유럽 심장 안전 서비스 시장, 서비스별(ECG/홀터 측정, 혈압 측정 , 체외 심장 안전 평가 서비스, 심혈관 영상, 실시간 원격 측정 모니터링, ECGS 중앙 재검토, 비침습적 심장 영상, 생리학적 스트레스 테스트, 철저한 QT 연구, TQT 및 노출 반응 모델링, 혈소판 응집 및 기타 서비스), 단계별(1단계, 2단계 및 3단계), 유형(통합 서비스 및 독립형 서비스), 최종 사용자( 제약 및 바이오제약 회사, 계약 연구 기관 및 학술 및 연구 기관) 산업 동향 및 2029년까지의 예측.
시장 분석 및 통찰력
유럽 심장 안전 서비스 시장은 임상 시험 수의 증가, 주요 시장 참여자의 증가, 수요를 증가시키는 기술 혁신과 같은 요인에 의해 주도되고 있으며, 연구 개발에 대한 투자 증가는 시장 성장으로 이어집니다. 현재 다양한 연구 조사가 진행 중이며, 이는 제조업체가 새롭고 혁신적인 심장 안전 서비스 시스템을 개발할 수 있는 경쟁 우위를 창출할 것으로 예상되며, 이는 심장 안전 서비스 시장에서 다양한 다른 기회를 제공할 것으로 예상됩니다. 그러나 승인에 대한 엄격한 정부 규정은 성장을 방해할 것으로 예상됩니다.
유럽 심장 안전 서비스 시장 보고서는 시장 점유율, 새로운 개발 및 제품 파이프라인 분석, 국내 및 지역 시장 참여자의 영향, 새로운 수익 주머니, 시장 규정의 변화, 제품 승인, 전략적 의사 결정, 제품 출시, 지리적 확장 및 시장의 기술 혁신 측면에서의 분석 기회에 대한 세부 정보를 제공합니다. 분석 및 시장 시나리오를 이해하려면 분석가 브리핑을 위해 저희에게 연락하세요. 저희 팀은 원하는 목표를 달성하기 위한 수익 영향 솔루션을 만드는 데 도움을 드릴 것입니다. 다양한 지역의 개발도상국에 있는 소매점의 확장성과 사업 확장, 기계 및 약물 제품의 안전한 유통을 위한 공급업체와의 파트너십은 예측 기간 동안 시장 수요를 촉진한 주요 원동력입니다.
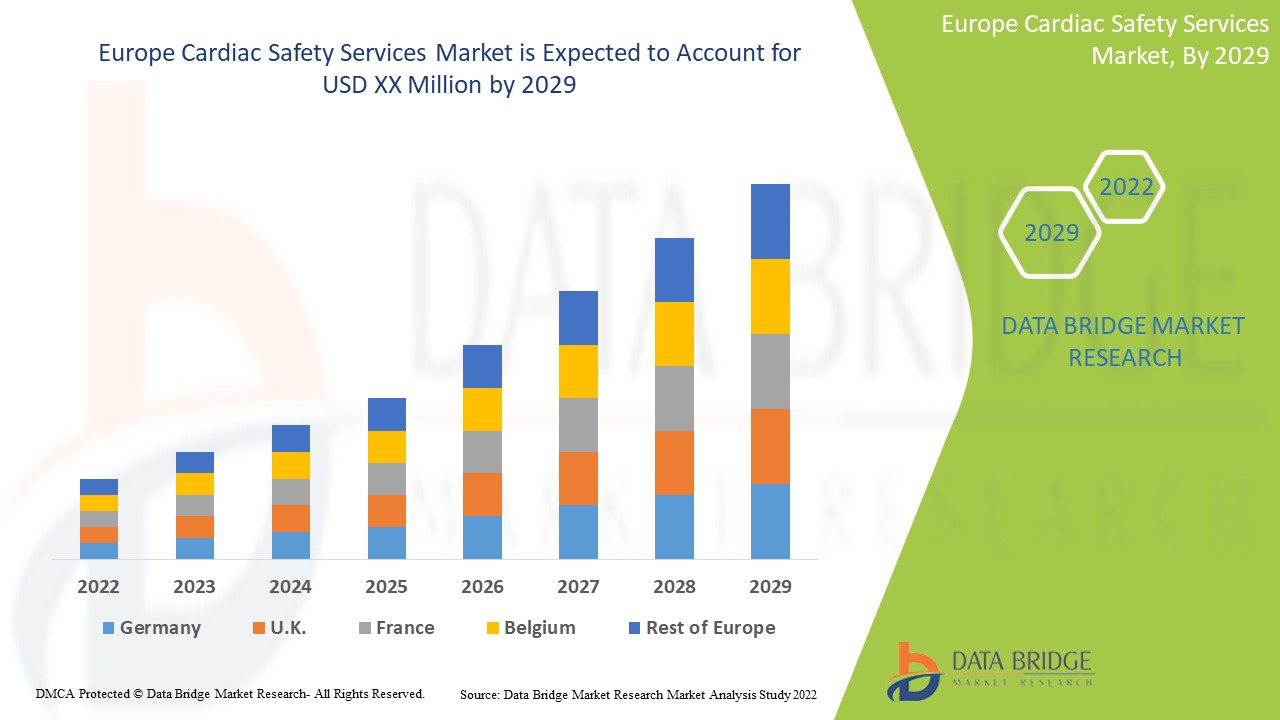
유럽 심장 안전 서비스 시장은 지지적이며 질병의 진행을 줄이는 것을 목표로 합니다. Data Bridge Market Research는 유럽 심장 안전 서비스 시장이 2022년에서 2029년까지의 예측 기간 동안 14.8%의 CAGR로 성장할 것이라고 분석합니다.
|
보고서 메트릭 |
세부 |
|
예측 기간 |
2022년부터 2029년까지 |
|
기준 연도 |
2021 |
|
역사적 연도 |
2020 (2019-2014까지 사용자 정의 가능) |
|
양적 단위 |
수익은 백만 달러, 가격은 미화로 표시됨 |
|
다루는 세그먼트 |
서비스별(ECG/홀터 측정, 혈압 측정 , 체외 심장 안전성 평가 서비스, 심혈관 영상, 실시간 원격 측정 모니터링, ECGS의 중앙 오버리드, 비침습적 심장 영상, 생리학적 스트레스 테스트, 철저한 QT 연구, TQT 및 노출 반응 모델링, 혈소판 응집 및 기타 서비스), 단계별(1단계, 2단계 및 3단계), 유형별(통합 서비스 및 독립형 서비스), 최종 사용자( 제약 및 생물제약 회사, 계약 연구 기관 및 학술 및 연구 기관) |
|
적용 국가 |
독일, 프랑스, 영국, 이탈리아, 스페인, 터키, 러시아, 네덜란드, 스위스, 벨기에 및 기타 유럽 |
|
시장 참여자 포함 |
Koninklijke Philips NV, Laboratory Corporation of America Holdings, IQVIA, Medpace, Ncardia, Certara, Eurofins Scientific, SGS SA, Banook 및 Celerion |
시장 정의:
심장 안전 서비스는 일반적으로 심장 안전을 모니터링하는 데 필요한 임상 시험 및 기타 연구를 지원하고 설계하는 데 도움이 됩니다. 심장 안전 서비스 시장에 대한 수요는 선진국과 개발도상국 모두에서 증가했으며 그 이유는 임상 시험과 제품 출시의 증가 때문입니다. 심장 안전 서비스 시장은 혁신적인 제품의 도입, 기술 제품의 증가, 가처분 소득의 증가로 인해 성장하고 있습니다. 시장은 신흥 시장 탐색, 시장 참여자의 전략적 이니셔티브, 의료비 지출 증가로 인해 예측 기간 동안 성장할 것입니다.
유럽 심장 안전 서비스 시장 동향
운전자
- 임상시험 수의 증가
임상 시험은 수백 년 전부터 시작된 체계적인 시스템으로, 여전히 약물 승인에 필요한 규제 요건의 중추입니다. 최근 임상 시험 분야에서 많은 진전이 있어 임상 시험의 수가 늘어났고 시장 성장을 촉진할 것으로 예상됩니다.
임상시험 규제에 다양한 변화가 있어 임상시험의 수가 늘어나고 긍정적인 결과도 나오고 있다.
예를 들어,
- Medical News 기사에 따르면, 모든 직원의 의무적 교육과 같은 임상 시험의 질 향상으로 인해 시험 수가 상당히 증가했습니다. 또한, NIH는 2017년에 NIH가 자금을 지원하는 시험에서 모든 연구자와 직원이 GCP(Good Clinical Practice)에 대한 교육을 받아야 한다고 밝혔습니다.
의료비 지출 및 자금 증가
국가가 의료에 사용하는 자금의 규모와 시간에 따른 성장률은 자금 조달 방식과 의료 시스템 조직의 구조를 포함한 다양한 경제적, 사회적 요인에 따라 달라집니다.
선진국과 신흥 경제권에서 사람들의 가처분 소득이 증가함에 따라 의료비 지출이 증가했습니다. 의료비에 더 많은 돈을 쓸수록 국가의 인구는 더 건강해집니다.
기회
- 신약개발 증가
임상 시험은 질병 치료를 위한 새로운 약물을 발견하고 개발하는 데 필수적입니다. 연구자들이 인간에게 어떤 치료법이 효과가 있고 없는지 알아낼 수 있는 가장 좋은 방법입니다. 약물 개발은 암, 내분비, 대사 등과 같은 다양한 질병을 치료하기 위한 의약품이나 장치로서 새로운 치료법을 형성하는 것으로 특징지어집니다.
- 따라서 임상시험은 의약품이 시장에 출시되기 전과 인체에 투여되기 전에 안전성과 효능을 확인하는 가장 효과적인 방법으로, 의료제품이 시장에 출시되기 전에 필수적인 부분인 심장 안전성 평가도 포함된다.
제지/도전
임상 심장 안전 데이터의 적절한 평가 및 보고는 필수적입니다. 모든 의료 제품에 대한 승인 및 제품 리콜은 심장 안전 평가에 따라 달라집니다. 따라서 법적 절차에 따라 심장 안전 평가를 제공하고 실시하는 것이 필요합니다. 그렇지 않으면 제품 승인이 늦어져 시장 성장이 제한될 것으로 예상됩니다.
예를 들어,
- IQVIA의 기사에 따르면 1957년에서 2007년 사이에 약물의 시판 후 철수가 47건 발생했으며, 이 중 45%가 심혈관 독성에 대한 우려 때문이었습니다. 마찬가지로, 지난 20년 동안 전임상 단계에서 실패한 잠재적 신약 분자의 27%는 필요한 규제 기준을 충족하지 못해 심혈관 독성 때문이었습니다.
유럽 심장 안전 서비스 시장 세분화
유럽 심장 안전 서비스 시장은 유형, 서비스, 단계 및 최종 사용자로 분류됩니다. 세그먼트 간 성장은 틈새 성장 포켓과 시장에 접근하고 핵심 응용 분야와 타겟 시장의 차이점을 파악하기 위한 전략을 분석하는 데 도움이 됩니다.
서비스
- ECG/홀터 측정
- 혈압 측정
- 시험관 내 심장 안전성 평가 서비스
- 심혈관 영상
- 실시간 원격 측정 모니터링
- ECGS의 중앙 과독
- 비침습적 심장 영상
- 생리학적 스트레스 테스트
- 철저한 QT 연구
- TQT 및 노출 반응 모델링
- 혈소판 응집
- 기타 서비스
서비스를 기준으로 심장 안전 서비스 시장은 ECG /홀터 측정, 혈압 측정, 체외 심장 안전 평가 서비스, 심혈관 영상, 실시간 원격 측정 모니터링, ECGS 중앙 오버리드, 비침습적 심장 영상, 생리학적 스트레스 테스트, 철저한 QT 연구, TQT 및 노출 반응 모델링, 혈소판 응집 및 기타 서비스로 세분화됩니다.
단계
- 1단계
- 2단계
- 3단계
심장 안전 서비스 시장은 단계별로 1단계, 2단계, 3단계로 구분됩니다.
유형
- 통합 서비스
- 독립형 서비스
심장 안전 서비스 시장은 유형을 기준으로 통합 서비스와 독립형 서비스로 구분됩니다.
최종 사용자
- 제약 및 바이오제약 회사
- 계약 연구 기관
- 학술 및 연구 기관
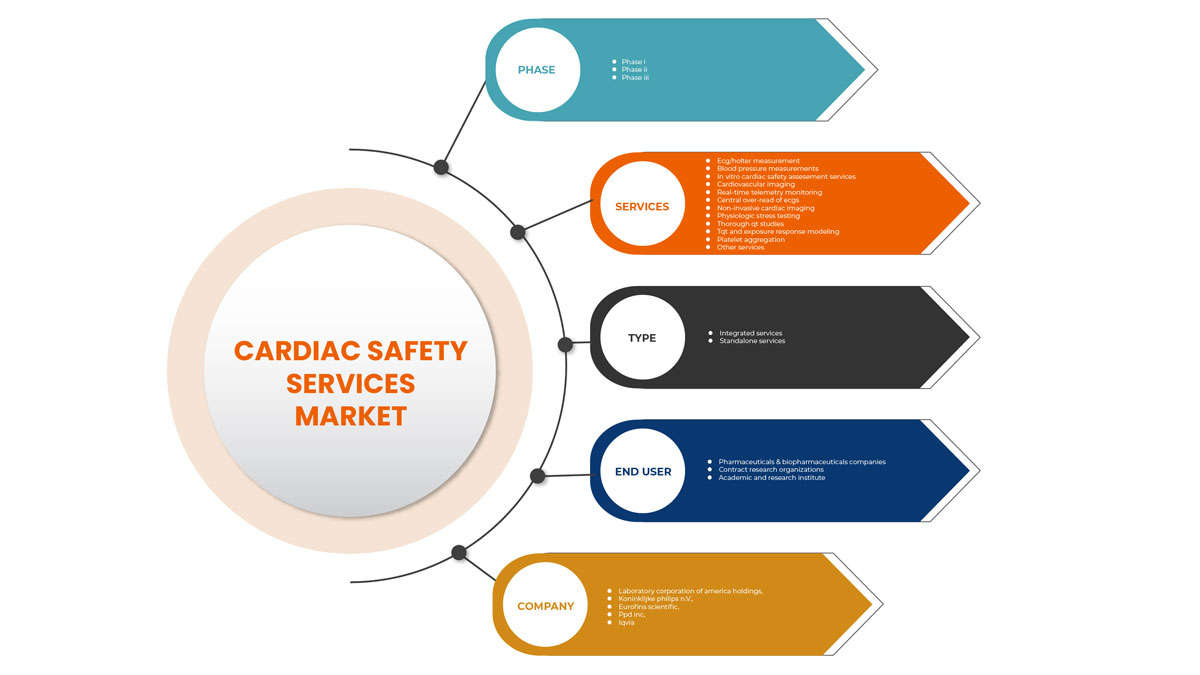
최종 사용자를 기준으로 볼 때, 심장 안전 서비스 시장은 제약 및 생물약학 회사, 계약 연구 기관, 학계 및 연구 기관으로 구분됩니다.
심장 안전 서비스 지역 분석/통찰력
심장 안전 서비스를 분석하고, 위에 언급된 대로 유형, 서비스, 단계 및 최종 사용자별로 시장 규모에 대한 통찰력과 추세를 제공합니다.
심장 안전 서비스 보고서에서 다루는 국가는 독일, 프랑스, 영국, 이탈리아, 스페인, 터키, 러시아, 네덜란드, 스위스, 벨기에와 기타 유럽 국가입니다.
독일은 신약 개발과 의료비 지출이 늘어나면서 우위를 점할 것으로 예상됩니다.
보고서의 국가 섹션은 또한 현재 및 미래 시장 추세에 영향을 미치는 개별 시장 영향 요인과 시장 규제의 변화를 제공합니다. 다운스트림 및 업스트림 가치 사슬 분석, 기술 추세 및 포터의 5가지 힘 분석, 사례 연구와 같은 데이터 포인트는 개별 국가의 시장 시나리오를 예측하는 데 사용되는 몇 가지 포인터입니다. 또한 유럽 브랜드의 존재 및 가용성과 지역 및 국내 브랜드와의 대규모 또는 희소한 경쟁으로 인해 직면한 과제, 국내 관세 및 무역 경로의 영향은 국가 데이터에 대한 예측 분석을 제공하는 동안 고려됩니다.
경쟁 환경 및 심장 안전 서비스 분석
유럽 심장 안전 서비스 시장 경쟁 구도는 경쟁자에 대한 세부 정보를 제공합니다. 포함된 세부 정보는 회사 개요, 회사 재무, 창출된 수익, 시장 잠재력, 연구 개발 투자, 새로운 시장 이니셔티브, 유럽의 입지, 생산 현장 및 시설, 생산 용량, 회사의 강점과 약점, 제품 출시, 제품 폭과 범위, 애플리케이션 우세입니다. 위에 제공된 데이터 포인트는 회사의 심장 안전 서비스 시장에 대한 초점과만 관련이 있습니다.
시장의 주요 기업으로는 Koninklijke Philips NV, Laboratory Corporation of America Holdings, IQVIA, Medpace, Ncardia, Certara, Eurofins Scientific, SGS SA, Banook, Celerion 등이 있습니다.
연구 방법론
데이터 수집 및 기준 연도 분석은 대규모 샘플 크기의 데이터 수집 모듈을 사용하여 수행됩니다. 시장 데이터는 시장 통계 및 일관된 모델을 사용하여 분석 및 추정됩니다. 또한 시장 점유율 분석 및 주요 추세 분석은 시장 보고서의 주요 성공 요인입니다. DBMR 연구팀에서 사용하는 주요 연구 방법론은 데이터 마이닝, 시장에 대한 데이터 변수의 영향 분석 및 기본(산업 전문가) 검증을 포함하는 데이터 삼각 측량입니다. 이 외에도 데이터 모델에는 공급업체 포지셔닝 그리드, 시장 타임라인 분석, 시장 개요 및 가이드, 회사 포지셔닝 그리드, 회사 시장 점유율 분석, 측정 표준, 유럽 대 지역 및 공급업체 점유율 분석이 포함됩니다. 추가 문의 사항이 있는 경우 분석가 전화를 요청하십시오.
SKU-
세계 최초의 시장 정보 클라우드 보고서에 온라인으로 접속하세요
- 대화형 데이터 분석 대시보드
- 높은 성장 잠재력 기회를 위한 회사 분석 대시보드
- 사용자 정의 및 질의를 위한 리서치 분석가 액세스
- 대화형 대시보드를 통한 경쟁자 분석
- 최신 뉴스, 업데이트 및 추세 분석
- 포괄적인 경쟁자 추적을 위한 벤치마크 분석의 힘 활용
목차
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF EUROPE CARDIAC SAFETY SERVICES MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 SERVICES LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 MARKET END USER GRID
2.11 VENDOR SHARE ANALYSIS
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PORTERS FIVE FORCES
4.2 PESTEL ANALYSIS
5 EPIDEMIOLOGY
5.1 INCIDENCE OF ALL BY GENDER
5.2 TREATMENT RATE
5.3 MORTALITY RATE
5.4 DRUG ADHERENCE AND THERAPY SWITCH MODEL
6 INDUSTRY INSIGHT
6.1 DEMOGRAPHIC TRENDS: IMPACTS ON ALL INCIDENCE RATES
6.2 PATENT ANALYSIS
6.3 PATENT FLOW DIAGRAM
6.4 KEY PATIENT ENROLLMENT STRATEGIES
6.5 PRICING STRATEGY
7 EUROPE CARDIAC SAFETY SERVICES MARKET: REGULATIONS
8 MARKET OVERVIEW
8.1 DRIVERS
8.1.1 INCREASE IN THE NUMBER OF CLINICAL TRIALS
8.1.2 INCREASE IN HEALTHCARE EXPENDITURE AND FUNDING
8.1.3 INCREASE IN STRATEGIC INITIATIVES BY MAJOR MARKET PLAYERS
8.1.4 INCREASE IN R&D ACTIVITIES
8.2 RESTRAINTS
8.2.1 HIGH COST OF CARDIAC SAFETY EVALUATION
8.2.2 STRICT REGULATORY
8.3 OPPORTUNITIES:
8.3.1 INCREASE IN NEW DRUG DEVELOPMENT
8.3.2 RISE IN THE EXPANSION OF THE CARDIAC SAFETY SERVICES
8.4 CHALLENGES
8.4.1 TIME-CONSUMING PROCEDURE
8.4.2 LACK OF SKILLED PERSON TO OPERATE DEVICES DURING CARDIAC SAFETY EVALUATION
9 EUROPE CARDIAC SAFETY SERVICES MARKET, BY SERVICES
9.1 OVERVIEW
9.2 ECG/HOLTER MEASUREMENTS
9.3 BLOOD PRESSURE MEASUREMENTS
9.4 IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES
9.4.1 HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS
9.4.1.1 1 CONCENTRATIONS
9.4.1.2 4 CONCENTRATIONS
9.4.2 COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA)
9.4.2.1 3 CONCENTRATIONS
9.4.2.2 5 CONCENTRATIONS
9.4.3 IN VITRO HERG ASSAY
9.4.4 OTHERS
9.5 CARDIOVASCULAR IMAGING
9.6 REAL TIME TELEMETRY MONITORING
9.7 CENTRAL OVER-READ OF ECGS
9.8 NON-INVASIVE CARDIAC IMAGING
9.9 PHYSIOLOGIC STRESS TESTING
9.1 THOROUGH QT STUDIES
9.11 TQT AND EXPOSURE RESPONSE MODELLING
9.12 PLATELET AGGREGATION
9.13 OTHERS
10 EUROPE CARDIAC SAFETY SERVICES MARKET, BY PHASE
10.1 OVERVIEW
10.2 PHASE I
10.3 PHASE II
10.4 PHASE III
11 EUROPE CARDIAC SAFETY SERVICES MARKET, BY TYPE
11.1 OVERVIEW
11.2 INTEGRATED SERVICES
11.3 STANDALONE SERVICES
12 EUROPE CARDIAC SAFETY SERVICES MARKET, BY END USER
12.1 OVERVIEW
12.2 PHARMACEUTICALS & BIOPHARMACEUTICALS COMPANIES
12.3 CONTRACT RESEARCH ORGANIZATIONS
12.4 ACADEMIC AND RESEARCH INSTITUTE
13 EUROPE CARDIAC SAFETY SERVICES MARKET, BY REGION
13.1 EUROPE
13.1.1 GERMANY
13.1.2 FRANCE
13.1.3 U.K.
13.1.4 ITALY
13.1.5 SPAIN
13.1.6 TURKEY
13.1.7 RUSSIA
13.1.8 NETHERLANDS
13.1.9 SWITZERLAND
13.1.10 BELGIUM
13.1.11 REST OF EUROPE
14 EUROPE CARDIAC SAFETY SERVICES MARKET: COMPANY LANDSCAPE
14.1 COMPANY SHARE ANALYSIS: EUROPE
15 COMPANY PROFILE
15.1 EUROFINS SCIENTIFIC
15.1.1 COMPANY SNAPSHOT
15.1.2 REVENUE ANALYSIS
15.1.3 COMPANY SHARE ANALYSIS
15.1.4 PRODUCT PORTFOLIO
15.1.5 RECENT DEVELOPMENTS
15.1.5.1 AGREEMENTS
15.1.5.2 ACQUISITION
15.2 PPD INC. (SUBSIDIARY OF THERMO FISHER SCIENTIFIC INC)
15.2.1 COMPANY SNAPSHOT
15.2.2 REVENUE ANALYSIS
15.2.3 COMPANY SHARE ANALYSIS
15.2.4 PRODUCT PORTFOLIO
15.2.5 RECENT DEVELOPMENT
15.2.5.1 INVESTMENT
15.3 KONINKLIJKE PHILIPS N.V.
15.3.1 COMPANY SNAPSHOT
15.3.2 REVENUE ANALYSIS
15.3.3 COMPANY SHARE ANALYSIS
15.3.4 PRODUCT PORTFOLIO
15.3.5 RECENT DEVELOPMENT
15.3.5.1 ACQUISITION
15.4 IQVIA
15.4.1 COMPANY SNAPSHOT
15.4.2 REVENUE ANALYSIS
15.4.3 COMPANY SHARE ANALYSIS
15.4.4 PRODUCT PORTFOLIO
15.4.5 RECENT DEVELOPMENTS
15.4.5.1 ACQUISITION
15.5 LABORATORY CORPORATION OF AMERICA HOLDINGS
15.5.1 COMPANY SNAPSHOT
15.5.2 REVENUE ANALYSIS
15.5.3 COMPANY SHARE ANALYSIS
15.5.4 PRODUCT PORTFOLIO
15.5.5 RECENT DEVELOPMENTS
15.5.5.1 NEW LABORATORY
15.5.5.2 ACQUISITION
15.6 BANOOK
15.6.1 COMPANY SNAPSHOT
15.6.2 PRODUCT PORTFOLIO
15.6.3 RECENT DEVELOPMENT
15.6.3.1 AGREEMENT
15.7 BIOTRIAL
15.7.1 COMPANY SNAPSHOT
15.7.2 PRODUCT PORTFOLIO
15.7.3 RECENT DEVELOPMENT
15.7.3.1 NEW CENTER OPENING
15.8 CELERION
15.8.1 COMPANY SNAPSHOT
15.8.2 PRODUCT PORTFOLIO
15.8.3 RECENT DEVELOPMENT
15.8.3.1 NEW CENTER OPENING
15.9 CERTARA
15.9.1 COMPANY SNAPSHOT
15.9.2 REVENUE ANALYSIS
15.9.3 PRODUCT PORTFOLIO
15.9.4 RECENT DEVELOPMENTS
15.9.4.1 CONTRACT
15.9.4.2 ACQUISITION
15.1 CLARIO
15.10.1 COMPANY SNAPSHOT
15.10.2 PRODUCT PORTFOLIO
15.10.3 RECENT DEVELOPMENT
15.10.3.1 PRODUCT EXPANSION
15.11 MEDPACE
15.11.1 COMPANY SNAPSHOT
15.11.2 REVENUE ANALYSIS
15.11.3 PRODUCT PORTFOLIO
15.11.4 RECENT DEVELOPMENTS
15.11.4.1 ACQUISITION
15.12 NCARDIA
15.12.1 COMPANY SNAPSHOT
15.12.2 PRODUCT PORTFOLIO
15.12.3 RECENT DEVELOPMENT
15.12.3.1 PARTNERSHIP
15.13 NEXEL CO., LTD
15.13.1 COMPANY SNAPSHOT
15.13.2 PRODUCT PORTFOLIO
15.13.3 RECENT DEVELOPMENTS
15.13.3.1 JOINT VENTURE
15.13.3.2 PARTNERSHIP
15.14 PHYSIOSTIM
15.14.1 COMPANY SNAPSHOT
15.14.2 PRODUCT PORTFOLIO
15.14.3 RECENT DEVELOPMENT
15.14.3.1 PARTNERSHIP
15.15 RICHMOND PHARMACOLOGY
15.15.1 COMPANY SNAPSHOT
15.15.2 PRODUCT PORTFOLIO
15.15.3 RECENT DEVELOPMENT
15.15.3.1 EVENT
15.16 SGS SA
15.16.1 COMPANY SNAPSHOT
15.16.2 REVENUE ANALYSIS
15.16.3 PRODUCT PORTFOLIO
15.16.4 RECENT DEVELOPMENT
15.16.4.1 ACQUISITION
15.17 SHANGHAI MEDICILON INC.
15.17.1 COMPANY SNAPSHOT
15.17.2 PRODUCT PORTFOLIO
15.17.3 RECENT DEVELOPMENTS
15.17.3.1 PARTNERSHIP
15.17.3.2 PARTNERSHIP
16 QUESTIONNAIRE
17 RELATED REPORTS
표 목록
TABLE 1 PROPORTION OF WOMEN IN CLINICAL STUDIES, ACCORDING TO DEVELOPMENT PHASE
TABLE 2 PROBABILITY OF SUCCESS BY CLINICAL TRIAL PHASE TO THERAPEUTIC AREA
TABLE 3 MORTALITY RATES FROM CLINICAL TRIALS AND EUROPEAN SAFETY AND EXPOSURE SURVEY (ESES), DEATHS PER 100 (PYE)
TABLE 4 ADHERENCE RATE TO COMMON CARDIOVASCULAR MEDICATION
TABLE 5 PROPORTION OF WOMEN IN CLINICAL STUDIES, ACCORDING TO DEVELOPMENT PHASE
TABLE 6 INITIATIVES TO INCREASE ENROLLMENT IN CLINICAL TRIALS AMONG UNDERREPRESENTED POPULATIONS
TABLE 7 ESTIMATED COST OF CARDIAC SAFETY EVALUATION DEVICES
TABLE 8 EUROPE CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 9 EUROPE ECG/HOLTER MEASUREMENTS IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 10 EUROPE BLOOD PRESSURE MEASUREMENTS IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 11 EUROPE IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 12 EUROPE IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 13 EUROPE HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 14 EUROPE COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 15 EUROPE CARDIOVASCULAR IMAGING IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 16 EUROPE REAL TIME TELEMETRY MONITORING IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 17 EUROPE CENTRAL OVER-READ OF ECGS IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 18 EUROPE NON-INVASIVE CARDIAC IMAGING IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 19 EUROPE PHYSIOLOGIC STRESS TESTING IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 20 EUROPE THOROUGH QT STUDIES IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 21 EUROPE TQT AND EXPOSURE RESPONSE MODELLING IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 22 EUROPE PLATELET AGGREGATION IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 23 EUROPE OTHERS IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 24 EUROPE CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 25 EUROPE PHASE I IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 26 EUROPE PHASE II IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 27 EUROPE PHASE III IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 28 EUROPE CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 29 EUROPE INTEGRATED SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 30 EUROPE STANDALONE SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 31 EUROPE CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 32 EUROPE PHARMACEUTICALS & BIOPHARMACEUTICALS COMPANIES IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 33 EUROPE CONTRACT RESEARCH ORGANIZATIONS IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 34 EUROPE ACADEMIC AND RESEARCH INSTITUTE IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 35 EUROPE CARDIAC SAFETY SERVICES MARKET, BY COUNTRY, 2020-2029 (USD MILLION)
TABLE 36 EUROPE CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 37 EUROPE IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 38 EUROPE HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 39 EUROPE COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 40 EUROPE CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 41 EUROPE CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 42 EUROPE CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 43 GERMANY CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 44 GERMANY IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 45 GERMANY HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 46 GERMANY COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 47 GERMANY CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 48 GERMANY CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 49 GERMANY CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 50 FRANCE CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 51 FRANCE IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 52 FRANCE HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 53 FRANCE COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 54 FRANCE CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 55 FRANCE CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 56 FRANCE CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 57 U.K. CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 58 U.K. IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 59 U.K. HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 60 U.K. COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 61 U.K. CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 62 U.K. CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 63 U.K. CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 64 ITALY CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 65 ITALY IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 66 ITALY HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 67 ITALY COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 68 ITALY CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 69 ITALY CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 70 ITALY CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 71 SPAIN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 72 SPAIN IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 73 SPAIN HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 74 SPAIN COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 75 SPAIN CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 76 SPAIN CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 77 SPAIN CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 78 TURKEY CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 79 TURKEY IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 80 TURKEY HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 81 TURKEY COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 82 TURKEY CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 83 TURKEY CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 84 TURKEY CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 85 RUSSIA CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 86 RUSSIA IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 87 RUSSIA HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 88 RUSSIA COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 89 RUSSIA CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 90 RUSSIA CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 91 RUSSIA CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 92 NETHERLANDS CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 93 NETHERLANDS IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 94 NETHERLANDS HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 95 NETHERLANDS COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 96 NETHERLANDS CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 97 NETHERLANDS CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 98 NETHERLANDS CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 99 SWITZERLAND CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 100 SWITZERLAND IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 101 SWITZERLAND HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 102 SWITZERLAND COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 103 SWITZERLAND CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 104 SWITZERLAND CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 105 SWITZERLAND CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 106 BELGIUM CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 107 BELGIUM IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 108 BELGIUM HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 109 BELGIUM COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 110 BELGIUM CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 111 BELGIUM CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 112 BELGIUM CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 113 REST OF EUROPE CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
그림 목록
FIGURE 1 EUROPE CARDIAC SAFETY SERVICES MARKET: SEGMENTATION
FIGURE 2 EUROPE CARDIAC SAFETY SERVICES MARKET: DATA TRIANGULATION
FIGURE 3 EUROPE CARDIAC SAFETY SERVICES MARKET: DROC ANALYSIS
FIGURE 4 EUROPE CARDIAC SAFETY SERVICES MARKET: EUROPE VS COUNTRY MARKET ANALYSIS
FIGURE 5 EUROPE CARDIAC SAFETY SERVICES MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 EUROPE CARDIAC SAFETY SERVICES MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 EUROPE CARDIAC SAFETY SERVICES MARKET: DBMR MARKET POSITION GRID
FIGURE 8 EUROPE CARDIAC SAFETY SERVICES MARKET: MARKET END USER GRID
FIGURE 9 EUROPE CARDIAC SAFETY SERVICES MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 EUROPE CARDIAC SAFETY SERVICES MARKET: SEGMENTATION
FIGURE 11 THE INCREASE IN DEMAND FOR CARDIAC SAFETY SERVICES ARE EXPECTED TO DRIVE THE EUROPE CARDIAC SAFETY SERVICES MARKET IN THE FORECAST PERIOD OF 2022 TO 2029
FIGURE 12 ECG/HOLTER MEASUREMENTS SUBSTITUTE IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE EUROPE CARDIAC SAFETY SERVICES MARKET IN 2022 & 2029
FIGURE 13 PATIENT FLOW DIAGRAM FOR ANY RANDOM DRUG
FIGURE 14 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF THE EUROPE CARDIAC SAFETY SERVICES MARKET
FIGURE 15 EUROPE CARDIAC SAFETY SERVICES MARKET: BY SERVICES, 2021
FIGURE 16 EUROPE CARDIAC SAFETY SERVICES MARKET: BY SERVICES, 2022-2029 (USD MILLION)
FIGURE 17 EUROPE CARDIAC SAFETY SERVICES MARKET: BY SERVICES, CAGR (2022-2029)
FIGURE 18 EUROPE CARDIAC SAFETY SERVICES MARKET: BY SERVICES, LIFELINE CURVE
FIGURE 19 EUROPE CARDIAC SAFETY SERVICES MARKET: BY PHASE, 2021
FIGURE 20 EUROPE CARDIAC SAFETY SERVICES MARKET: BY PHASE, 2022-2029 (USD MILLION)
FIGURE 21 EUROPE CARDIAC SAFETY SERVICES MARKET: BY PHASE, CAGR (2022-2029)
FIGURE 22 EUROPE CARDIAC SAFETY SERVICES MARKET: BY PHASE, LIFELINE CURVE
FIGURE 23 EUROPE CARDIAC SAFETY SERVICES MARKET: BY TYPE, 2021
FIGURE 24 EUROPE CARDIAC SAFETY SERVICES MARKET: BY TYPE, 2022-2029 (USD MILLION)
FIGURE 25 EUROPE CARDIAC SAFETY SERVICES MARKET: BY TYPE, CAGR (2022-2029)
FIGURE 26 EUROPE CARDIAC SAFETY SERVICES MARKET: BY TYPE, LIFELINE CURVE
FIGURE 27 EUROPE CARDIAC SAFETY SERVICES MARKET: BY END USER, 2021
FIGURE 28 EUROPE CARDIAC SAFETY SERVICES MARKET: BY END USER, 2022-2029 (USD MILLION)
FIGURE 29 EUROPE CARDIAC SAFETY SERVICES MARKET: BY END USER, CAGR (2022-2029)
FIGURE 30 EUROPE CARDIAC SAFETY SERVICES MARKET: BY END USER, LIFELINE CURVE
FIGURE 31 EUROPE CARDIAC SAFETY SERVICES MARKET: SNAPSHOT (2021)
FIGURE 32 EUROPE CARDIAC SAFETY SERVICES MARKET: BY COUNTRY (2021)
FIGURE 33 EUROPE CARDIAC SAFETY SERVICES MARKET: BY COUNTRY (2022 & 2029)
FIGURE 34 EUROPE CARDIAC SAFETY SERVICES MARKET: BY COUNTRY (2021 & 2029)
FIGURE 35 EUROPE CARDIAC SAFETY SERVICES MARKET: BY SERVICES (2022-2029)
FIGURE 36 EUROPE CARDIAC SAFETY SERVICES MARKET: COMPANY SHARE 2021 (%)

연구 방법론
데이터 수집 및 기준 연도 분석은 대규모 샘플 크기의 데이터 수집 모듈을 사용하여 수행됩니다. 이 단계에는 다양한 소스와 전략을 통해 시장 정보 또는 관련 데이터를 얻는 것이 포함됩니다. 여기에는 과거에 수집한 모든 데이터를 미리 검토하고 계획하는 것이 포함됩니다. 또한 다양한 정보 소스에서 발견되는 정보 불일치를 검토하는 것도 포함됩니다. 시장 데이터는 시장 통계 및 일관된 모델을 사용하여 분석하고 추정합니다. 또한 시장 점유율 분석 및 주요 추세 분석은 시장 보고서의 주요 성공 요인입니다. 자세한 내용은 분석가에게 전화를 요청하거나 문의 사항을 드롭하세요.
DBMR 연구팀에서 사용하는 주요 연구 방법론은 데이터 마이닝, 시장에 대한 데이터 변수의 영향 분석 및 주요(산업 전문가) 검증을 포함하는 데이터 삼각 측량입니다. 데이터 모델에는 공급업체 포지셔닝 그리드, 시장 타임라인 분석, 시장 개요 및 가이드, 회사 포지셔닝 그리드, 특허 분석, 가격 분석, 회사 시장 점유율 분석, 측정 기준, 글로벌 대 지역 및 공급업체 점유율 분석이 포함됩니다. 연구 방법론에 대해 자세히 알아보려면 문의를 통해 업계 전문가에게 문의하세요.
사용자 정의 가능
Data Bridge Market Research는 고급 형성 연구 분야의 선두 주자입니다. 저희는 기존 및 신규 고객에게 목표에 맞는 데이터와 분석을 제공하는 데 자부심을 느낍니다. 보고서는 추가 국가에 대한 시장 이해(국가 목록 요청), 임상 시험 결과 데이터, 문헌 검토, 재생 시장 및 제품 기반 분석을 포함하도록 사용자 정의할 수 있습니다. 기술 기반 분석에서 시장 포트폴리오 전략에 이르기까지 타겟 경쟁업체의 시장 분석을 분석할 수 있습니다. 귀하가 원하는 형식과 데이터 스타일로 필요한 만큼 많은 경쟁자를 추가할 수 있습니다. 저희 분석가 팀은 또한 원시 엑셀 파일 피벗 테이블(팩트북)로 데이터를 제공하거나 보고서에서 사용 가능한 데이터 세트에서 프레젠테이션을 만드는 데 도움을 줄 수 있습니다.