Asia Pacific Molecular Diagnostics Services Market
시장 규모 (USD 10억)
연평균 성장률 :
% 
 USD
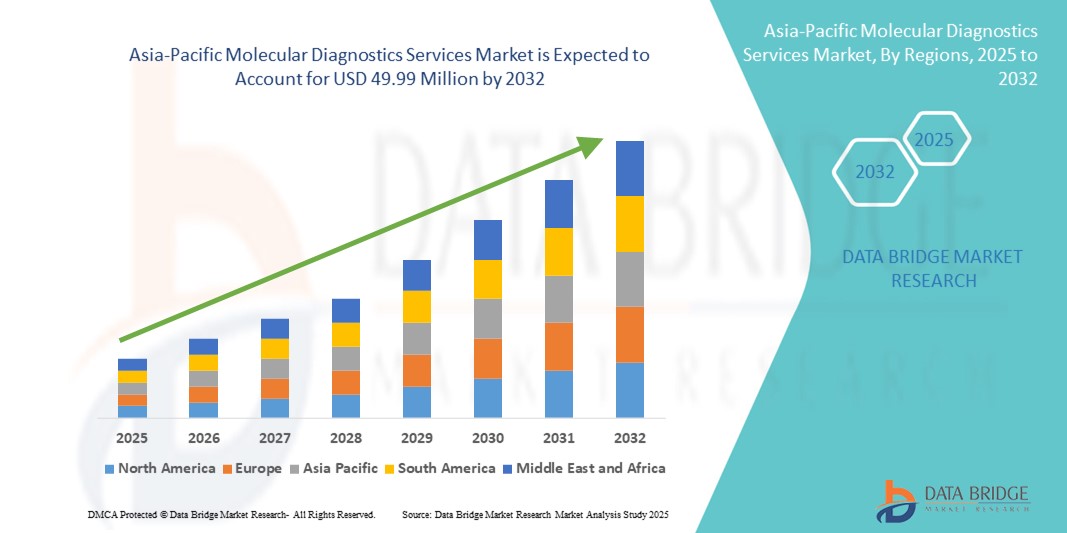
25.84 Million
USD
49.99 Million
2024
2032
USD
25.84 Million
USD
49.99 Million
2024
2032
| 2025 –2032 | |
| USD 25.84 Million | |
| USD 49.99 Million | |
|
|
|
|
تجزئة سوق خدمات التشخيص الجزيئي في منطقة آسيا والمحيط الهادئ، حسب نوع الخدمة (خدمات إصلاح الأجهزة، خدمات التدريب، خدمات الامتثال، خدمات المعايرة ، خدمات الصيانة، خدمات الأتمتة القابلة للتطوير، خدمات تسليم المفتاح، خدمات نقل الأجهزة، تخصيص الأجهزة، خدمات ضمان الأداء، خدمات التصميم والتطوير، حلول سلسلة التوريد، خدمات طرح المنتجات الجديدة، خدمات التصنيع، الخدمات البيئية والتنظيمية، شهادات وتدقيق أنظمة الإدارة الطبية ، خدمات البحوث السريرية، الخدمات الاستشارية، وغيرها من الخدمات)، التكنولوجيا (تفاعل البوليميراز المتسلسل، تفاعل البوليميراز المتسلسل في الوقت الحقيقي، تسلسل الجيل التالي، وغيرها من التقنيات)، المستخدم النهائي ( المستشفيات ومراكز التشخيص والمؤسسات الأكاديمية والبحثية وغيرها) اتجاهات الصناعة والتوقعات حتى عام 2030.
حجم سوق خدمات التشخيص الجزيئي في منطقة آسيا والمحيط الهادئ
- تم تقييم حجم سوق خدمات التشخيص الجزيئي في منطقة آسيا والمحيط الهادئ بـ 25.84 مليون دولار أمريكي في عام 2024 ومن المتوقع أن يصل إلى 49.99 مليون دولار أمريكي بحلول عام 2032 ، بمعدل نمو سنوي مركب قدره 8.60٪ خلال الفترة المتوقعة.
- يُعزى نمو السوق بشكل كبير إلى زيادة الوعي، وزيادة فرص الحصول على الرعاية الصحية، والتطورات في تقنيات التشخيص في جميع أنحاء منطقة آسيا والمحيط الهادئ، مما يُمكّن من الكشف المبكر عن مجموعة واسعة من الأمراض الوراثية والمعدية والمزمنة وإدارتها. تشهد دول مثل الهند والصين وإندونيسيا طفرة في تطوير البنية التحتية للرعاية الصحية، مما يُسهم في تزايد اعتماد خدمات التشخيص الجزيئي.
- علاوة على ذلك، فإن تزايد الاستثمارات في مرافق المختبرات، وتوسيع خدمات التشخيص في المناطق الريفية وشبه الحضرية، وتزايد الشراكات بين القطاعين العام والخاص، كلها عوامل تدفع عجلة الابتكار وتوفر تقنيات الاختبار الجزيئي المتقدمة. وتُعزز المبادرات الصحية الحكومية، إلى جانب الحضور المتنامي لشركات التشخيص الدولية وقدرات التصنيع المحلية، نمو سوق خدمات التشخيص الجزيئي في منطقة آسيا والمحيط الهادئ بشكل كبير.
تحليل سوق خدمات التشخيص الجزيئي في منطقة آسيا والمحيط الهادئ
- يشهد سوق خدمات التشخيص الجزيئي في منطقة آسيا والمحيط الهادئ نموًا ملحوظًا، مدفوعًا بزيادة الاستثمارات في منصات التشخيص المتقدمة، وارتفاع معدل انتشار الأمراض المعدية والوراثية، والاعتماد المتزايد على مبادرات الطب الدقيق. وتعمل دول مثل الصين والهند واليابان وكوريا الجنوبية على توسيع بنيتها التحتية للمختبرات وقدراتها البحثية، مما يساهم في زيادة الطلب على خدمات التشخيص الجزيئي عالية الجودة.
- يُدعم التركيز المتزايد على الرعاية الصحية الشخصية والبحوث السريرية في المنطقة من خلال زيادة التمويل الحكومي، وتنامي استثمارات القطاع الخاص في الرعاية الصحية، وتوسع مراكز التشخيص المتخصصة. ويساهم تزايد الوعي بالكشف المبكر عن الأمراض، إلى جانب التطورات في تقنيات الاختبار الجزيئي مثل تفاعل البوليميراز المتسلسل (PCR)، وتفاعل البوليميراز المتسلسل في الوقت الفعلي (Real Time PCR)، وتسلسل الجيل التالي (NGS)، في توسيع نطاق اعتماد هذه التقنيات في المستشفيات ومعاهد البحث.
- سيطرت الصين على سوق خدمات التشخيص الجزيئي في منطقة آسيا والمحيط الهادئ، حيث استحوذت على أكبر حصة من الإيرادات بنسبة 35.1% في عام 2024، مدفوعة بشبكتها الراسخة من المستشفيات، وعدد كبير من المرضى، والتكامل السريع لتقنيات التشخيص الجزيئي المتقدمة في سير العمل السريري والبحثي.
- من المتوقع أن تُسجل الهند أسرع معدل نمو سنوي مركب بنسبة 13.6% في سوق خدمات التشخيص الجزيئي في منطقة آسيا والمحيط الهادئ خلال فترة التوقعات، مدفوعًا بتوسيع نطاق الوصول إلى الرعاية الصحية، وتزايد الطلب على الاختبارات الجزيئية بأسعار معقولة، وتنامي الاستثمار في البنية التحتية للمختبرات. تُسرّع مبادرات مثل برامج التشخيص المدعومة حكوميًا وتزايد التعاون مع القطاع الخاص من اعتماد التشخيص الجزيئي في المناطق الحضرية وشبه الحضرية.
- سيطرت خدمات تفاعل البوليميراز المتسلسل (PCR) على سوق خدمات التشخيص الجزيئي في منطقة آسيا والمحيط الهادئ بنسبة 36.5% في عام 2024، وذلك بفضل اعتمادها الواسع في الاختبارات الجزيئية للأمراض المعدية، والفحوصات الجينية، وتطبيقات التشخيص الروتينية. ولا تزال تقنية تفاعل البوليميراز المتسلسل (PCR) تُعدّ تقنية أساسية بفضل موثوقيتها، وسعرها المناسب، وقدرتها على التكيف مع مختلف سير العمل السريري.
نطاق التقرير وتقسيم سوق خدمات التشخيص الجزيئي في منطقة آسيا والمحيط الهادئ
|
صفات |
رؤى رئيسية حول سوق خدمات التشخيص الجزيئي في منطقة آسيا والمحيط الهادئ |
|
القطاعات المغطاة |
|
|
الدول المغطاة |
آسيا والمحيط الهادئ
|
|
اللاعبون الرئيسيون في السوق |
|
|
فرص السوق |
|
|
مجموعات معلومات البيانات ذات القيمة المضافة |
بالإضافة إلى الرؤى حول سيناريوهات السوق مثل القيمة السوقية ومعدل النمو والتجزئة والتغطية الجغرافية واللاعبين الرئيسيين، فإن تقارير السوق التي تم تنظيمها بواسطة Data Bridge Market Research تشمل أيضًا تحليلًا متعمقًا من الخبراء وتحليل التسعير وتحليل حصة العلامة التجارية واستطلاع رأي المستهلكين وتحليل التركيبة السكانية وتحليل سلسلة التوريد وتحليل سلسلة القيمة ونظرة عامة على المواد الخام / المواد الاستهلاكية ومعايير اختيار البائعين وتحليل PESTLE وتحليل Porter والإطار التنظيمي. |
اتجاهات سوق خدمات التشخيص الجزيئي في منطقة آسيا والمحيط الهادئ
تطوير العلاجات وتوسيع نطاق البحث السريري في منطقة آسيا والمحيط الهادئ
- من الاتجاهات المهمة والمتسارعة في سوق خدمات التشخيص الجزيئي في منطقة آسيا والمحيط الهادئ التركيز المتزايد على الابتكارات العلاجية والبحوث السريرية، لا سيما في مجالات مثل علم الأورام والأمراض المعدية والاضطرابات الوراثية. تُمكّن الاختبارات الجزيئية المتقدمة من التشخيص المبكر واتباع أساليب علاجية أكثر استهدافًا.
- على سبيل المثال، تستثمر العديد من شركات التشخيص ومعاهد البحث في منطقة آسيا والمحيط الهادئ في تقنيات تسلسل الجيل التالي (NGS)، والاختبارات القائمة على تفاعل البوليميراز المتسلسل (PCR)، ولوحات المؤشرات الحيوية المتعددة. تهدف هذه التطورات إلى تقديم نتائج تشخيصية أسرع وأكثر دقة وفعالية من حيث التكلفة، وهي أمور بالغة الأهمية للطب الشخصي وإدارة الأمراض.
- يُمكّن الاعتماد المتزايد على نماذج الطب الدقيق في المستشفيات والعيادات التخصصية من إجراء تدخلات علاجية أكثر فعالية. تعتمد هذه النماذج على التحليل الجزيئي المتقدم ونظم المعلومات الحيوية لتوجيه اختيار العلاج، ومراقبة تطور المرض، والتنبؤ باستجابة المريض.
- وتساعد الشراكات بين شركات تكنولوجيا التشخيص ومراكز البحوث الأكاديمية والبرامج المدعومة من الحكومة أيضًا على توسيع نطاق الوصول إلى الاختبارات الجزيئية من خلال تحسين أطر السداد وتوحيد ممارسات المختبر وتعزيز تدريب الأطباء.
- مع استمرار منطقة آسيا والمحيط الهادئ في إعطاء الأولوية للرعاية الصحية الدقيقة والنتائج القائمة على القيمة، فإن سوق خدمات التشخيص الجزيئي مهيأة للنمو المستدام - مدفوعًا بالابتكار وتحسين دقة التشخيص والطلب المتزايد على الكشف المبكر والاستراتيجيات العلاجية الشخصية.
ديناميكيات سوق خدمات التشخيص الجزيئي في منطقة آسيا والمحيط الهادئ
سائق
الحاجة المتزايدة بسبب ارتفاع معدلات التشخيص والتقدم في مجال البحوث الجينية
- إن الانتشار المتزايد للأمراض المعقدة والمزمنة في منطقة آسيا والمحيط الهادئ، مدعومًا بتنامي الوعي وتحسين القدرات التشخيصية، يُسهم بشكل كبير في نمو السوق. وتعمل دول مثل الصين والهند واليابان وكوريا الجنوبية على تحسين بنيتها التحتية للرعاية الصحية وبرامجها التشخيصية، مما يُمكّن من الكشف المبكر والتدخل في الوقت المناسب لحالات مثل السرطان والأمراض المعدية والاضطرابات الوراثية.
- على سبيل المثال، في أبريل 2024، أعلنت شركة Anavex Life Sciences عن تقدم إيجابي في تجربتها السريرية للمرحلة الثالثة لدواء Anavex 2-73 (بلاركامسين)، الذي يستهدف الاضطرابات العصبية التنكسية من خلال التشخيص الدقيق والمؤشرات الحيوية الجزيئية. ومن المتوقع أن تُحفز هذه الابتكارات اعتماد التشخيص الجزيئي المتقدم، مما يُسرع نمو سوق خدمات التشخيص الجزيئي في منطقة آسيا والمحيط الهادئ خلال الفترة المتوقعة.
- إن الاهتمام المتزايد بالطب الشخصي وتوافر التقنيات الجزيئية من الجيل التالي - بما في ذلك تفاعل البوليميراز المتسلسل، وتفاعل البوليميراز المتسلسل في الوقت الحقيقي، والتسلسل من الجيل التالي - يدفع السوق إلى التحول من طرق التشخيص التقليدية إلى حلول اختبار أكثر دقة ومخصصة للمريض.
- تدعم الهيئات التنظيمية في جميع أنحاء منطقة آسيا والمحيط الهادئ، مثل وكالة الأدوية والأجهزة الطبية (PMDA) في اليابان والإدارة الوطنية للمنتجات الطبية (NMPA) في الصين، بشكل متزايد الابتكار التشخيصي من خلال الموافقات السريعة ودعم التجارب السريرية وإرشادات الامتثال المبسطة، مما يعزز الوصول السريع إلى السوق لخدمات التشخيص الجزيئي المتقدمة.
- تُعزز التعاونات بين شركات التكنولوجيا الحيوية الإقليمية ومراكز البحوث الأكاديمية وجمعيات الرعاية الصحية منظومة الابتكار في منطقة آسيا والمحيط الهادئ. تُسهم هذه الشراكات بشكل كبير في توسيع نطاق وصول المرضى إلى التشخيصات الجزيئية المتقدمة، وتوسيع نطاق مبادرات البحث السريري، وتعزيز الوعي بالكشف المبكر عن الأمراض والفحوصات الجينية بين مختلف الفئات السكانية.
ضبط النفس/التحدي
البنية التحتية المحدودة والتنوع في التبني السريري
- تشكل التكلفة العالية المرتبطة بخدمات التشخيص الجزيئي المتقدمة - بما في ذلك الأجهزة المتطورة والكواشف والتسلسل عالي الإنتاجية - عائقًا كبيرًا أمام التبني على نطاق واسع، وخاصة في المناطق الريفية أو التي تعاني من نقص التمويل.
- حتى عندما يتم دعمها بحوافز حكومية، فإن الاختبارات التشخيصية الجزيئية تنطوي عادةً على سير عمل معقدة، وموظفين متخصصين، ومراقبة جودة صارمة، مما يجعلها أقل سهولة في الوصول إليها بالنسبة لأنظمة الرعاية الصحية ذات الميزانيات المحدودة.
- علاوة على ذلك، غالبًا ما تتركز مرافق المختبرات المتخصصة والموظفين المدربين في المراكز الحضرية، مما يجبر المرضى في المناطق النائية على السفر لمسافات طويلة أو مواجهة تأخيرات في الاختبار والإبلاغ.
- من التحديات الأخرى عدم وجود بروتوكولات موحدة لبعض التحاليل الجزيئية والاختبارات الجينية. تُسهم الخبرة السريرية المحدودة وتفاوت قدرات المختبرات في عدم اتساق تبني هذه التقنيات بين مقدمي الرعاية الصحية.
- وللتغلب على هذه التحديات، فإن إصلاحات السياسات، وتعزيز التمويل الحكومي، والتعاون البحثي عبر الحدود، وإنشاء مراكز تشخيص جزيئي مخصصة في جميع أنحاء منطقة آسيا والمحيط الهادئ ستكون ضرورية لتوسيع نطاق الوصول وتحقيق النمو المستدام في سوق خدمات التشخيص الجزيئي في منطقة آسيا والمحيط الهادئ.
نطاق سوق خدمات التشخيص الجزيئي في منطقة آسيا والمحيط الهادئ
يتم تقسيم السوق على أساس نوع الخدمة والتكنولوجيا والمستخدم النهائي.
- حسب نوع الخدمة
بناءً على نوع الخدمة، يُقسّم سوق خدمات التشخيص الجزيئي في منطقة آسيا والمحيط الهادئ إلى خدمات إصلاح الأجهزة، وخدمات التدريب، وخدمات الامتثال، وخدمات المعايرة، وخدمات الصيانة، وخدمات الأتمتة القابلة للتطوير، والخدمات الجاهزة للاستخدام، وخدمات نقل الأجهزة، وتخصيص الأجهزة، وخدمات ضمان الأداء، وخدمات التصميم والتطوير، وحلول سلسلة التوريد، وخدمات طرح المنتجات الجديدة، وخدمات التصنيع، والخدمات البيئية والتنظيمية، وشهادات وتدقيق أنظمة الإدارة الطبية، وخدمات البحث السريري، والخدمات الاستشارية، وغيرها من الخدمات. هيمنت خدمات الصيانة على السوق بحصة إيرادات بلغت 23.7% في عام 2024، نظرًا لدورها الأساسي في ضمان استمرارية ودقة عمل أجهزة التشخيص الجزيئي. وتُعد هذه الخدمات بالغة الأهمية لتقليل فترات التوقف عن العمل، وإطالة عمر المعدات، والحفاظ على دقة تشخيصية ثابتة عبر شبكات المستشفيات والمختبرات.
من المتوقع أن تشهد خدمات البحث السريري أعلى معدل نمو سنوي مركب بنسبة 9.1% بين عامي 2025 و2032، مدفوعًا بالتركيز المتزايد على أبحاث الطب الانتقالي والدقيق. ويدعم هذا النمو زيادة الاستثمارات في اكتشاف المؤشرات الحيوية، وعلم الجينوم، ومبادرات الرعاية الصحية الشخصية، بالإضافة إلى توسيع التعاون بين مؤسسات البحث ومقدمي خدمات التشخيص.
- حسب التكنولوجيا
بناءً على التكنولوجيا، يُقسّم سوق خدمات التشخيص الجزيئي في منطقة آسيا والمحيط الهادئ إلى تفاعل البوليميراز المتسلسل (PCR)، وتفاعل البوليميراز المتسلسل في الوقت الفعلي (Real Time PCR)، وتسلسل الجيل التالي (NGS)، وتقنيات أخرى. وقد تصدرت خدمات تفاعل البوليميراز المتسلسل السوق بحصة بلغت 36.5% في عام 2024، بفضل اعتمادها الواسع في الاختبارات الجزيئية للأمراض المعدية، والفحوصات الجينية، وتطبيقات التشخيص الروتينية. ولا تزال تقنية تفاعل البوليميراز المتسلسل (PCR) تقنية أساسية بفضل موثوقيتها، وسعرها المناسب، وقدرتها على التكيف مع سير العمل السريري المتعدد.
من المتوقع أن ينمو تسلسل الجيل التالي (NGS) بأسرع معدل نمو سنوي مركب قدره 14.2% بين عامي 2025 و2032، مدفوعًا بالطلب المتزايد على التحليلات الجينومية عالية الإنتاجية، وعلم الأورام الدقيق، والتحليل الشامل للأمراض. يُمكّن تسلسل الجيل التالي الباحثين والأطباء من إجراء دراسات جينومية واسعة النطاق بدقة مُحسّنة، مما يدعم التشخيص المبكر، وتصنيف العلاج، والنهج العلاجية المُخصصة.
- حسب المستخدم النهائي
بناءً على المستخدم النهائي، يُقسّم سوق خدمات التشخيص الجزيئي في منطقة آسيا والمحيط الهادئ إلى مستشفيات ومراكز تشخيص ومؤسسات أكاديمية وبحثية وغيرها. واستحوذت المستشفيات على الحصة الأكبر بنسبة 41.8% في عام 2024، مدعومةً بأعداد مرضاها الكبيرة، وبنيتها التحتية المتكاملة للمختبرات، واعتمادها المستمر على منصات التشخيص الجزيئي المتقدمة. ولا تقتصر استخدامات المستشفيات لهذه الخدمات على التشخيص الروتيني فحسب، بل تشمل أيضًا دعم أقسام متخصصة مثل الأورام والأمراض المعدية والاستشارات الوراثية.
من المتوقع أن تنمو المؤسسات الأكاديمية والبحثية بأسرع معدل نمو سنوي مركب قدره 10.3% خلال فترة التوقعات، مدفوعةً بتزايد مبادرات البحث الجزيئي، والتمويل الحكومي والخاص لدراسات الجينوم والدراسات السريرية، واعتماد تقنيات التشخيص المتطورة في البحوث التطبيقية. وتلعب هذه المؤسسات دورًا محوريًا في دفع عجلة الابتكار والتحقق من صحة حلول التشخيص الجزيئي الجديدة قبل تسويقها.
تحليل إقليمي لسوق خدمات التشخيص الجزيئي في منطقة آسيا والمحيط الهادئ
- هيمنت منطقة آسيا والمحيط الهادئ على سوق خدمات التشخيص الجزيئي العالمي، محققةً أكبر حصة إيرادات بلغت 32.5% في عام 2024، مدفوعةً بتوسع البنية التحتية للرعاية الصحية في المنطقة، وتزايد انتشار الأمراض المزمنة والمعدية، والتبني السريع لتقنيات التشخيص الجزيئي المتقدمة. كما أن الاستثمارات في المرافق الطبية، وتزايد عدد مراكز التشخيص، والمبادرات الحكومية التي تُشجع على الكشف المبكر عن الأمراض، تُعزز نمو السوق.
- تُعزز الأطر التنظيمية القوية، والتغطية التأمينية الواسعة، والوعي العالي لدى المرضى النمو في قطاعي الرعاية الصحية العام والخاص. كما أن زيادة التمويل الحكومي لبرامج التشخيص، إلى جانب الشراكات بين القطاعين العام والخاص ومبادرات الرعاية الصحية لما بعد الجائحة، تُسرّع من اعتماد خدمات التشخيص الجزيئي المتقدمة.
- علاوة على ذلك، تستضيف منطقة آسيا والمحيط الهادئ العديد من مقدمي خدمات التشخيص الرائدين والمؤسسات الأكاديمية ومراكز البحث والتطوير، مما يسهل الابتكار المستمر في تطوير الاختبارات والتقييم السريري ودمج تقنيات الجيل التالي.
نظرة عامة على سوق خدمات التشخيص الجزيئي في الصين ومنطقة آسيا والمحيط الهادئ
استحوذ سوق خدمات التشخيص الجزيئي في الصين على أكبر حصة سوقية في منطقة آسيا والمحيط الهادئ بنسبة 35.1% في عام 2024، مدفوعًا بقاعدة سكانية كبيرة، وتزايد انتشار الأمراض المعدية والوراثية، وتوسيع نطاق الوصول إلى مراكز التشخيص المتخصصة. وتشجع إصلاحات الرعاية الصحية الحكومية، وزيادة التغطية التأمينية، وسياسات السداد المواتية، مقدمي الخدمات المحليين والدوليين على توسيع نطاق خدماتهم في مجال التشخيص الجزيئي. كما تستثمر الشركات المحلية بكثافة في البحث والتطوير لتلبية الطلب المتزايد على حلول التشخيص المتقدمة.
نظرة عامة على سوق خدمات التشخيص الجزيئي في منطقة آسيا والمحيط الهادئ في اليابان
استحوذ سوق خدمات التشخيص الجزيئي في اليابان على 21.5% من حصة سوق آسيا والمحيط الهادئ في عام 2024، مدعومًا ببنيته التحتية المتطورة للرعاية الصحية، وتغطية تأمينية قوية، وبيئة مختبرية متطورة تكنولوجيًا. ويعزز تزايد اعتماد تقنيات تسلسل الجيل التالي (NGS)، والاختبارات القائمة على تفاعل البوليميراز المتسلسل (PCR)، وغيرها من تقنيات التشخيص الجزيئي، لا سيما في المستشفيات ومراكز الأبحاث، الطلب في السوق. كما أن تركيز اليابان على الكشف المبكر عن الأمراض والطب الدقيق يعزز مكانتها الرائدة في المنطقة.
نظرة عامة على سوق خدمات التشخيص الجزيئي في الهند ومنطقة آسيا والمحيط الهادئ
من المتوقع أن يكون سوق خدمات التشخيص الجزيئي في الهند أسرع الأسواق نموًا في منطقة آسيا والمحيط الهادئ، بمعدل نمو سنوي مركب قدره 13.6% بين عامي 2025 و2032، مدفوعًا بتزايد الوعي الصحي، وتزايد إمكانية الوصول إلى خدمات التشخيص، وارتفاع الدخل المتاح. وتُسهم البرامج الوطنية للكشف عن الأمراض، وتوسيع البنية التحتية للتشخيص في المدن الكبرى، وتنامي مشاركة القطاع الخاص، في تسريع وتيرة تبني هذه الخدمات. كما تبرز الهند كمركز رائد للتشخيص الجزيئي منخفض التكلفة، مما يعزز تنافسيتها الإقليمية.
حصة سوق خدمات التشخيص الجزيئي في منطقة آسيا والمحيط الهادئ
وتقود صناعة خدمات التشخيص الجزيئي في منطقة آسيا والمحيط الهادئ في المقام الأول شركات راسخة، بما في ذلك:
- شركة إف. هوفمان-لا روش المحدودة (سويسرا)
- شركة داناهر (الولايات المتحدة)
- بيوميرو (فرنسا)
- شركة QIAGEN NV (هولندا)
- شركة ثيرمو فيشر العلمية (الولايات المتحدة)
- مختبرات بيو-راد، المحدودة (الولايات المتحدة)
- أبوت (الولايات المتحدة)
- دياسورين سبا (إيطاليا)
- شركة هولوجيك (الولايات المتحدة)
أحدث التطورات في سوق خدمات التشخيص الجزيئي في منطقة آسيا والمحيط الهادئ
- في أغسطس 2025 ، تزايد اعتماد شركات البحث والتطوير الصيدلانية الصينية على الكواشف المخبرية من موردين محليين، مثل شانغهاي تيتان ساينتيفيك ونانجينغ فازايم بيوتيك، لخفض التكاليف وتقصير أوقات التسليم. جاء هذا التحول استجابةً لارتفاع رسوم الاستيراد والمخاوف بشأن موثوقية سلسلة التوريد في ظل التوترات التجارية المستمرة مع الولايات المتحدة. بعد أن كانت تهيمن عليها سابقًا شركات غربية مثل ثيرمو فيشر وميرك، شهد سوق الكواشف الصيني، الذي تبلغ قيمته 5.76 مليار دولار أمريكي، تحولًا ملحوظًا نحو الشركات المحلية.
- في مارس 2025 ، أعلنت شركة Qiagen أنها ستتوقف عن إنتاج أنظمة اختبار تفاعل البوليميراز المتسلسل المتكاملة NeuMoDx 96 و288 نظرًا لتطورات السوق وتغير احتياجات العملاء بعد جائحة كوفيد-19. وبدأت الشركة مناقشات مع العملاء لفهم تأثير ذلك على مبيعات عام 2024، وستدعم العملاء الحاليين حتى عام 2025.
- في فبراير 2024 ، عرضت PlexBio تقنيتها المتقدمة للكشف عن سرطان الرئة في Medlab Dubai، مسلطة الضوء على التزامها بتوسيع قدرات التشخيص الجزيئي في علم الأورام
- في يناير 2024 ، زادت شركة Revvity، التي كانت سابقًا جزءًا من شركة PerkinElmer، استثماراتها بشكل كبير في البحث والبرمجيات والعمليات الداخلية بعد انقسامها وإعادة تسميتها. ركزت الشركة على تعزيز قطاعي علوم الحياة والتشخيص، بهدف زيادة الإنفاق على البحث والتطوير والاستثمار في الكفاءات التشغيلية، بما في ذلك منصة جديدة للتجارة الإلكترونية وتحسين سلسلة التوريد.
SKU-
세계 최초의 시장 정보 클라우드 보고서에 온라인으로 접속하세요
- 대화형 데이터 분석 대시보드
- 높은 성장 잠재력 기회를 위한 회사 분석 대시보드
- 사용자 정의 및 질의를 위한 리서치 분석가 액세스
- 대화형 대시보드를 통한 경쟁자 분석
- 최신 뉴스, 업데이트 및 추세 분석
- 포괄적인 경쟁자 추적을 위한 벤치마크 분석의 힘 활용
연구 방법론
데이터 수집 및 기준 연도 분석은 대규모 샘플 크기의 데이터 수집 모듈을 사용하여 수행됩니다. 이 단계에는 다양한 소스와 전략을 통해 시장 정보 또는 관련 데이터를 얻는 것이 포함됩니다. 여기에는 과거에 수집한 모든 데이터를 미리 검토하고 계획하는 것이 포함됩니다. 또한 다양한 정보 소스에서 발견되는 정보 불일치를 검토하는 것도 포함됩니다. 시장 데이터는 시장 통계 및 일관된 모델을 사용하여 분석하고 추정합니다. 또한 시장 점유율 분석 및 주요 추세 분석은 시장 보고서의 주요 성공 요인입니다. 자세한 내용은 분석가에게 전화를 요청하거나 문의 사항을 드롭하세요.
DBMR 연구팀에서 사용하는 주요 연구 방법론은 데이터 마이닝, 시장에 대한 데이터 변수의 영향 분석 및 주요(산업 전문가) 검증을 포함하는 데이터 삼각 측량입니다. 데이터 모델에는 공급업체 포지셔닝 그리드, 시장 타임라인 분석, 시장 개요 및 가이드, 회사 포지셔닝 그리드, 특허 분석, 가격 분석, 회사 시장 점유율 분석, 측정 기준, 글로벌 대 지역 및 공급업체 점유율 분석이 포함됩니다. 연구 방법론에 대해 자세히 알아보려면 문의를 통해 업계 전문가에게 문의하세요.
사용자 정의 가능
Data Bridge Market Research는 고급 형성 연구 분야의 선두 주자입니다. 저희는 기존 및 신규 고객에게 목표에 맞는 데이터와 분석을 제공하는 데 자부심을 느낍니다. 보고서는 추가 국가에 대한 시장 이해(국가 목록 요청), 임상 시험 결과 데이터, 문헌 검토, 재생 시장 및 제품 기반 분석을 포함하도록 사용자 정의할 수 있습니다. 기술 기반 분석에서 시장 포트폴리오 전략에 이르기까지 타겟 경쟁업체의 시장 분석을 분석할 수 있습니다. 귀하가 원하는 형식과 데이터 스타일로 필요한 만큼 많은 경쟁자를 추가할 수 있습니다. 저희 분석가 팀은 또한 원시 엑셀 파일 피벗 테이블(팩트북)로 데이터를 제공하거나 보고서에서 사용 가능한 데이터 세트에서 프레젠테이션을 만드는 데 도움을 줄 수 있습니다.