, 시장에 대한 데이터 변수의 영향 분석 및 주요(산업 전문가) 검증을 포함하는 데이터 삼각 측량입니다. 데이터 모델에는 공급업체 포지셔닝 그리드, 시장 타임라인 분석, 시장 개요 및 가이드, 회사 포지셔닝 그리드, 특허 분석, 가격 분석, 회사 시장 점유율 분석, 측정 기준, 글로벌 대 지역 및 공급업체 점유율 분석이 포함됩니다. 연구 방법론에 대해 자세히 알아보려면 문의를 통해 업계 전문가에게 문의하세요.
사용자 정의 가능
Data Bridge Market Research는 고급 형성 연구 분야의 선두 주자입니다. 저희는 기존 및 신규 고객에게 목표에 맞는 데이터와 분석을 제공하는 데 자부심을 느낍니다. 보고서는 추가 국가에 대한 시장 이해(국가 목록 요청), 임상 시험 결과 데이터, 문헌 검토, 재생 시장 및 제품 기반 분석을 포함하도록 사용자 정의할 수 있습니다. 기술 기반 분석에서 시장 포트폴리오 전략에 이르기까지 타겟 경쟁업체의 시장 분석을 분석할 수 있습니다. 귀하가 원하는 형식과 데이터 스타일로 필요한 만큼 많은 경쟁자를 추가할 수 있습니다. 저희 분석가 팀은 또한 원시 엑셀 파일 피벗 테이블(팩트북)로 데이터를 제공하거나 보고서에서 사용 가능한 데이터 세트에서 프레젠테이션을 만드는 데 도움을 줄 수 있습니다.