Global Diagnostic Tests Market
시장 규모 (USD 10억)
연평균 성장률 :
% 
 USD
286.36 Billion
USD
545.94 Billion
2024
2032
USD
286.36 Billion
USD
545.94 Billion
2024
2032
| 2025 –2032 | |
| USD 286.36 Billion | |
| USD 545.94 Billion | |
|
|
|
|
글로벌 진단 검사 시장 세분화, 유형별(일상 검사 및 특수 검사), 구성 요소(서비스 및 제품), 기술(면역 분석 기반, PCR 기반, 차세대 시퀀싱, 분광법 기반, 크로마토그래피 기반, 미세유체학 및 기타), 검사 모드(처방 기반 검사 및 OTC 검사), 응용 분야(종양학, 심장학, 정형외과, 위장병학, 산부인과, 신경학, 치과학 및 기타), 샘플 유형(혈액, 소변, 타액, 땀, 모발 및 기타), 검사 장소(실험실 기반 검사 및 재택 검사), 검사 유형(생화학, 혈액학, 미생물학, 조직병리학 및 기타), 연령(노인, 성인 및 소아), 최종 사용자(병원, 진단 센터, 외래 수술 센터(ASC), 전문 클리닉, 재택 치료, 혈액 은행, 연구) 연구소 및 기타), 유통 채널(직접 입찰, 소매 판매 및 온라인 판매) - 2032년까지의 산업 동향 및 예측
진단 테스트 시장 규모
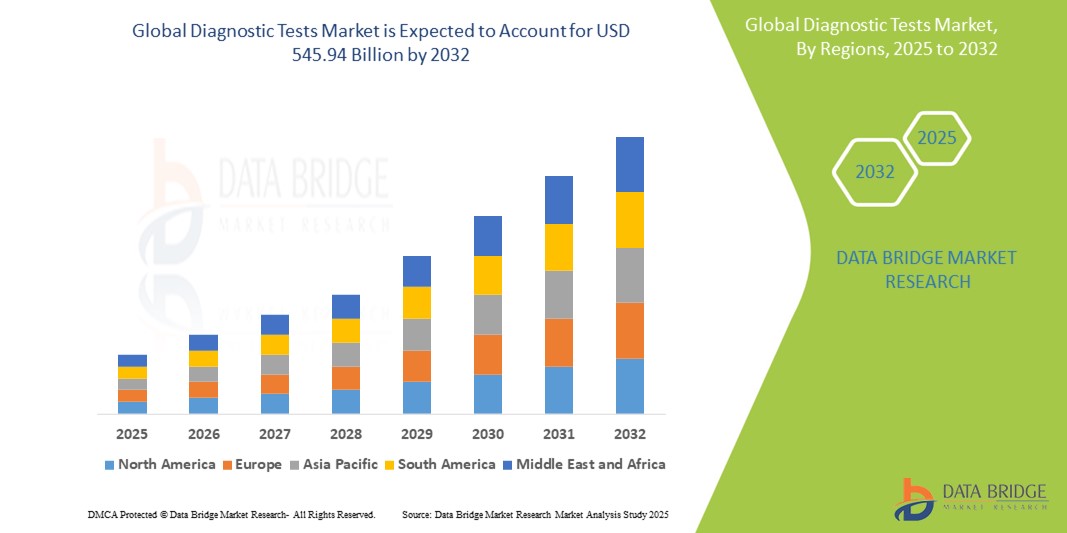
- 글로벌 진단 테스트 시장 규모는 2024년에 2,863억 6천만 달러 로 평가되었으며 예측 기간 동안 8.4%의 CAGR 로 2032년까지 5,459억 4천만 달러에 도달할 것으로 예상됩니다 .
- 시장 확장은 만성 및 감염성 질환 의 유병률 증가로 인해 전 세계 의료 시스템 전반에서 조기에 정확한 진단 도구에 대한 수요가 증가함에 따라 강력하게 촉진되었습니다.
- 더욱이, 현장 진단(POC), AI 통합 진단, 분자 진단과 같은 기술 혁신은 진단 시장의 지형을 변화시키고 정확성과 접근성을 향상시키고 있습니다. 이러한 추세는 진단 검사 시장의 도입을 가속화하고 견고한 성장을 촉진하고 있습니다.
진단 테스트 시장 분석
- 다양한 질병 및 상태에 대한 실험실 기반 또는 진료소 탐지를 제공하는 진단 테스트는 향상된 정확도, 빠른 결과, 디지털 건강 플랫폼과의 원활한 통합으로 인해 임상 및 가정 환경 모두에서 현대 의료 시스템의 점점 더 중요한 구성 요소가 되고 있습니다.
- 진단 테스트에 대한 수요 증가는 주로 만성 및 감염성 질환의 전 세계적 유병률 증가, 조기 질병 탐지에 대한 중요성 증가, 개인화 및 예방적 의료 솔루션에 대한 선호도 증가에 의해 촉진됩니다.
- 북미는 2024년 42.5%의 가장 큰 수익 점유율로 진단 검사 시장을 장악했으며, 첨단 의료 인프라, 높은 의료비 지출, 핵심 산업 참여자들의 강력한 입지를 특징으로 합니다. 특히 미국은 PCR 및 차세대 시퀀싱 기술의 혁신에 힘입어 병원 및 전문 진료소를 중심으로 분자 및 유전자 검사 분야에서 상당한 성장을 경험했습니다.
- 아시아 태평양 지역은 의료 접근성 증가와 진단 인프라에 대한 공공 및 민간 투자 증가로 인해 예측 기간 동안 진단 테스트 시장에서 가장 빠르게 성장하는 지역이 될 것으로 예상됩니다.
- 일상 검사 부문은 다양한 의료 환경에서 정기 건강 검진, 조기 질병 탐지 및 만성 질환 모니터링에 널리 사용되면서 2024년 진단 검사 시장 점유율 63.82%로 시장을 장악했습니다.
보고서 범위 및 진단 테스트 시장 세분화
|
속성 |
진단 테스트 주요 시장 통찰력 |
|
다루는 세그먼트 |
|
|
포함 국가 |
북아메리카
유럽
아시아 태평양
중동 및 아프리카
남아메리카
|
|
주요 시장 참여자 |
|
|
시장 기회 |
|
|
부가가치 데이터 정보 세트 |
Data Bridge Market Research에서 큐레이팅한 시장 보고서에는 시장 가치, 성장률, 세분화, 지리적 적용 범위, 주요 기업 등 시장 시나리오에 대한 통찰력 외에도 심층적인 전문가 분석, 가격 분석, 브랜드 점유율 분석, 소비자 설문 조사, 인구 통계 분석, 공급망 분석, 가치 사슬 분석, 원자재/소모품 개요, 공급업체 선택 기준, PESTLE 분석, Porter 분석 및 규제 프레임워크가 포함되어 있습니다. |
진단 검사 시장 동향
“AI와 재택 진단 통합의 발전”
- 글로벌 진단 테스트 시장에서 중요하고 가속화되는 추세는 디지털 진단 플랫폼과 재택 테스트 솔루션에 인공 지능(AI)을 통합하여 다양한 치료 환경에서 진단 정확도, 속도 및 접근성을 크게 향상시키는 것입니다.
- 예를 들어 PathAI 및 Aidoc과 같은 AI 기반 플랫폼은 복잡한 영상 및 병리학 데이터를 빠르게 해석하여 임상의를 지원하는 반면 Cue Health 및 LetsGetChecked와 같은 가정용 진단 도구는 감염성 질환 및 일반 건강 모니터링을 위한 스마트폰 지원 테스트 키트를 제공합니다.
- 진단 분야의 AI는 예측 분석, 실시간 결과 해석, 임상 의사 결정 지원 등의 기능을 제공하여 진단 정확도를 높이고 처리 시간을 단축합니다. 일부 플랫폼은 환자 데이터를 기반으로 적응하고 학습하여 검사 권장 사항을 개선하고 개인 맞춤형 치료를 강화합니다.
- AI와 원격 검사 장비의 통합은 분산형 진단을 용이하게 하여 사용자가 최소한의 임상적 개입으로 당뇨병, 콜레스테롤, 감염 등의 상태를 자가 모니터링할 수 있도록 지원합니다. 검사 결과는 연결된 앱이나 원격 의료 플랫폼을 통해 의료 서비스 제공자와 원활하게 공유할 수 있습니다.
- 이러한 추세는 착용형 센서, AI 알고리즘 및 모바일 진단이 융합되어 사전 예방적이고 개인화된 건강 관리 모니터링을 제공하는 지능형 진단 생태계 개발을 촉진하고 있습니다.
- 의료 시스템이 조기 발견, 환자 권한 부여, 확장 가능한 디지털 건강 솔루션으로 전환함에 따라 AI가 강화되고 사용하기 쉬운 진단 도구에 대한 수요가 임상 및 가정 환경 모두에서 빠르게 증가하고 있습니다.
진단 테스트 시장 동향
운전사
만성 질환 증가 및 예방 의료에 대한 관심 증가로 수요 증가
- 만성 및 감염성 질환의 세계적 부담이 증가하고 예방 의료 관행으로의 강력한 전환이 결합되어 임상 및 가정 기반 환경에서 진단 테스트에 대한 수요가 증가하는 주요 원동력이 되었습니다.
- 예를 들어, 2024년 3월, 로슈 진단(Roche Diagnostics)은 만성 질환 및 감염원 검사 속도를 높이고 진단 접근성과 효율성을 향상시키는 새로운 고처리량 PCR 플랫폼을 출시했습니다. 이러한 선도 기업들의 발전은 향후 몇 년 동안 시장 확대를 촉진할 것으로 예상됩니다.
- 의료 시스템이 질병 부담과 치료 비용을 줄이기 위해 조기 발견과 시기적절한 개입을 우선시함에 따라 진단 검사는 모든 연령대의 질병 검진, 모니터링 및 관리를 위한 필수 도구가 되었습니다.
- 또한, 유전체학 및 바이오마커 테스트의 혁신으로 인해 개인화된 의료에 대한 강조가 커지면서 진단이 맞춤형 치료 계획의 핵심 구성 요소로 자리 잡고 있습니다.
- 현대 진단 기술의 편리성과 정확성, 그리고 실시간으로 실행 가능한 통찰력을 제공하는 능력은 환자와 의료 제공자 모두에게 정기적인 검사를 도입하도록 장려하고 있습니다. 빠르고 사용자 친화적인 검사와 모바일 기반 진단 기술의 증가는 외딴 지역과 서비스 취약 지역의 접근성을 확대하고 있습니다.
제지/도전
"데이터 프라이버시 우려와 첨단 진단 기술의 높은 비용"
- 데이터 프라이버시와 민감한 건강 정보의 안전한 처리에 대한 우려가 커지면서 특히 AI나 클라우드 기반 플랫폼과 통합된 고급 진단 솔루션의 광범위한 채택에 상당한 과제가 제기되고 있습니다.
- 예를 들어, 미국의 HIPAA 및 EU의 GDPR을 포함한 여러 규제 기관은 환자 데이터 사용 및 저장에 대한 엄격한 지침을 도입하여 진단 회사가 강력한 사이버 보안 및 규정 준수 프레임워크를 구현하도록 요구했습니다.
- 안전한 데이터 전송, 저장 및 환자 동의 관리를 보장하는 것은 사용자 신뢰를 얻는 데 필수적입니다. 기업들은 진단 분야의 데이터 보안을 강화하기 위해 암호화 기술과 블록체인 기반 시스템에 점점 더 많은 투자를 하고 있습니다.
- 또한, 차세대 염기서열 분석(NGS), 분자 분석, AI 기반 플랫폼과 같은 최첨단 진단 기술의 높은 비용은 특히 저소득 및 중소득 국가와 저보험 인구에서 접근성을 제한합니다. 기본적인 검사 비용은 여전히 저렴하지만, 고급 솔루션은 상당한 장비 및 운영 비용이 소요되는 경우가 많습니다.
- 규제 조화, 비용 효율적인 혁신, 확장 가능한 인프라를 통해 이러한 격차를 해소하는 것이 다양한 의료 환경에서 진단 접근성을 확대하고 지속 가능한 성장을 유지하는 데 중요합니다.
진단 테스트 시장 범위
시장은 유형, 구성 요소, 기술, 테스트 모드, 응용 프로그램, 샘플 유형, 테스트 장소, 테스트 유형, 연령대, 최종 사용자 및 유통 채널을 기준으로 세분화됩니다.
- 유형별
진단 검사 시장은 유형별로 일반 검사와 특수 검사로 구분됩니다. 일반 검사 부문은 2024년 기준 63.82%의 시장 점유율로 가장 큰 시장을 장악했으며, 이는 일반적인 건강 검진 및 비용 효율성 측면에서 널리 활용되기 때문입니다. 일반 검사는 표준화와 조기 질병 발견 및 모니터링 효율성 덕분에 다양한 의료 환경에서 널리 사용되고 있습니다.
개인화된 의학의 발전과 복잡한 질병에 대한 정확한 진단에 대한 수요 증가로 인해 전문 테스트는 예측 기간 동안 가장 빠른 성장률을 보일 것으로 예상됩니다.
- 구성 요소별
시장은 구성 요소를 기준으로 서비스와 제품으로 세분화됩니다. 서비스 부문은 병원 및 진단 센터에서 진단 검사 서비스에 대한 수요가 증가함에 따라 2024년에 가장 큰 시장 점유율을 기록했습니다.
진단 시약, 기기, 다양한 테스트 절차에 필수적인 소모품의 혁신에 힘입어 제품 부문은 예측 기간 동안 가장 빠른 성장을 보일 것으로 예상됩니다.
- 기술로
기술 기준으로 시장은 면역분석 기반, PCR 기반, 차세대 시퀀싱, 분광법 기반, 크로마토그래피 기반, 미세유체역학 등으로 세분화됩니다. 면역분석 기반 기술은 높은 민감도와 바이오마커 검출에 대한 폭넓은 적용성으로 2024년 시장을 주도했습니다.
CR 기반 기술은 예측 기간 동안 가장 빠른 성장을 보일 것으로 예상되며, 특히 팬데믹 이후 분자 진단 및 감염병 탐지 분야에서 중요한 역할을 할 것으로 기대됩니다.
- 테스트 모드별
검사 방식을 기준으로 시장은 처방 기반 검사와 일반 의약품 검사로 구분됩니다. 처방 기반 검사는 전문적인 감독이 필요한 임상 환경에서 널리 사용되기 때문에 2024년에는 시장 점유율이 더 높았습니다.
그러나 소비자 인식 증가, 편의성에 대한 수요, 정확한 가정용 테스트 키트를 가능하게 하는 기술 발전으로 인해 OTC 테스트가 예측 기간 동안 가장 빠른 성장을 보일 것으로 예상됩니다.
- 응용 프로그램별
시장은 응용 분야별로 종양학, 심장학, 정형외과, 위장병학, 부인과, 신경학, 치과학 등으로 세분화됩니다. 종양학은 암 유병률 증가와 조기 발견 및 모니터링의 필요성으로 인해 2024년에 가장 큰 비중을 차지했습니다.
심장학과 기타 만성 질환 관련 진단 검사는 의료비 지출 증가와 인구 고령화에 힘입어 가장 빠른 성장을 보일 것으로 예상됩니다.
- 샘플 유형별
시장은 샘플 유형에 따라 혈액, 소변, 타액, 땀, 모발 등으로 세분화됩니다. 혈액 샘플은 다양한 진단 검사에서 신뢰성과 광범위한 사용으로 2024년 시장을 장악했습니다.
침과 소변 검사는 비침습적 대안으로 2025년에서 2032년 사이에 가장 빠른 성장을 보일 것으로 예상되며, 환자의 준수도를 높이고 샘플 수집을 용이하게 할 것입니다.
- 테스트 사이트별
검사 장소를 기준으로 시장은 실험실 기반 검사와 가정 검사로 구분됩니다. 실험실 기반 검사는 첨단 장비와 전문 지식의 가용성으로 인해 2024년에 시장 점유율이 가장 높았습니다.
그러나 기술 발전에 힘입어 편의성과 빠른 결과에 대한 소비자들의 수요 증가로 인해 가정 검사가 예측 기간 동안 가장 빠른 성장을 보일 것으로 예상됩니다.
- 테스트 유형별
검사 유형을 기준으로 시장은 생화학, 혈액학, 미생물학, 조직병리학 등으로 세분화됩니다. 생화학은 대사, 장기 기능 및 기타 전신 질환 진단에 있어 포괄적인 역할을 수행하여 2024년 시장을 주도했습니다.
감염성 질환 관리에 대한 지속적인 필요성으로 인해 미생물학 검사는 예측 기간 동안 가장 빠른 성장을 보일 것으로 예상됩니다.
- 연령별
연령을 기준으로 시장은 노인, 성인, 소아 연령대로 구분됩니다. 성인 부문은 질병 부담이 높고 진단에 대한 필요성이 높기 때문에 2024년에 가장 큰 점유율을 기록했습니다.
소아과 시장은 선천적, 전염성 질환에 대한 인식이 높아지고 검진이 확대됨에 따라 2025년부터 2032년까지 연평균 성장률이 더 높을 것으로 예상됩니다.
- 최종 사용자별
최종 사용자 기준으로 시장은 병원, 진단 센터, 외래 수술 센터(ASC), 전문 클리닉, 재택 치료, 혈액은행, 연구실 및 기관 등으로 세분화됩니다. 병원과 진단 센터는 진단 검사 서비스의 주요 시설로서 2024년에 가장 높은 매출 점유율을 기록하며 시장을 장악했습니다.
가정 기반 진단 서비스와 원격 진료가 전 세계적으로 확대됨에 따라 가정 간병 부문은 예측 기간 동안 가장 빠른 성장을 보일 것으로 예상됩니다.
- 유통 채널별
유통 채널을 기준으로 시장은 직접 입찰, 소매 판매, 온라인 판매로 구분됩니다. 2024년에는 의료기관의 대량 조달로 인해 직접 입찰이 시장을 주도했습니다.
예측 기간 동안 온라인 판매는 전자상거래 확장과 가정용 진단 키트 직접 구매에 대한 소비자 선호도 증가에 힘입어 빠르게 성장하고 있습니다.
진단 테스트 시장 지역 분석
- 북미는 2024년 42.5%의 가장 큰 매출 점유율로 진단 테스트 시장을 장악했으며, 이는 첨단 의료 인프라, 높은 의료비 지출, 핵심 산업 참여자들의 강력한 입지에 힘입은 것입니다.
- 이 지역의 소비자들은 분자 및 유전자 검사를 포함한 첨단 진단 기술이 제공하는 정확성, 속도 및 편의성을 높이 평가합니다.
- 이러한 광범위한 채택은 강력한 정부 이니셔티브, 예방 의료에 대한 인식 제고, 만성 질환 유병률 증가, 병원, 진단 센터 및 가정 간병 환경에서 진단 테스트를 현대 의료의 필수 구성 요소로 확립함으로써 더욱 뒷받침됩니다.
미국 진단 검사 시장 통찰력
미국 진단 검사 시장은 2024년 북미에서 79%의 매출 점유율을 기록하며 가장 큰 매출 점유율을 기록했습니다. 이는 첨단 의료 인프라와 최첨단 진단 기술의 광범위한 도입에 힘입은 것입니다. 예방 의료 및 만성 질환 관리에 대한 인식이 높아짐에 따라 정기적이고 전문적인 진단 검사에 대한 수요가 증가하고 있습니다. 진단에 AI와 디지털 헬스케어 도구가 통합되는 추세와 개인 맞춤 의료에 대한 집중적인 관심은 시장 성장을 더욱 가속화하고 있습니다. 주요 진단 기업의 진출과 증가하는 재택 검사 옵션 또한 미국 시장 성장에 기여하고 있습니다.
유럽 진단 테스트 시장 통찰력
유럽 진단 검사 시장은 엄격한 규제 체계와 증가하는 의료비 지출에 힘입어 꾸준히 성장할 것으로 예상됩니다. 이 지역의 인구 고령화와 만성 질환 유병률 증가는 조기에 정확한 진단에 대한 수요를 증가시키고 있습니다. 분자 진단에 대한 투자 증가와 보험급여 정책 확대는 병원과 진단 센터 전반의 도입을 뒷받침하고 있습니다. 또한, 디지털 헬스케어를 장려하고 진단 검사를 예방 의료 프로그램에 통합하는 정부 정책은 유럽 시장의 성장을 촉진하고 있습니다.
영국 진단 테스트 시장 통찰력
영국 진단 검사 시장은 조기 질병 발견 및 예방 치료를 강조하는 국가 보건 전략에 힘입어 탄탄한 성장세를 보일 것으로 예상됩니다. 환자 인식 제고와 첨단 진단 기술에 대한 정부 지원이 시장 확대를 견인하고 있습니다. 영국의 선진 의료 시스템과 특히 OTC 진단 검사의 증가에 따른 가정용 검사 도입 증가는 꾸준한 수요 증가에 기여하고 있습니다. 또한, 원격 의료 서비스 확대는 진단 솔루션에 대한 접근성을 높여줍니다.
독일 진단 테스트 시장 통찰력
독일의 진단 검사 시장은 탄탄한 의료 인프라와 연구 및 혁신 투자 증가에 힘입어 크게 성장할 것으로 예상됩니다. 독일의 정밀 의학에 대한 집중과 차세대 염기서열 분석 및 분자 진단에 대한 수요 증가가 시장을 견인하고 있습니다. 만성 질환 부담 증가와 적극적인 보험급여 정책은 병원 및 전문 진료소의 도입을 촉진하고 있습니다. 데이터 보안 및 규정 준수에 대한 강조는 소비자의 기대에 부응하여 시장 성장을 강화하고 있습니다.
아시아 태평양 진단 검사 시장 통찰력
아시아 태평양 진단 검사 시장은 급속한 도시화, 의료비 지출 증가, 그리고 조기 질병 진단에 대한 인식 제고에 힘입어 예측 기간 동안 가장 빠른 연평균 성장률(CAGR)을 기록할 것으로 예상됩니다. 중국, 인도, 일본 등지의 의료 인프라 확충과 생활 습관병 유병률 증가는 정기 검사 및 전문 검사 수요를 견인하고 있습니다. 의료 접근성 향상 및 디지털 헬스케어 도입을 위한 정부 정책은 시장 침투율을 더욱 높이고 있습니다. 또한, 이 지역은 비용 효율적인 제조와 가정 기반 검사의 증가로 수혜를 받고 있습니다.
일본 진단 검사 시장 통찰력
일본의 진단 검사 시장은 만성 질환 관리를 위한 첨단 진단 기술이 필요한 고령 인구 증가에 힘입어 꾸준히 성장하고 있습니다. 일본 정부는 진단 검사를 스마트 헬스케어 시스템 및 IoT 기기와 통합하는 데 주력하고 있으며, 이러한 추세는 도입률을 높이고 있습니다. 의료 혁신 및 예방 의학 프로그램에 대한 정부 지원 확대는 성장을 촉진하고 있습니다. 특히 노인 의료 및 종양학 분야에서 비침습적이고 신속한 진단 검사 수요가 증가하고 있습니다.
인도 진단 테스트 시장 통찰력
인도는 의료 인프라 확충과 예방 의료에 대한 관심 증가로 2024년 아시아 태평양 지역에서 가장 큰 시장 점유율을 기록했습니다. 중산층 인구 증가, 도시화, 그리고 정부 의료 제도는 접근성 높은 진단 서비스에 대한 수요를 촉진합니다. 민간 진단 센터의 증가와 저렴한 진단 기술의 보급률 증가는 시장 확대를 촉진합니다. 또한, 조기 질병 진단에 대한 인식 제고와 원격 진료의 성장은 인도의 진단 검사 시장이 빠르게 성장하는 데 기여하고 있습니다.
진단 테스트 시장 점유율
진단 검사 산업은 주로 다음을 포함한 잘 확립된 회사들이 주도하고 있습니다.
- 애벗(미국)
- F. 호프만-라 로슈 유한회사(스위스)
- Siemens Healthineers AG(독일)
- 다나허 코퍼레이션(미국)
- Thermo Fisher Scientific Inc.(미국)
- BD(미국)
- Bio-Rad Laboratories, Inc. (미국)
- 비오메리유(프랑스)
- QIAGEN(네덜란드)
- 시스멕스 주식회사(일본)
- 정형외과 임상진단(미국)
- Hologic, Inc. (미국)
- 세페이드(미국)
- 일루미나(Illumina, Inc.) (미국)
- Genomic Health, Inc. (미국)
- Myriad Genetics, Inc. (미국)
- DiaSorin SpA(이탈리아)
- THERADIAG(프랑스)
- Exact Sciences Corporation(미국)
- Beckman Coulter, Inc.(미국)
글로벌 진단 검사 시장의 최근 동향은 무엇인가?
- 2024년 9월, 시카고 대학교 프리츠커 분자공학과와 UCLA 사뮤엘리 공과대학 연구진은 전계효과 트랜지스터와 종이 기반 분석 카트리지를 통합한 새로운 진단 시스템을 발표했습니다. 머신러닝 알고리즘으로 강화된 이 하이브리드 바이오센서는 혈청 샘플의 콜레스테롤 수치 측정에서 97% 이상의 정확도를 보였습니다. 이 혁신은 트랜지스터 기반 검출의 높은 민감도와 종이 기반 검사의 경제성 및 간편성을 결합하여 다양한 건강 상태에 대한 가정용 진단에 혁명을 일으킬 잠재력을 가지고 있습니다.
- 2024년 9월, ARCpoint Inc.는 MD Care Group과의 협력을 확대하여 원격 진료 의료진이 ARCpoint의 MyARCpointLabs 플랫폼을 통해 직접 진단 검사를 주문할 수 있도록 애플리케이션 프로그래밍 인터페이스(API)를 개선했습니다. 이러한 통합을 통해 가상 의료 제공자가 진단 검사를 용이하게 하는 프로세스가 간소화되어 환자가 시기적절하고 편리한 건강 평가를 받을 수 있게 되었습니다.
- 2024년 8월, 퀴아젠(Qiagen)과 아스트라제네카(AstraZeneca)는 종양학 분야를 넘어 만성 질환에 대한 동반 진단법 개발을 위한 파트너십 확대를 발표했습니다. 퀴아젠의 QIAstat-Dx 플랫폼을 활용한 이번 협력은 일상적인 임상 평가에서 표적 치료에 적합한 환자를 식별할 수 있는 유전형 분석법을 개발하는 것을 목표로 하며, 만성 질환 관리 분야에서 개인 맞춤 의학을 향한 중요한 진전을 의미합니다.
- 2024년 6월, 인공지능(AI) 애플리케이션 전문 바이오테크 기업인 BioAI는 Genomic Testing Cooperative와 파트너십을 맺고 AI 기반 디지털 병리학 솔루션을 개발했습니다. 이번 협력은 유전체 바이오마커 스크리닝 알고리즘 및 분석법 개발, 첨단 컴퓨팅 도구를 통한 임상 연구 및 진단 애플리케이션 향상에 중점을 두고 있습니다.
- 2024년 6월, 세계보건기구(WHO)는 태국에서 열린 지역 협의 회의에서 아세안 회원국들과 협력하여 양질의 진단 검사 접근성을 강화했습니다. 이 이니셔티브는 국가 필수 진단 목록(NEDL)의 개발 및 시행을 지원하여 동남아시아 전역에서 필수 진단 검사의 이용 및 접근성을 확보하는 것을 목표로 했습니다.
SKU-
세계 최초의 시장 정보 클라우드 보고서에 온라인으로 접속하세요
- 대화형 데이터 분석 대시보드
- 높은 성장 잠재력 기회를 위한 회사 분석 대시보드
- 사용자 정의 및 질의를 위한 리서치 분석가 액세스
- 대화형 대시보드를 통한 경쟁자 분석
- 최신 뉴스, 업데이트 및 추세 분석
- 포괄적인 경쟁자 추적을 위한 벤치마크 분석의 힘 활용
연구 방법론
데이터 수집 및 기준 연도 분석은 대규모 샘플 크기의 데이터 수집 모듈을 사용하여 수행됩니다. 이 단계에는 다양한 소스와 전략을 통해 시장 정보 또는 관련 데이터를 얻는 것이 포함됩니다. 여기에는 과거에 수집한 모든 데이터를 미리 검토하고 계획하는 것이 포함됩니다. 또한 다양한 정보 소스에서 발견되는 정보 불일치를 검토하는 것도 포함됩니다. 시장 데이터는 시장 통계 및 일관된 모델을 사용하여 분석하고 추정합니다. 또한 시장 점유율 분석 및 주요 추세 분석은 시장 보고서의 주요 성공 요인입니다. 자세한 내용은 분석가에게 전화를 요청하거나 문의 사항을 드롭하세요.
DBMR 연구팀에서 사용하는 주요 연구 방법론은 데이터 마이닝, 시장에 대한 데이터 변수의 영향 분석 및 주요(산업 전문가) 검증을 포함하는 데이터 삼각 측량입니다. 데이터 모델에는 공급업체 포지셔닝 그리드, 시장 타임라인 분석, 시장 개요 및 가이드, 회사 포지셔닝 그리드, 특허 분석, 가격 분석, 회사 시장 점유율 분석, 측정 기준, 글로벌 대 지역 및 공급업체 점유율 분석이 포함됩니다. 연구 방법론에 대해 자세히 알아보려면 문의를 통해 업계 전문가에게 문의하세요.
사용자 정의 가능
Data Bridge Market Research는 고급 형성 연구 분야의 선두 주자입니다. 저희는 기존 및 신규 고객에게 목표에 맞는 데이터와 분석을 제공하는 데 자부심을 느낍니다. 보고서는 추가 국가에 대한 시장 이해(국가 목록 요청), 임상 시험 결과 데이터, 문헌 검토, 재생 시장 및 제품 기반 분석을 포함하도록 사용자 정의할 수 있습니다. 기술 기반 분석에서 시장 포트폴리오 전략에 이르기까지 타겟 경쟁업체의 시장 분석을 분석할 수 있습니다. 귀하가 원하는 형식과 데이터 스타일로 필요한 만큼 많은 경쟁자를 추가할 수 있습니다. 저희 분석가 팀은 또한 원시 엑셀 파일 피벗 테이블(팩트북)로 데이터를 제공하거나 보고서에서 사용 가능한 데이터 세트에서 프레젠테이션을 만드는 데 도움을 줄 수 있습니다.