글로벌 계약 제약품 포장 시장, 유형별(1차 포장 시스템, 2차 포장 시스템, 3차 포장 시스템), 원자재(플라스틱 및 폴리머, 유리, 금속, 종이 및 판지, 고무, 면 및 기타), 응용 분야(점막 약물 전달 포장, 폐 약물 전달 포장, 비강 약물 전달 포장, 국소 약물 전달 포장, 주사 포장, 경구 약물 전달 포장 및 기타) - 업계 동향 및 2030년까지의 예측.
계약 제약 포장 시장 분석 및 규모
계약 제약 포장 시장은 제약 회사의 포장 서비스로 구성된 제약 산업 내의 중요한 부문입니다. 이 시장은 효율적인 포장 솔루션에 대한 수요 증가와 엄격한 규정에 의해 주도되고 있으며, 환자 안전과 혁신 기술에 대한 강조가 커지면서 계약 제약 포장 부문은 꾸준한 성장을 향해 나아가고 있습니다.
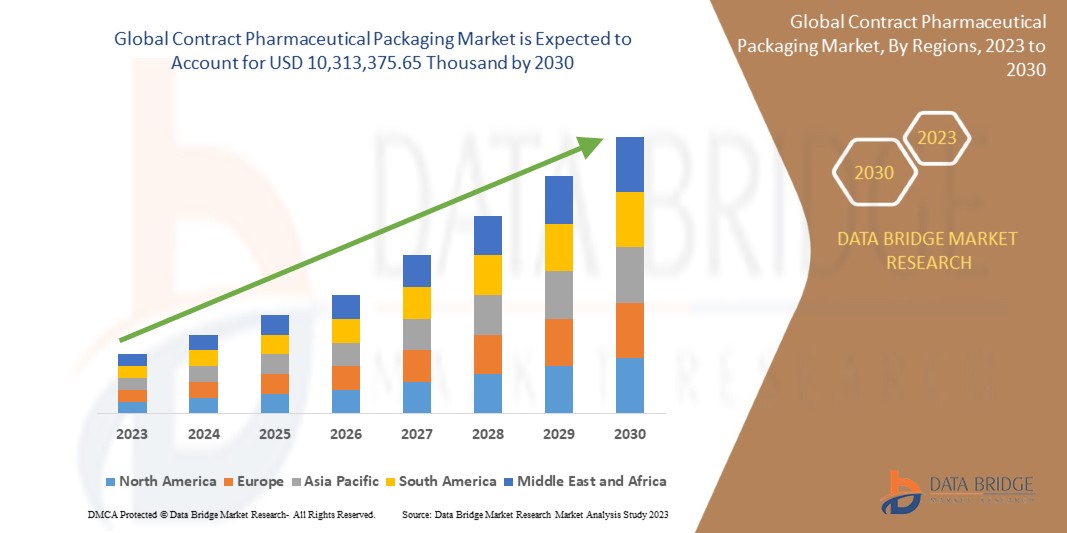
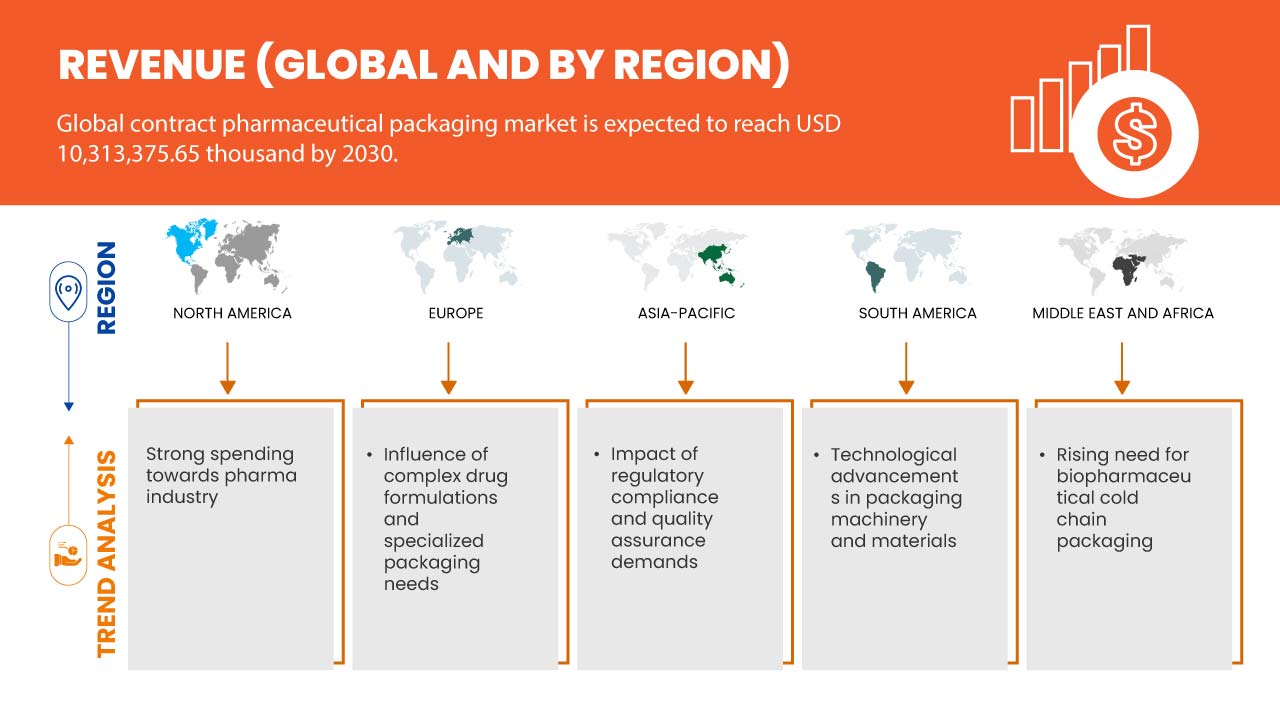
Data Bridge Market Research에 따르면 글로벌 계약 제약품 포장 시장은 2030년까지 10,313,375.65달러 규모에 도달할 것으로 예상되며, 이는 예측 기간 동안 7.4%의 CAGR로 성장할 것으로 예상됩니다.
|
보고서 메트릭 |
세부 |
|
예측 기간 |
2023년부터 2030년까지 |
|
기준 연도 |
2022 |
|
역사적 해 |
2021 (2015-2020까지 사용자 정의 가능) |
|
양적 단위 |
수익 (USD 천) |
|
다루는 세그먼트 |
유형(1차 포장 시스템, 2차 포장 시스템 및 3차 포장 시스템), 원료(플라스틱 및 폴리머, 유리, 금속, 종이 및 보드, 고무, 면 및 기타), 응용 분야(점막 약물 전달 포장, 폐 약물 전달 포장, 비강 약물 전달 포장, 국소 약물 전달 포장, 주사형 포장, 경구 약물 전달 포장 및 기타) |
|
적용 국가 |
미국, 캐나다, 멕시코, 독일, 영국, 프랑스, 이탈리아, 스페인, 네덜란드, 벨기에, 러시아, 터키, 스위스, 룩셈부르크, 유럽의 나머지 지역, 중국, 인도, 일본, 한국, 호주 및 뉴질랜드, 인도네시아, 싱가포르, 말레이시아, 태국, 필리핀, 아시아 태평양의 나머지 지역, 브라질, 아르헨티나, 남미의 나머지 지역, 아랍에미리트, 남아프리카, 이집트, 사우디 아라비아, 이스라엘, 중동 및 아프리카의 나머지 지역 |
|
시장 참여자 포함 |
AbbVie Inc, PCI Pharma Services, Nelipak Healthcare, Sharp Services, LLC, Aphena Pharma Solutions, ROPACK INC., SilganUnicep 2, Reed-Lane, Jones Healthcare Group, Wasdell Packaging Group, SternMaid &Co. KG, Sepha, Tripak Pharmaceuticals, Assemblies Unlimited, Inc., AmeriPac, Tjoapack 등이 있습니다. |
시장 정의
계약 제약 포장 시장은 제약 산업 내의 전문 분야를 포괄하며 맞춤형 포장 솔루션에 중점을 둡니다. 여기에는 1차, 2차 및 3차 포장의 설계, 개발 및 생산과 제약 제품을 위한 라벨링 및 아트워크 관리 솔루션이 포함됩니다. 주요 시장 동인에는 수요 증가, 혁신적인 제품으로 인한 복잡한 포장의 필요성, 엄격한 규정이 포함됩니다. 주목할 만한 추세로는 효율성과 낭비 감소를 위한 연기 포장 채택, 데이터 무결성 및 공급망 개선을 위한 직렬화, 환경 영향을 최소화하기 위한 친환경 소재로의 전환이 있습니다. 이 시장은 산업 규정 및 지침을 준수하는 동시에 제품 무결성, 사용자 친화성 및 시장성을 보존하는 것을 목표로 합니다.
글로벌 계약 제약 포장 시장 역학
이 섹션에서는 시장 동인, 이점, 기회, 제약 및 과제를 이해하는 것을 다룹니다. 이 모든 내용은 아래에서 자세히 설명합니다.
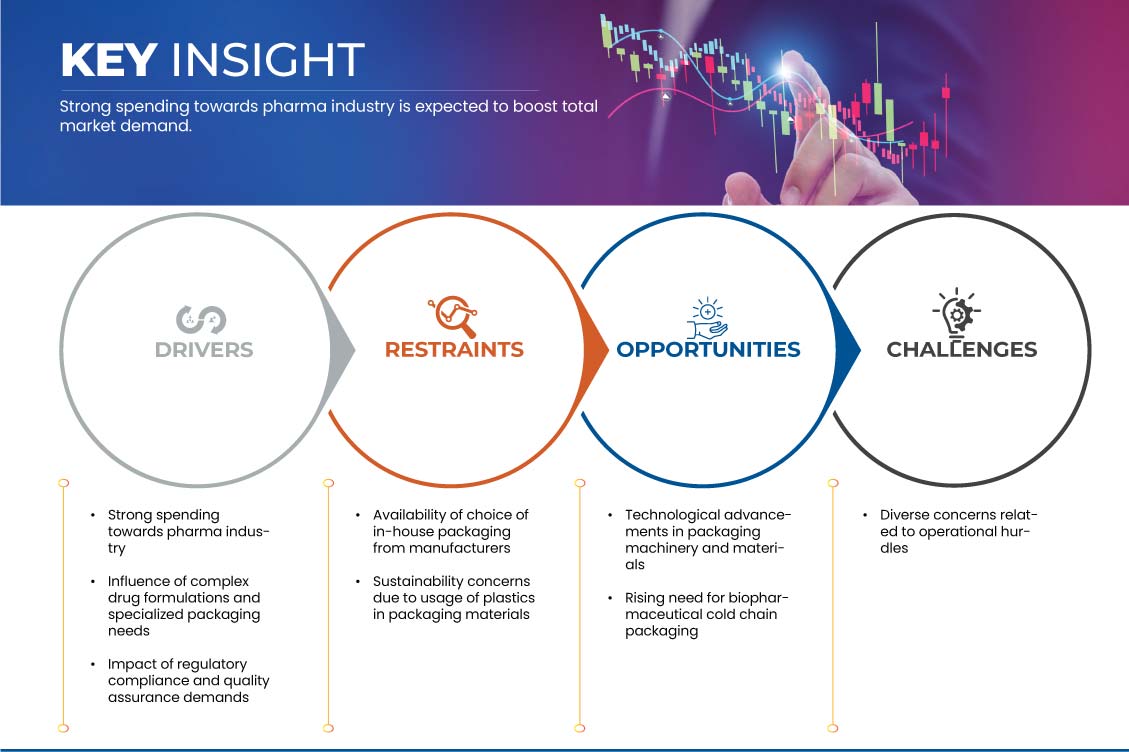
운전자:
- 제약 산업에 대한 높은 지출
최근 몇 년 동안 제약 회사는 약물 효능을 높이고, 환자 준수를 개선하고, 부작용을 줄이기 위해 복잡한 약물 제형을 점점 더 개발해 왔습니다. 이러한 제형은 종종 정확한 투여량, 특수한 전달 메커니즘 및 제어 방출 프로필을 필요로 합니다. 따라서 제약 포장은 단순한 블리스터 팩과 병을 넘어 발전했습니다. 이러한 복잡한 제형의 무결성을 보존하면서 정확한 투여량과 투여를 보장할 수 있는 특수 포장에 대한 수요가 급증했습니다. 이러한 변화는 이러한 복잡한 포장 요구 사항을 처리할 수 있는 기술적 전문 지식을 보유한 계약 포장 공급업체에게 기회를 열어주었습니다.
- 복잡한 약물 제형 및 특수 포장 요구 사항의 영향
제약 산업 내 재정 할당의 상당한 증가는 글로벌 계약 제약 포장 시장 확장의 중요한 촉매로 부상했습니다. 이러한 투자 급증은 포장 부문의 성장을 주도하는 여러 요인에 기인할 수 있습니다. 인구 고령화 및 만성 질환 유병률과 같은 요인에 의해 주도되는 제약 제품에 대한 수요 증가로 인해 생산량이 증가했습니다. 이러한 생산 급증은 자연스럽게 포장 서비스에 대한 비례적 수요를 수반합니다. 계약 제약 포장 공급업체는 다양한 제품 포트폴리오와 수량에 맞는 확장 가능한 솔루션을 제공하여 이러한 수요를 충족합니다.
기회
- 포장 기계 및 재료의 기술적 발전
포장 기계 및 재료의 기술적 발전은 글로벌 계약 제약 포장 시장의 풍경을 형성하는 데 중요한 역할을 했습니다. 자동화, 재료 과학 및 지속 가능성의 혁신에 의해 주도되는 이러한 발전은 시장 성장을 위한 상당한 기회를 창출할 준비가 되어 있습니다. 기술 발전의 한 가지 핵심 측면은 포장 프로세스의 자동화입니다. 현대 포장 기계는 정교한 로봇 시스템, 컴퓨터화된 제어 및 실시간 모니터링 기능을 갖추고 있습니다. 이는 포장 프로세스에서 향상된 정밀도, 효율성 및 감소된 인적 오류로 이어집니다.
- 생물제약 냉장사슬 포장에 대한 수요 증가
생물제약 콜드체인 포장에 대한 수요가 급증하면서 제약 산업의 핵심 동인이 되었고, 글로벌 계약 제약 포장 시장 성장에 상당한 기회가 생겼습니다. 이러한 급증은 복잡한 공급망 전반에 걸쳐 온도에 민감한 생물제약 제품의 효능과 무결성을 유지해야 하는 절실한 필요성에 의해 뒷받침됩니다. 백신, 유전자 치료 및 특수 약물을 포함한 생물제약은 종종 온도 변화에 취약하여 보관 및 운송 중에 엄격한 온도 제어가 필요합니다.
제약/도전
- 제조업체에서 제공하는 자체 포장 선택 가능
계약 포장 분야에서 제조업체의 자체 포장 선택이 중요한 요소로 부상했습니다. 기업은 효율성을 높이고 생산 및 포장 공정을 최적화할 수 있는 방법을 끊임없이 모색하고 있습니다. 자체 포장 공정을 자동화하는 데 투자하는 추세가 상당한 추진력을 얻었습니다. 자체 포장 제품의 전망은 새로운 시장 영역을 모색하거나 기존 운영을 간소화하려는 기업에 수익성 있는 기회를 제공합니다. 여기에는 자본 포장 장비를 취득하고 회사 시설 내에서 포장 공정을 수행하는 것이 포함됩니다. 즉, 자체 포장은 장기적으로 좋은 수익을 낼 수 있으며 지속적인 비용은 인건비, 유지 관리 및 마모된 부품 교체로 제한됩니다.
- 포장재에 플라스틱 사용으로 인한 지속 가능성 문제
제약 포장에 일회용 플라스틱을 광범위하게 사용하는 것은 제품 무결성과 환자 안전을 유지하는 목적에 부합하지만, 전 세계적으로 증가하는 지속 가능성 우려와 모순됩니다. 제약 부문에서 GMP(Good Manufacturing Practices)를 준수하는 것은 약물의 품질과 안전을 보장하는 데 필수적이지만, 이러한 규정은 종종 지속 가능한 포장 관행에 대한 명확한 지침이 부족합니다. 이로 인해 플라스틱 라이너와 타이를 사용한 이중 포장, 세척 비용을 피하기 위해 장비의 내부 라이닝을 플라스틱으로 하는 관행이 생깁니다. 이러한 관행은 GMP와 일치하지만 지속 가능한 목표에 반하여 환경적 문제를 초래합니다. GMP는 환자 안전과 제품 품질을 보장하지만 환경적 지속 가능성을 우선시하지 않습니다. 이러한 모순은 산업이 환자 웰빙과 환경적 책임의 균형을 맞추기 위해 고군분투하고 있음을 보여줍니다.
- 운영상의 장애물과 관련된 다양한 우려
계약 제약 포장은 장점이 있지만 운영상의 장애물과 관련된 다양한 우려에서 비롯된 광범위한 과제에 직면합니다. 포장 역학이 끊임없이 변화하는 환경에서 포장 공급망의 복잡성은 우려의 문제가 되었습니다. 접이식 상자를 설계하고 일관된 라벨 라인 운영을 보장하는 포장 엔지니어의 필수적인 역할은 매우 중요합니다. 그러나 경쟁력 있는 가격을 추구하는 것은 종종 적시에 완전한 주문에 대한 생산 팀의 요구 사항을 충족해야 하는 필요성과 충돌합니다.
한 가지 과제는 인구가 밀집된 지역과 고가 부동산 지역에서 생산을 위한 가용 공간이 부족하다는 것입니다. 제한된 공간 내에서 수익성을 극대화해야 한다는 필수 조건은 생산성을 높이기 위해 계약 포장 장비에 엄청난 압력을 가합니다. 또 다른 어려운 측면인 교체는 효율성과 수익성에 직접적인 위협이 됩니다.
최근 개발 사항
- 2022년 12월, Nelipak Corporation은 노스캐롤라이나주 윈스턴-세일럼에 최첨단 유연 포장 생산 시설을 설립했습니다. 이 전략적 움직임은 Nelipak의 의료용 포장 역량을 유럽에서 아메리카로 확장하여 이 지역의 증가하는 수요에 부응할 것입니다. ISO-7 클린룸 공간과 ISO 13485 인증을 갖춘 110,000제곱피트 규모의 시설은 2,000만 달러의 투자를 의미하며 향후 5년 동안 약 80개의 일자리를 창출할 것으로 예상됩니다.
- 2022년 10월, Aphena Pharma Solutions Inc.는 테네시주 쿠크빌에서 2019년 중반에 시작한 2,000만 달러 규모의 확장 및 리노베이션이 완료되어 대량의 고형 복용량 포장 용량이 추가되었다고 발표했습니다. 이 추가 공간을 통해 Aphena는 회사의 병 및 블리스터 포장 용량을 크게 늘릴 수 있었으며, 4개의 고속 병입 라인과 고형 기반 제품을 위한 2개의 고속 블리스터 라인을 추가로 구축하여 Aphena를 모든 제네릭 또는 OTC 제약 회사의 강력한 전략적 성장 파트너로 만들었습니다.
- 2022년 9월, Nelipak Corporation은 애리조나주 피닉스와 펜실베이니아주 화이트홀에 있는 시설에 대해 International Sustainability and Carbon Certification(ISCC) PLUS를 획득했습니다. 이 인정은 재활용 및 바이오 기반 재료를 통합하여 순환 공급망을 활용하는 Nelipak의 지속 가능한 관행에 대한 헌신을 강조했습니다.
- 2020년 10월, Alnylam Pharmaceuticals는 Sharp와 협력하여 유럽 시장에서 승인된 RNAi 치료제를 포장했습니다. 벨기에에 있는 Sharp의 Hamont-Achel 사이트는 유럽 국가 전역에서 Alnylam의 제품 포장을 담당합니다. 이 협력은 벨기에에서 Alnylam에 대한 상당한 국제적 투자를 의미하며, 이 지역에서의 입지를 강화하고 운영의 핵심 허브로 자리매김하려는 전략과 일치합니다.
- 2020년 5월, 샤프는 펜실베이니아주 매컨지에 있는 제약 포장 시설을 인수했습니다. 이전에 Quality Packaging Specialists International, LLC(QPSI)가 소유했던 이 시설은 160,000제곱피트 규모이며 병입, 블리스터링, 바이알 라벨링, 의료 기기 키팅, 일련번호 지정 서비스를 포함한 1차 및 2차 제약 포장을 위한 완벽한 설비를 갖추고 있습니다. 전략적 인수는 증가하는 고객 볼륨 수요에 대응하여 샤프가 효율적인 포장 솔루션을 제공할 수 있는 역량을 강화했습니다.
글로벌 계약 제약 포장 시장 범위
글로벌 계약 제약 포장 시장은 유형, 원자재 및 응용 분야로 세분화됩니다. 이러한 세그먼트 간의 성장은 산업의 주요 성장 세그먼트를 분석하고 사용자에게 귀중한 시장 개요와 시장 통찰력을 제공하여 핵심 시장 응용 분야를 식별하기 위한 전략적 결정을 내리는 데 도움이 됩니다.
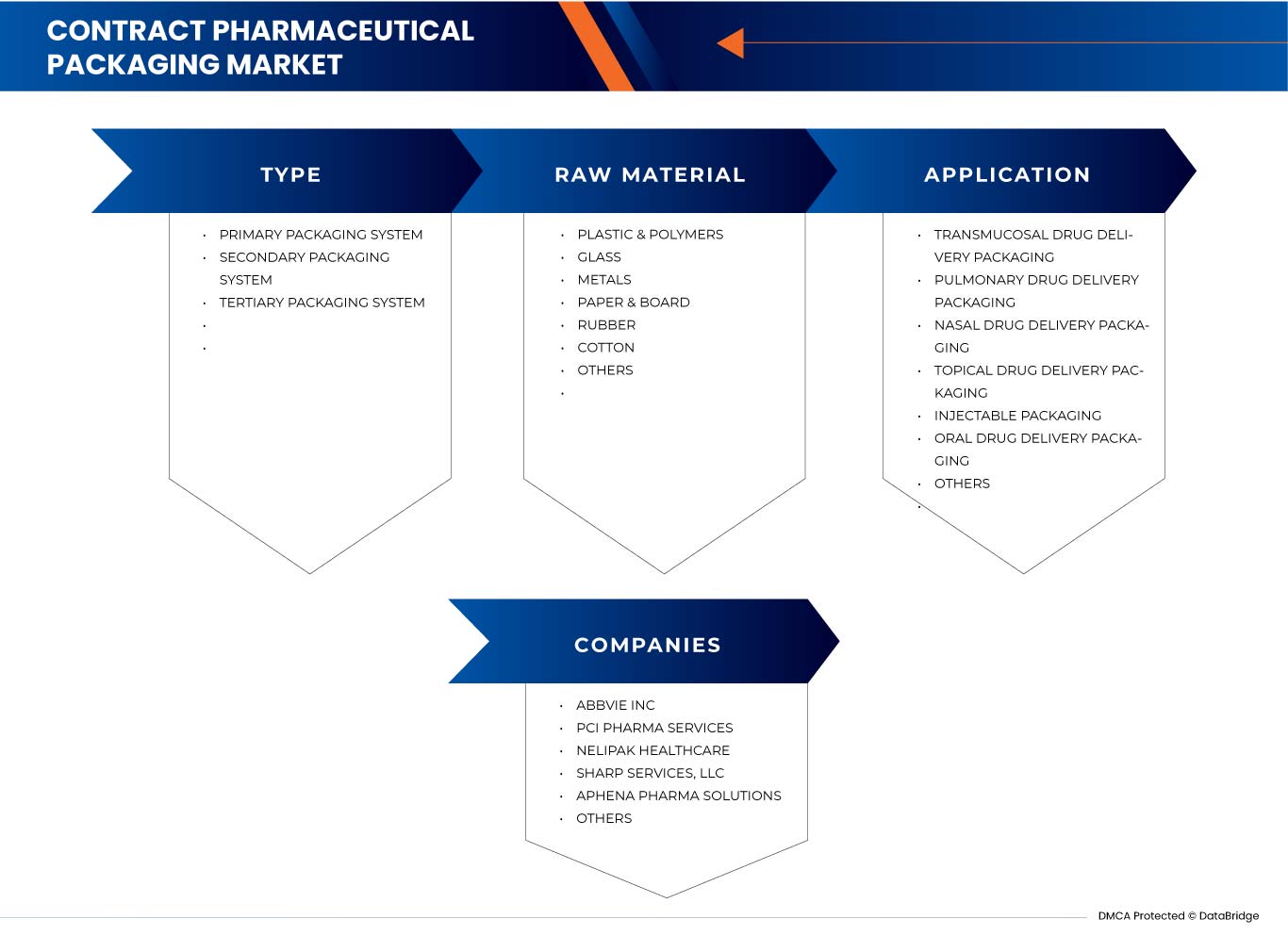
유형
- 1차 포장 시스템
- 2차 포장 시스템
- 3차 포장 시스템
시장은 유형을 기준으로 1차 포장 시스템, 2차 포장 시스템, 3차 포장 시스템으로 구분됩니다.
원료
- 플라스틱 및 폴리머
- 유리
- 궤조
- 종이 및 판지
- 고무
- 면
- 기타
원자재를 기준으로 시장은 플라스틱 및 폴리머, 유리, 금속, 종이 및 판지, 고무, 면 및 기타로 구분됩니다.
애플리케이션
- 점막 약물 전달 포장
- 폐 약물 전달 포장
- 비강 약물 전달 포장
- 국소 약물 전달 포장
- 주입형 포장
- 경구 약물 전달 포장
- 기타
시장은 응용 분야별로 점막 약물 전달 포장, 폐 약물 전달 포장, 비강 약물 전달 포장, 국소 약물 전달 포장, 주사형 포장, 경구 약물 전달 포장 및 기타 포장으로 구분됩니다.
글로벌 계약 제약 포장 시장 지역 분석/통찰력
글로벌 계약 제약품 포장 시장을 분석하고, 위에 언급된 유형, 원재료, 응용 분야를 기준으로 국가별 시장 규모에 대한 통찰력과 추세를 제공합니다.
글로벌 계약 제약품 포장 시장 보고서에서 다루는 국가는 미국, 캐나다, 멕시코, 독일, 영국, 프랑스, 이탈리아, 스페인, 네덜란드, 벨기에, 러시아, 터키, 스위스, 룩셈부르크, 유럽의 나머지 지역, 중국, 인도, 일본, 한국, 호주, 뉴질랜드, 인도네시아, 싱가포르, 말레이시아, 태국, 필리핀, 아시아 태평양의 나머지 지역, 브라질, 아르헨티나, 남미의 나머지 지역, 아랍에미리트, 남아프리카, 이집트, 사우디 아라비아, 이스라엘, 중동 및 아프리카의 나머지 지역입니다.
북미 지역은 효율적인 포장 솔루션에 대한 수요 증가와 엄격한 규제로 인해 시장을 주도하고 성장할 것으로 예상됩니다. 미국은 빠르게 변화하는 소비자 선호도와 증가하는 환경 문제를 충족하기 위한 혁신적인 포장 방법의 채택이 확대됨에 따라 북미 지역에서 우위를 점할 것으로 예상됩니다. 독일은 소비와 수요가 증가함에 따라 유럽 지역에서 우위를 점할 것으로 예상됩니다. 중국은 전 세계 다양한 제약 산업에서 상당한 개발과 투자가 이루어짐에 따라 아시아 태평양 지역에서 우위를 점할 것으로 예상됩니다.
보고서의 국가 섹션은 또한 현재 및 미래 시장 추세에 영향을 미치는 개별 시장 영향 요인과 시장 규제의 변화를 제공합니다. 다운스트림 및 업스트림 가치 사슬 분석, 기술 추세 및 포터의 5가지 힘 분석, 사례 연구와 같은 데이터 포인트는 개별 국가의 시장 시나리오를 예측하는 데 사용되는 몇 가지 포인터입니다. 또한 글로벌 브랜드의 존재 및 가용성과 지역 및 국내 브랜드와의 대규모 또는 희소한 경쟁으로 인해 직면한 과제, 국내 관세 및 무역 경로의 영향은 국가 데이터에 대한 예측 분석을 제공하는 동안 고려됩니다.
경쟁 환경 및 글로벌 계약 제약 포장 시장 점유율 분석
글로벌 계약 제약 포장 시장 경쟁 구도는 경쟁자별 세부 정보를 제공합니다. 포함된 세부 정보는 회사 개요, 회사 재무, 창출된 수익, 시장 잠재력, 연구 개발 투자, 새로운 시장 이니셔티브, 글로벌 입지, 생산 현장 및 시설, 생산 용량, 회사의 강점과 약점, 제품 출시, 제품 폭과 범위, 응용 분야 우세입니다. 위에 제공된 데이터 포인트는 회사의 시장 집중과만 관련이 있습니다.
글로벌 계약 제약품 포장 시장의 주요 기업으로는 AbbVie Inc, PCI Pharma Services Nelipak Healthcare, Sharp Services, LLC, Aphena Pharma Solutions, ROPACK INC., SilganUnicep 2, Reed-Lane, Jones Healthcare Group, Wasdell Packaging Group, SternMaid & Co. KG, Sepha, Tripak Pharmaceuticals, Assemblies Unlimited, Inc., AmeriPac, Tjoapack 등이 있습니다.
SKU-
세계 최초의 시장 정보 클라우드 보고서에 온라인으로 접속하세요
- 대화형 데이터 분석 대시보드
- 높은 성장 잠재력 기회를 위한 회사 분석 대시보드
- 사용자 정의 및 질의를 위한 리서치 분석가 액세스
- 대화형 대시보드를 통한 경쟁자 분석
- 최신 뉴스, 업데이트 및 추세 분석
- 포괄적인 경쟁자 추적을 위한 벤치마크 분석의 힘 활용
목차
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELING
2.7 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.8 DBMR MARKET POSITION GRID
2.9 MARKET APPLICATION COVERAGE GRID
2.1 DBMR VENDOR SHARE ANALYSIS
2.11 SECONDARY SOURCES
2.12 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTLE ANALYSIS
4.1.1 POLITICAL FACTORS:
4.1.2 ECONOMIC FACTORS:
4.1.3 SOCIAL FACTORS:
4.1.4 TECHNOLOGICAL FACTORS:
4.1.5 LEGAL FACTORS:
4.1.6 ENVIRONMENTAL FACTORS:
4.2 PORTER’S FIVE FORCES
4.3 VENDOR SELECTION CRITERIA
5 DYNAMICS OF RAW MATERIAL PREFERENCES
5.1 PLASTIC AND POLYMERS:
5.1.1 HDPE (HIGH-DENSITY POLYETHYLENE):
5.1.2 LDPE (LOW-DENSITY POLYETHYLENE):
5.1.3 PET (POLYETHYLENE TEREPHTHALATE):
5.1.4 PVC (POLYVINYL CHLORIDE):
5.1.5 PP (POLYPROPYLENE):
5.1.6 COC (CYCLIC OLEFIN COPOLYMER):
5.2 GLASS:
5.2.1 TYPE IV-NP (GENERAL PURPOSE SODA LIME GLASS):
5.2.2 TYPE I (BOROSILICATE GLASS):
5.2.3 TYPE III (REGULAR SODA-LIME GLASS):
5.2.4 TREATED SODA LIME GLASS:
5.3 METAL:
5.3.1 TIN:
5.3.2 IRON:
5.3.3 ALUMINIUM:
5.3.4 LEAD:
5.4 PAPER AND BOARD:
5.4.1 SOLID BOARD:
5.4.2 CHIPBOARD:
5.4.3 CARDBOARD:
5.4.4 FIBER BOARD:
5.5 RUBBER:
5.5.1 NATURAL RUBBER IN CONTRACT PHARMACEUTICAL PACKAGING:
5.5.2 NEOPRENE RUBBER IN CONTRACT PHARMACEUTICAL PACKAGING:
5.5.3 BUTYL RUBBER IN CONTRACT PHARMACEUTICAL PACKAGING:
6 PARTNERSHIP DETAILS
7 TECHNOLOGICAL ADVANCEMENTS
8 MARKET OVERVIEW
8.1 DRIVERS
8.1.1 STRONG SPENDING TOWARDS PHARMA INDUSTRY
8.1.2 IMPACT OF COMPLEX DRUG FORMULATIONS AND SPECIALIZED PACKAGING NEEDS
8.1.3 IMPACT OF REGULATORY COMPLIANCE AND QUALITY ASSURANCE DEMANDS
8.2 RESTRAINTS
8.2.1 AVAILABILITY OF CHOICE OF IN-HOUSE PACKAGING FROM MANUFACTURERS
8.2.2 SUSTAINABILITY CONCERNS DUE TO USAGE OF PLASTICS IN PACKAGING MATERIALS
8.3 OPPORTUNITIES
8.3.1 TECHNOLOGICAL ADVANCEMENTS IN PACKAGING MACHINERY AND MATERIALS
8.3.2 RISING NEED FOR BIOPHARMACEUTICAL COLD CHAIN PACKAGING
8.4 CHALLENGE
8.4.1 DIVERSE CONCERNS RELATED TO OPERATIONAL HURDLES
9 GLOBAL CONTRACT PHARMACEUTICAL PACKAGING MARKET: BY REGION
9.1 OVERVIEW
9.2 EUROPE
9.3 NORTH AMERICA
9.4 SOUTH AMERICA
9.5 ASIA-PACIFIC
9.6 MIDDLE EAST & AFRICA
10 GLOBAL CONTRACT PHARMACEUTICAL PACKAGING MARKET: COMPANY LANDSCAPE
10.1 COMPANY SHARE ANALYSIS: GLOBAL
10.2 COMPANY SHARE ANALYSIS: EUROPE
10.3 COMPANY SHARE ANALYSIS: NORTH AMERICA
10.4 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
11 SWOT ANALYSIS
12 COMPANY PROFILE
12.1 ABBVIE INC.
12.1.1 COMPANY SNAPSHOT
12.1.2 REVENUE ANALYSIS
12.1.3 COMPANY SHARE ANALYSIS
12.1.4 SERVICE PORTFOLIO
12.1.5 RECENT DEVELOPMENTS
12.2 PCI PHARMA SERVICES
12.2.1 COMPANY SNAPSHOT
12.2.2 COMPANY SHARE ANALYSIS
12.2.3 SERVICE PORTFOLIO
12.2.4 RECENT DEVELOPMENTS
12.3 NELIPAK CORPORATION
12.3.1 COMPANY SNAPSHOT
12.3.2 COMPANY SHARE ANALYSIS
12.3.3 PRODUCT PORTFOLIO
12.3.4 RECENT DEVELOPMENTS
12.4 SHARP SERVICES, LLC
12.4.1 COMPANY SNAPSHOT
12.4.2 COMPANY SHARE ANALYSIS
12.4.3 PRODUCT PORTFOLIO
12.4.4 RECENT DEVELOPMENTS
12.5 APHENA PHARMA SOLUTIONS
12.5.1 COMPANY SNAPSHOT
12.5.2 COMPANY SHARE ANALYSIS
12.5.3 SERVICE PORTFOLIO
12.5.4 RECENT DEVELOPMENT
12.6 AMERIPAC
12.6.1 COMPANY SNAPSHOT
12.6.2 PRODUCT PORTFOLIO
12.6.3 RECENT DEVELOPMENT
12.7 ASSEMBLIES UNLIMITED, INC.
12.7.1 COMPANY SNAPSHOT
12.7.2 SERVICE PORTFOLIO
12.7.3 RECENT DEVELOPMENT
12.8 JONES HEALTHCARE GROUP
12.8.1 COMPANY SNAPSHOT
12.8.2 PRODUCT PORTFOLIO
12.8.3 RECENT DEVELOPMENT
12.9 REED-LANE
12.9.1 SNAPSHOT
12.9.2 PRODUCT PORTFOLIO
12.9.3 RECENT DEVELOPMENT
12.1 ROPACK INC.
12.10.1 COMPANY SNAPSHOT
12.10.2 PRODUCT PORTFOLIO
12.10.3 RECENT DEVELOPMENT
12.11 SEPHA
12.11.1 COMPANY SNAPSHOT
12.11.2 PRODUCT PORTFOLIO
12.11.3 RECENT DEVELOPMENTS
12.12 SILGANUNICEP
12.12.1 COMPANY SNAPSHOT
12.12.2 SERVICE PORTFOLIO
12.12.3 RECENT DEVELOPMENTS
12.13 STERNMAID GMBH & CO. KG
12.13.1 COMPANY SNAPSHOT
12.13.2 SERVICE PORTFOLIO
12.13.3 RECENT DEVELOPMENTS
12.14 TJOAPACK
12.14.1 COMPANY SNAPSHOT
12.14.2 SERVICE PORTFOLIO
12.14.3 RECENT DEVELOPMENT
12.15 TRIPAK PHARMACEUTICALS
12.15.1 COMPANY SNAPSHOT
12.15.2 SERVICE PORTFOLIO
12.15.3 RECENT DEVELOPMENTS
12.16 WASDELL PACKAGING GROUP
12.16.1 COMPANY SNAPSHOT
12.16.2 PRODUCT PORTFOLIO
12.16.3 RECENT DEVELOPMENT
13 QUESTIONNAIRE
14 RELATED REPORTS
그림 목록
FIGURE 1 GLOBAL CONTRACT PHARMACEUTICAL PACKAGING MARKET: SEGMENTATION
FIGURE 2 GLOBAL CONTRACT PHARMACEUTICAL PACKAGING MARKET: DATA TRIANGULATION
FIGURE 3 GLOBAL CONTRACT PHARMACEUTICAL PACKAGING MARKET: DROC ANALYSIS
FIGURE 4 GLOBAL CONTRACT PHARMACEUTICAL PACKAGING MARKET: GLOBAL VS REGIONAL MARKET ANALYSIS
FIGURE 5 GLOBAL CONTRACT PHARMACEUTICAL PACKAGING MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 GLOBAL CONTRACT PHARMACEUTICAL PACKAGING MARKET: MULTIVARIATE MODELLING
FIGURE 7 GLOBAL CONTRACT PHARMACEUTICAL PACKAGING MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 8 GLOBAL CONTRACT PHARMACEUTICAL PACKAGING MARKET: DBMR MARKET POSITION GRID
FIGURE 9 GLOBAL CONTRACT PHARMACEUTICAL PACKAGING MARKET: APPLICATION COVERAGE GRID
FIGURE 10 GLOBAL CONTRACT PHARMACEUTICAL PACKAGING MARKET: VENDOR SHARE ANALYSIS
FIGURE 11 GLOBAL CONTRACT PHARMACEUTICAL PACKAGING MARKET: SEGMENTATION
FIGURE 12 NORTH AMERICA IS EXPECTED TO DOMINATE THE GLOBAL CONTRACT PHARMACEUTICAL PACKAGING MARKET, WHILE ASIA-PACIFIC IS EXPECTED TO GROW WITH THE HIGHEST CAGR IN THE FORECAST PERIOD
FIGURE 13 STRONG SPENDING TOWARDS PHARMA INDUSTRY IS EXPECTED TO DRIVE THE GROWTH OF THE GLOBAL CONTRACT PHARMACEUTICAL PACKAGING MARKET IN THE FORECAST PERIOD
FIGURE 14 THE PRIMARY PACKAGING SYSTEM SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST MARKET SHARE OF THE GLOBAL CONTRACT PHARMACEUTICAL PACKAGING MARKET IN 2023 AND 2030
FIGURE 15 ASIA-PACIFIC IS THE FASTEST-GROWING MARKET FOR THE GLOBAL CONTRACT PHARMACEUTICAL PACKAGING MARKET IN THE FORECAST PERIOD
FIGURE 16 VENDOR SELECTION CRITERIA
FIGURE 17 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF THE GLOBAL CONTRACT PHARMACEUTICAL PACKAGING MARKET
FIGURE 18 FDI INFLOWS IN DRUGS & PHARMACEUTICALS ACTIVITIES
FIGURE 19 GLOBAL CONTRACT PHARMACEUTICAL PACKAGING MARKET: SNAPSHOT (2022)
FIGURE 20 EUROPE CONTRACT PHARMACEUTICAL PACKAGING MARKET: SNAPSHOT (2022)
FIGURE 21 NORTH AMERICA CONTRACT PHARMACEUTICAL PACKAGING MARKET: SNAPSHOT (2022)
FIGURE 22 SOUTH AMERICA CONTRACT PHARMACEUTICAL PACKAGING MARKET: SNAPSHOT (2022)
FIGURE 23 ASIA-PACIFIC CONTRACT PHARMACEUTICAL PACKAGING MARKET: SNAPSHOT (2022)
FIGURE 24 MIDDLE EAST & AFRICA CONTRACT PHARMACEUTICAL PACKAGING MARKET: SNAPSHOT (2022)
FIGURE 25 GLOBAL CONTRACT PHARMACEUTICAL PACKAGING MARKET: COMPANY SHARE 2022 (%)
FIGURE 26 EUROPE CONTRACT PHARMACEUTICAL PACKAGING MARKET: COMPANY SHARE 2022 (%)
FIGURE 27 NORTH AMERICA CONTRACT PHARMACEUTICAL PACKAGING MARKET: COMPANY SHARE 2022 (%)
FIGURE 28 ASIA-PACIFIC CONTRACT PHARMACEUTICAL PACKAGING MARKET: COMPANY SHARE 2022 (%)

연구 방법론
데이터 수집 및 기준 연도 분석은 대규모 샘플 크기의 데이터 수집 모듈을 사용하여 수행됩니다. 이 단계에는 다양한 소스와 전략을 통해 시장 정보 또는 관련 데이터를 얻는 것이 포함됩니다. 여기에는 과거에 수집한 모든 데이터를 미리 검토하고 계획하는 것이 포함됩니다. 또한 다양한 정보 소스에서 발견되는 정보 불일치를 검토하는 것도 포함됩니다. 시장 데이터는 시장 통계 및 일관된 모델을 사용하여 분석하고 추정합니다. 또한 시장 점유율 분석 및 주요 추세 분석은 시장 보고서의 주요 성공 요인입니다. 자세한 내용은 분석가에게 전화를 요청하거나 문의 사항을 드롭하세요.
DBMR 연구팀에서 사용하는 주요 연구 방법론은 데이터 마이닝, 시장에 대한 데이터 변수의 영향 분석 및 주요(산업 전문가) 검증을 포함하는 데이터 삼각 측량입니다. 데이터 모델에는 공급업체 포지셔닝 그리드, 시장 타임라인 분석, 시장 개요 및 가이드, 회사 포지셔닝 그리드, 특허 분석, 가격 분석, 회사 시장 점유율 분석, 측정 기준, 글로벌 대 지역 및 공급업체 점유율 분석이 포함됩니다. 연구 방법론에 대해 자세히 알아보려면 문의를 통해 업계 전문가에게 문의하세요.
사용자 정의 가능
Data Bridge Market Research는 고급 형성 연구 분야의 선두 주자입니다. 저희는 기존 및 신규 고객에게 목표에 맞는 데이터와 분석을 제공하는 데 자부심을 느낍니다. 보고서는 추가 국가에 대한 시장 이해(국가 목록 요청), 임상 시험 결과 데이터, 문헌 검토, 재생 시장 및 제품 기반 분석을 포함하도록 사용자 정의할 수 있습니다. 기술 기반 분석에서 시장 포트폴리오 전략에 이르기까지 타겟 경쟁업체의 시장 분석을 분석할 수 있습니다. 귀하가 원하는 형식과 데이터 스타일로 필요한 만큼 많은 경쟁자를 추가할 수 있습니다. 저희 분석가 팀은 또한 원시 엑셀 파일 피벗 테이블(팩트북)로 데이터를 제공하거나 보고서에서 사용 가능한 데이터 세트에서 프레젠테이션을 만드는 데 도움을 줄 수 있습니다.