Asia Pacific Medical Device Testing Market
시장 규모 (USD 10억)
연평균 성장률 :
% 
 USD
527.22 Million
USD
1,237.03 Million
2021
2029
USD
527.22 Million
USD
1,237.03 Million
2021
2029
| 2022 –2029 | |
| USD 527.22 Million | |
| USD 1,237.03 Million | |
|
|
|
아시아 태평양 의료 기기 테스트 시장, 서비스 유형별(테스트 서비스, 검사 서비스 및 인증 서비스), 테스트 유형(물리적 테스트, 화학/생물학적 테스트, 사이버 보안 테스트, 미생물학 및 무균 테스트 및 기타), 단계(전임상 및 임상), 소싱 유형(사내 및 아웃소싱), 기기 클래스(클래스 I, 클래스 II 및 클래스 III), 제품(활성 임플란트 의료 기기, 활성 의료 기기, 비활성 의료 기기, 체외 진단 의료 기기, 안과 의료 기기, 정형외과 및 치과 의료 기기, 혈관 의료 기기 및 기타) 산업 동향 및 2029년까지의 예측
아시아 태평양 의료 기기 테스트 시장 분석 및 통찰력
의료 기기 테스트는 기기가 사용 중에 안정적이고 안전하게 수행된다는 것을 입증하는 프로세스입니다. 신제품 개발에서는 광범위한 설계 검증 테스트가 적용됩니다. 여기에는 성능 테스트, 독성, 화학 분석, 때로는 인적 요인 또는 임상 테스트가 포함됩니다. 지속적인 품질 보증 테스트는 일반적으로 더 제한적입니다. 여기에는 일반적으로 치수 검사, 일부 기능 테스트 및 패키징 검증이 포함됩니다. 검사 서비스, 인증 서비스 등과 같은 다양한 유형의 의료 테스트 서비스가 시중에 제공됩니다.
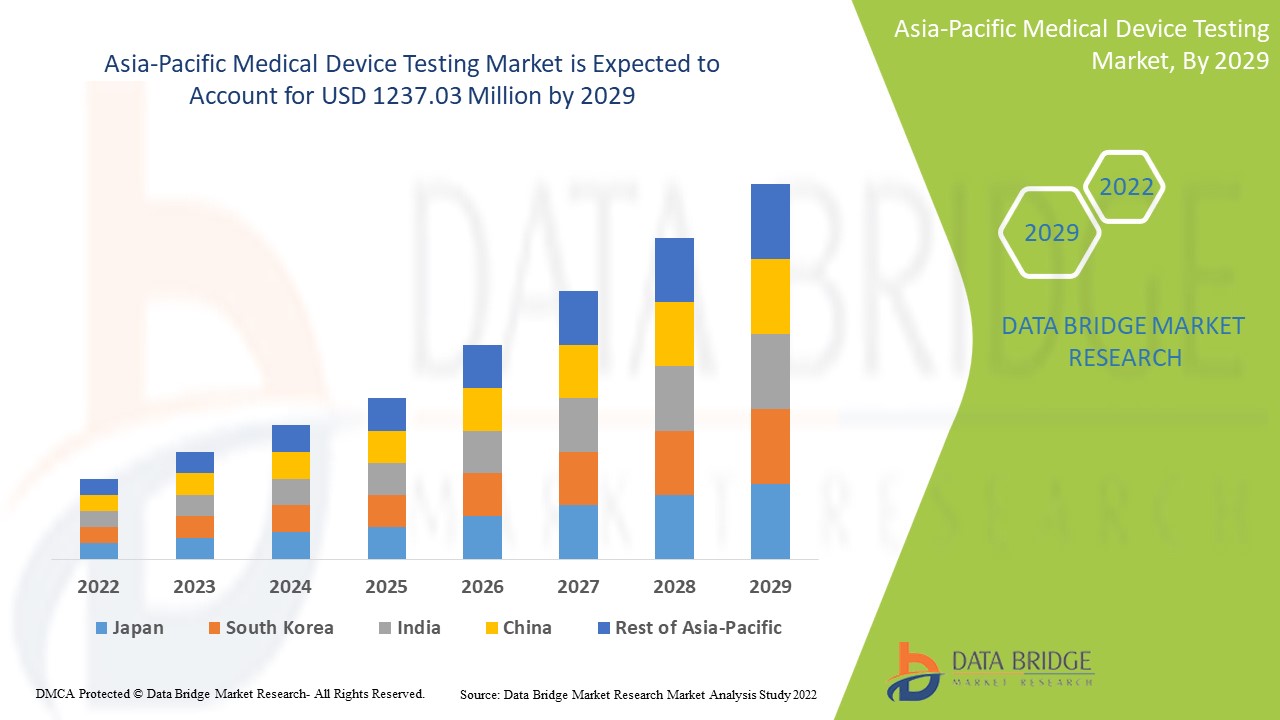
아시아 태평양 의료 기기 테스트 시장은 2022년부터 2029년까지의 예측 기간 동안 성장할 것으로 예상됩니다. Data Bridge Market Research는 시장이 2022년부터 2029년까지의 예측 기간 동안 11.7%의 CAGR로 성장하고 있으며 2021년 5억 2,722만 달러에서 2029년까지 1,237.03만 달러에 도달할 것으로 분석했습니다.
|
보고서 메트릭 |
세부 |
|
예측 기간 |
2022년부터 2029년까지 |
|
기준 연도 |
2021 |
|
역사적 연도 |
2020 (2019-2014로 사용자 정의 가능) |
|
양적 단위 |
수익은 백만 달러, 가격은 미화로 표시됨 |
|
다루는 세그먼트 |
서비스 유형별(테스트 서비스, 검사 서비스 및 인증 서비스), 테스트 유형별(물리적 테스트, 화학/생물학적 테스트, 사이버보안 테스트, 미생물학 및 무균 테스트 및 기타), 단계별(임상 전 및 임상), 소싱 유형(자체 및 아웃소싱), 기기 클래스별(클래스 I, 클래스 II 및 클래스 III), 제품별(활성 임플란트 의료 기기, 활성 의료 기기, 비활성 의료 기기, 체외 진단 의료 기기, 안과 의료 기기, 정형외과 및 치과 의료 기기, 혈관 의료 기기 및 기타) |
|
적용 국가 |
중국, 일본, 인도, 한국, 호주, 싱가포르, 태국, 말레이시아, 인도네시아, 필리핀, 아시아 태평양의 나머지 지역 |
|
시장 참여자 포함 |
Intertek Group plc, SGS SA, Bureau Veritas, TUV SUD, TUV Rheinland, Biomedical Device Labs, UL LLC, North American Science Associates, LLC, WuXi AppTec, NSF, Eurofins Scientific, Nelson Laboratories, LLC- Sotera Health company, Element Materials Technology, Medical Engineering Technologies Ltd., Bioneeds, Cigniti, Arbro Pharmaceuticals Private Limited & Auriga Research Private Limited, IMR Test Labs 등 |
시장 정의
의료 기기 테스트는 기기가 사용 중에 안정적이고 안전하게 수행된다는 것을 입증하는 프로세스입니다. 신제품 개발에서는 광범위한 설계 검증 테스트가 적용됩니다. 여기에는 성능 테스트, 독성 및 화학 분석, 때로는 인적 요소 또는 임상 테스트가 포함됩니다. 지속적인 품질 보증 테스트는 일반적으로 더 제한적입니다. 여기에는 일반적으로 치수 검사, 일부 기능 테스트 및 포장 검증이 포함됩니다. 검사 서비스, 인증 서비스 등과 같은 다양한 유형의 의료 테스트 서비스가 시중에 제공됩니다.
의료 기기 테스트 시장 동향
운전자
- 의료기기 검증 및 유효성 확인에 대한 필요성 증가
검증 및 확인 방법은 의료 산업에서 널리 사용되고 있습니다. 일반적으로 검증은 제품이 지정된 요구 사항을 준수하는지 여부를 확인하는 개발 단계인 반면, 검증은 의도된 용도가 충족되었는지, 따라서 사용성 세부 사항이 충족되는지 확인합니다. 의료 기기에 대한 가장 일반적인 검증 및 확인 유형은 설계, 프로세스 및 소프트웨어 검증 및 확인입니다. 의료 기기는 또한 점점 더 작고 설계가 복잡해지고 있으며, 때로는 고급 엔지니어링 플라스틱을 사용합니다. 이로 인해 검증 및 확인(V&V)이 더욱 중요해집니다. 그 결과 반복성이 향상되고 실수가 줄고 재작업 및 재설계가 줄어들고 시장 출시 시간이 빨라지고 경쟁력이 향상되고 생산 비용이 낮아집니다.
- 체외검사 수요 증가
체외 진단(IVD)은 인체에서 채취한 혈액이나 조직과 같은 샘플에 대한 검사입니다. 체외 진단은 질병이나 기타 상태를 감지할 수 있으며 질병을 치료, 치료 또는 예방하기 위해 개인의 전반적인 건강을 모니터링하는 데 사용할 수 있습니다. 체외 검사는 HIV 감염, 말라리아, 간염 등과 같은 다양한 질병을 감지하는 데 사용됩니다. 이러한 질병의 유병률은 전 세계적으로 빠르게 증가하고 있으며, 이로 인해 체외 검사 및 다양한 의료 기기에 대한 수요가 증가하고 있습니다.
기회
-
증가하는 의료비
다양한 국가의 사람들의 가처분 소득이 증가함에 따라 전 세계적으로 의료비가 증가했습니다. 게다가 인구 요구 사항을 충족하기 위해 정부 기관과 의료 기관은 의료비 지출을 가속화하여 주도권을 잡고 있습니다. 의료비 지출의 증가는 최근 몇 년 동안 의료 환경에서 의료 기기 테스트 서비스를 개선하는 데 도움이 됩니다.
또한, 주요 시장 참여자들이 취하는 전략적 이니셔티브는 2022~2029년 예측 기간 동안 의료 기기 테스트 시장에 구조적 무결성과 미래 기회를 제공할 것입니다.
제지/도전
- 의료기기 국산화의 장벽
그러나 일부 지역에서 의료 기기의 국내 개발에 대한 장벽과 의료 기기의 높은 비용은 의료 기기의 생산 감소를 방해하여 시장 성장을 저해할 수 있습니다. 또한 의료 기술 산업의 치열한 경쟁과 해외 자격에 대한 긴 리드타임은 시장 개발에 도전적인 요소가 될 수 있습니다.
이 의료 기기 테스트 시장 보고서는 최근의 새로운 개발, 무역 규정, 수출입 분석, 생산 분석, 가치 사슬 최적화, 시장 점유율, 국내 및 지역 시장 참여자의 영향, 새로운 수익 주머니, 시장 규정의 변화, 전략적 시장 성장 분석, 시장 규모, 범주 시장 성장, 응용 분야 틈새 시장 및 지배력, 제품 승인, 제품 출시, 지리적 확장, 시장의 기술 혁신에 대한 분석 기회를 제공합니다. 운동 실조증 시장에 대한 자세한 정보를 얻으려면 Data Bridge Market Research에 연락하여 분석가 브리핑을 받으세요. 저희 팀은 시장 성장을 달성하기 위한 정보에 입각한 시장 결정을 내리는 데 도움을 드립니다.
COVID-19 이후 의료 기기 테스트 시장에 미치는 영향
COVID-19는 시장에 긍정적인 영향을 미쳤습니다. MRI 스캐너, 인공호흡기 등과 같은 의료 기기의 사용이 그 해에 증가했습니다. 따라서 다양한 기기의 사용이 전 세계적으로 널리 증가했습니다. 따라서 팬데믹은 이 테스트 시장에 긍정적인 영향을 미쳤습니다.
최근 개발
- 2021년 4월, TÜV SÜD는 Medtec LIVE에 참가하여 의료 기기 시험의 원스톱 숍이 될 수 있는 역량을 선보였다고 발표했습니다. 이 서비스에는 전기 및 기능 안전, 사이버 보안 및 소프트웨어, EMC, 생체 적합성 시험이 포함되었습니다. TÜV SÜD의 전문가들은 다양한 강연, 라이브 해킹, 엘리베이터 피치를 통해 온라인 무역 박람회 및 의회 프로그램에 참여했습니다.
아시아 태평양 의료 기기 테스트 시장 범위
아시아 태평양 의료 기기 테스트 시장은 서비스 유형, 테스트 유형, 단계, 소싱 유형, 기기 클래스 및 제품으로 세분화됩니다. 이러한 세그먼트 간의 성장은 산업의 빈약한 성장 세그먼트를 분석하고 사용자에게 핵심 시장 응용 프로그램을 식별하기 위한 전략적 결정을 내릴 수 있는 귀중한 시장 개요와 시장 통찰력을 제공하는 데 도움이 됩니다.
서비스 유형
- 테스트 서비스
- 검사 서비스
- 인증 서비스
서비스 유형을 기준으로 아시아 태평양 의료기기 시험 시장은 시험 서비스, 검사 서비스, 인증 서비스로 구분됩니다.
테스트 유형
- 물리적 테스트
- 화학/생물학적 테스트
- 사이버 보안 테스트
- 미생물학 및 무균 테스트
- 기타
테스트 유형을 기준으로 볼 때, 아시아 태평양 의료기기 테스트 시장은 물리적 테스트, 화학/생물학적 테스트, 사이버보안 테스트, 미생물학 및 무균 테스트, 기타로 구분됩니다.
단계
- 전임상
- 객관적인
아시아 태평양 의료기기 시험 시장은 단계별로 전임상과 임상으로 구분됩니다.
소싱 유형
- 아웃소싱
- 사내에서
소싱 유형을 기준으로 볼 때 아시아 태평양 의료기기 테스트 시장은 자체 테스트와 아웃소싱으로 구분됩니다.
장치 클래스
- 1학년
- 2학년
- 3학년
아시아 태평양 의료기기 시험 시장은 기기 유형을 기준으로 1등급, 2등급, 3등급으로 구분됩니다.
제품
- 능동 임플란트 의료기기
- 활성 의료 기기
- 비활성 의료 기기
- 체외진단 의료기기
- 안과 의료 기기
- 정형외과 및 치과 의료기기
- 혈관 의료 기기
- 기타
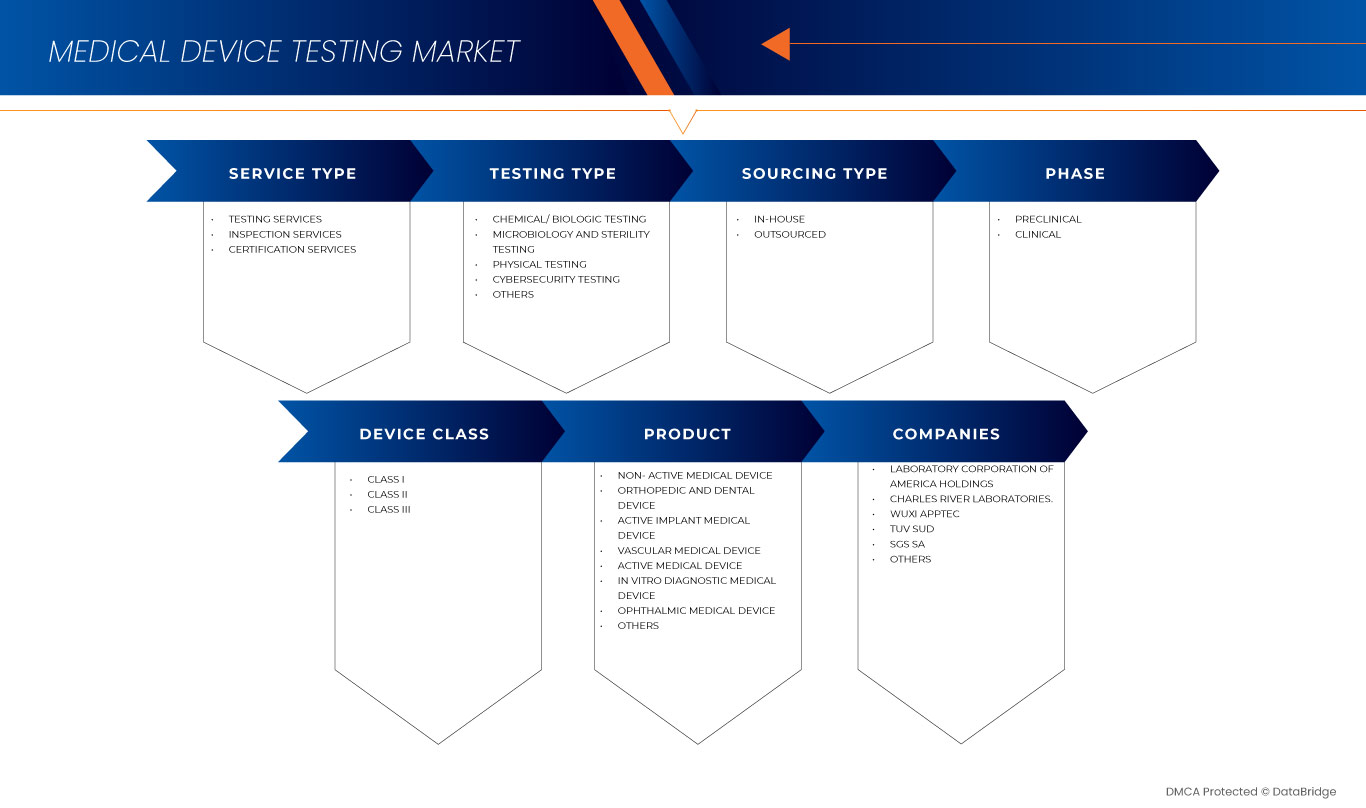
아시아 태평양 의료기기 시험 시장은 제품을 기준으로 활성 임플란트 의료기기, 활성 의료기기, 비활성 의료기기, 체외진단 의료기기, 안과 의료기기, 정형외과 및 치과 의료기기, 혈관 의료기기 및 기타로 구분됩니다.
의료 기기 테스트 시장 지역 분석/통찰력
의료 기기 테스트 시장을 분석하고, 위에 참조된 대로 국가, 서비스 유형, 테스트 유형, 단계, 조달 유형, 기기 종류 및 제품별로 시장 규모에 대한 통찰력과 추세를 제공합니다.
이 지역에 포함된 국가는 중국, 인도, 일본, 호주, 한국, 싱가포르, 인도네시아, 태국, 말레이시아, 필리핀 및 기타 아시아 태평양 국가입니다.
중국은 시장 점유율과 시장 수익 측면에서 의료 기기 테스트 시장을 지배하고 있으며 예측 기간 동안 지배력을 계속 확대할 것입니다. 이는 의료 기기에 대한 국가의 혁신과 기술의 확대 때문입니다.
보고서의 국가 섹션은 또한 현재 및 미래 시장 추세에 영향을 미치는 개별 시장 영향 요인과 시장 규정의 변화를 제공합니다. 신규 및 교체 판매, 국가 인구 통계, 질병 역학 및 수출입 관세와 같은 데이터 포인트는 개별 국가의 시장 시나리오를 예측하는 데 사용되는 주요 포인터 중 일부입니다. 또한 글로벌 브랜드의 존재 및 가용성과 국내 및 국내 브랜드와의 치열한 경쟁으로 인해 직면한 과제, 판매 채널의 영향이 국가 데이터에 대한 예측 분석을 제공하는 동안 고려됩니다.
경쟁 환경 및 의료 기기 테스트 시장 점유율 분석
의료 기기 테스트 시장 경쟁 구도는 경쟁업체의 세부 정보를 제공합니다. 포함된 세부 정보는 회사 개요, 회사 재무, 창출된 수익, 시장 잠재력, 연구 개발 투자, 새로운 시장 이니셔티브, 글로벌 입지, 생산 현장 및 시설, 생산 용량, 회사의 강점과 약점, 제품 출시, 제품 폭과 범위, 응용 분야 우세입니다. 위의 데이터 포인트는 회사의 의료 기기 테스트에 대한 집중과만 관련이 있습니다.
의료기기 테스트 시장의 주요 기업으로는 Intertek Group plc, SGS SA, Bureau Veritas, TUV SUD, TUV Rheinland, Biomedical Device Labs, UL LLC, North American Science Associates, LLC, WuXi AppTec, NSF, Eurofins Scientific, Nelson Laboratories, LLC- A Sotera Health company, Element Materials Technology, Medical Engineering Technologies Ltd., Bioneeds, Cigniti, Arbro Pharmaceuticals Private Limited & Auriga Research Private Limited, IMR Test Labs 등이 있습니다.
연구 방법론
데이터 수집 및 기준 연도 분석은 대규모 샘플 크기의 데이터 수집 모듈을 사용하여 수행됩니다. 시장 데이터는 시장 통계 및 일관된 모델을 사용하여 분석 및 추정됩니다. 또한 시장 점유율 분석 및 주요 추세 분석은 시장 보고서의 주요 성공 요인입니다. DBMR 연구팀에서 사용하는 주요 연구 방법론은 데이터 마이닝, 시장에 대한 데이터 변수의 영향 분석 및 기본(산업 전문가) 검증을 포함하는 데이터 삼각 측량입니다. 이 외에도 데이터 모델에는 공급업체 포지셔닝 그리드, 시장 타임라인 분석, 시장 개요 및 가이드, 회사 포지셔닝 그리드, 회사 시장 점유율 분석, 측정 표준, 아시아 태평양 대 지역 및 공급업체 점유율 분석이 포함됩니다. 추가 문의 사항이 있는 경우 분석가에게 전화를 요청하십시오.
SKU-
세계 최초의 시장 정보 클라우드 보고서에 온라인으로 접속하세요
- 대화형 데이터 분석 대시보드
- 높은 성장 잠재력 기회를 위한 회사 분석 대시보드
- 사용자 정의 및 질의를 위한 리서치 분석가 액세스
- 대화형 대시보드를 통한 경쟁자 분석
- 최신 뉴스, 업데이트 및 추세 분석
- 포괄적인 경쟁자 추적을 위한 벤치마크 분석의 힘 활용
목차
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF ASIA PACIFIC MEDICAL DEVICE TESTING MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 SERVICE TYPE LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 MARKET TESTING TYPE COVERAGE GRID
2.11 VENDOR SHARE ANALYSIS
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL ANALYSIS
4.2 PORTER'S FIVE FORCES
5 ASIA PACIFIC MEDICAL DEVICE TESTING MARKET: REGULATIONS
6 MARKET OVERVIEW
6.1 DRIVERS
6.1.1 RISING NEED FOR VERIFICATION VALIDATION OF MEDICAL DEVICES
6.1.2 INCREASING DEMAND FOR IN-VITRO TESTS
6.1.3 ESCALATION IN INNOVATION AND TECHNOLOGIES
6.2 RESTRAINTS
6.2.1 BARRIERS TO THE LOCAL DEVELOPMENT OF MEDICAL DEVICES
6.2.2 HIGH COST OF MEDICAL DEVICES
6.3 OPPORTUNITIES
6.3.1 RISE IN HEALTHCARE EXPENDITURE
6.3.2 DEVELOPMENT IN AI AND IOT IN VARIOUS MEDICAL DEVICES
6.3.3 STRATEGIC INITIATIVES OF KEY PLAYERS
6.4 CHALLENGES
6.4.1 HIGH COMPETITION IN MEDICAL TECHNOLOGY INDUSTRY
6.4.2 LONG LEAD TIME FOR OVERSEAS QUALIFICATION
7 ASIA PACIFIC MEDICAL DEVICE TESTING MARKET, BY SERVICE TYPE
7.1 OVERVIEW
7.2 TESTING SERVICES
7.3 INSPECTION SERVICES
7.4 CERTIFICATION SERVICES
8 ASIA PACIFIC MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE
8.1 OVERVIEW
8.2 CHEMICAL/BIOLOGICAL TESTING
8.3 MICROBIOLOGY AND STERILITY TESTING
8.3.1 STERILITY TEST & VALIDATION
8.3.2 BIO BURDEN DETERMINATION
8.3.3 ANTIMICROBIAL ACTIVITY TESTING
8.3.4 PYROGEN & ENDOTOXIN TESTING
8.3.5 OTHERS
8.4 PHYSICAL TESTING
8.4.1 ELECTRICAL SAFETY TESTING
8.4.2 FUNCTIONAL SAFETY TESTING
8.4.3 EMC TESTING
8.4.4 ENVIRONMENTAL TESTING
8.4.5 OTHERS
8.5 CYBERSECURITY TESTING
8.6 OTHERS
9 ASIA PACIFIC MEDICAL DEVICE TESTING MARKET, BY PHASE
9.1 OVERVIEW
9.2 PRECLINICAL
9.3 CLINICAL
10 ASIA PACIFIC MEDICAL DEVICE TESTING MARKET, BY SOURCING TYPE
10.1 OVERVIEW
10.2 OUTSOURCED
10.3 IN-HOUSE
11 ASIA PACIFIC MEDICAL DEVICE TESTING MARKET, BY DEVICE CLASS
11.1 OVERVIEW
11.2 CLASS I
11.3 CLASS III
11.4 CLASS II
12 ASIA PACIFIC MEDICAL DEVICE TESTING MARKET, BY PRODUCT
12.1 OVERVIEW
12.2 NON-ACTIVE MEDICAL DEVICE
12.3 ORTHOPEDIC AND DENTAL MEDICAL DEVICE
12.4 ACTIVE IMPLANT MEDICAL DEVICE
12.5 VASCULAR MEDICAL DEVICE
12.6 ACTIVE MEDICAL DEVICE
12.7 IN-VITRO DIAGNOSTICS MEDICAL DEVICE
12.8 OPTHALMIC MEDICAL DEVICE
12.9 OTHERS
13 ASIA PACIFIC MEDICAL DEVICE TESTING MARKET, BY GEOGRAPHY
13.1 ASIA-PACIFIC
13.1.1 CHINA
13.1.2 JAPAN
13.1.3 INDIA
13.1.4 SOUTH KOREA
13.1.5 AUSTRALIA
13.1.6 INDONESIA
13.1.7 THAILAND
13.1.8 PHILIPPINES
13.1.9 MALAYSIA
13.1.10 SINGAPORE
13.1.11 REST OF ASIA-PACIFIC
14 ASIA PACIFIC MEDICAL DEVICE TESTING MARKET: COMPANY LANDSCAPE
14.1 COMPANY SHARE ANALYSIS: ASIA PACIFIC
15 SWOT ANALYSIS
16 COMPANY PROFILE
16.1 LABCORP
16.1.1 COMPANY SNAPSHOT
16.1.2 REVENUE ANALYSIS
16.1.3 COMPANY SHARE ANALYSIS
16.1.4 PRODUCT PORTFOLIO
16.1.5 RECENT DEVELOPMENTS
16.2 CHARLES RIVER LABORATORIES.
16.2.1 COMPANY SNAPSHOT
16.2.2 REVENUE ANALYSIS
16.2.3 COMPANY SHARE ANALYSIS
16.2.4 PRODUCT PORTFOLIO
16.2.5 RECENT DEVELOPMENTS
16.3 TUV SUD
16.3.1 COMPANY SNAPSHOT
16.3.2 REVENUE ANALYSIS
16.3.3 COMPANY SHARE ANALYSIS
16.3.4 PRODUCT PORTFOLIO
16.3.5 RECENT DEVELOPMENTS
16.4 WUXI APPTEC
16.4.1 COMPANY SNAPSHOT
16.4.2 REVENUE ANALYSIS
16.4.3 COMPANY SHARE ANALYSIS
16.4.4 PRODUCT PORTFOLIO
16.4.5 RECENT DEVELOPMENTS
16.5 SGS SA
16.5.1 COMPANY SNAPSHOT
16.5.2 REVENUE ANALYSIS
16.5.3 COMPANY SHARE ANALYSIS
16.5.4 PRODUCT PORTFOLIO
16.5.5 RECENT DEVELOPMENTS
16.6 NORTH AMERICAN SCIENCE ASSOCIATES, LLC
16.6.1 COMPANY SNAPSHOT
16.6.2 PRODUCT PORTFOLIO
16.6.3 RECENT DEVELOPMENTS
16.7 HOHENSTEIN
16.7.1 COMPANY SNAPSHOT
16.7.2 PRODUCT PORTFOLIO
16.7.3 RECENT DEVELOPMENTS
16.8 ARBRO PHARMACEUTICALS PRIVATE LIMITED & AURIGA RESEARCH PRIVATE LIMITED
16.8.1 COMPANY SNAPSHOT
16.8.2 PRODUCT PORTFOLIO
16.8.3 RECENT DEVELOPMENTS
16.9 BIOMEDICAL DEVICE LABS
16.9.1 COMPANY SNAPSHOT
16.9.2 PRODUCT PORTFOLIO
16.9.3 RECENT DEVELOPMENTS
16.1 BIONEEDS
16.10.1 COMPANY SNAPSHOT
16.10.2 PRODUCT PORTFOLIO
16.10.3 RECENT DEVELOPMENTS
16.11 BUREAU VERITAS
16.11.1 COMPANY SNAPSHOT
16.11.2 REVENUE ANALYSIS
16.11.3 PRODUCT PORTFOLIO
16.11.4 RECENT DEVELOPMENTS
16.12 CIGNITI
16.12.1 COMPANY SNAPSHOT
16.12.2 REVENUE ANALYSIS
16.12.3 PRODUCT PORTFOLIO
16.12.4 RECENT DEVELOPMENTS
16.13 ELEMENT MATERIALS TECHNOLOGY
16.13.1 COMPANY SNAPSHOT
16.13.2 PRODUCT PORTFOLIO
16.13.3 RECENT DEVELOPMENTS
16.14 ENDOLAB MECHANICAL ENGINEERING GMBH
16.14.1 COMPANY SNAPSHOT
16.14.2 PRODUCT PORTFOLIO
16.14.3 RECENT DEVELOPMENTS
16.15 EUROFINS SCIENTIFIC
16.15.1 COMPANY SNAPSHOT
16.15.2 REVENUE ANALYSIS
16.15.3 PRODUCT PORTFOLIO
16.15.4 RECENT DEVELOPMENTS
16.16 GATEWAY ANALYTICAL.
16.16.1 COMPANY SNAPSHOT
16.16.2 PRODUCT PORTFOLIO
16.16.3 RECENT DEVELOPMENTS
16.17 IMR TEST LABS
16.17.1 COMPANY SNAPSHOT
16.17.2 PRODUCT PORTFOLIO
16.17.3 RECENT DEVELOPMENTS
16.18 INTERTEK GROUP PLC
16.18.1 COMPANY SNAPSHOT
16.18.2 REVENUE ANALYSIS
16.18.3 PRODUCT PORTFOLIO
16.18.4 RECENT DEVELOPMENTS
16.19 ITC ZLIN
16.19.1 COMPANY SNAPSHOT
16.19.2 PRODUCT PORTFOLIO
16.19.3 RECENT DEVELOPMENTS
16.2 MEDICAL ENGINEERING TECHNOLOGIES LTD.
16.20.1 COMPANY SNAPSHOT
16.20.2 PRODUCT PORTFOLIO
16.20.3 RECENT DEVELOPMENTS
16.21 MEDISTRI SA
16.21.1 COMPANY SNAPSHOT
16.21.2 PRODUCT PORTFOLIO
16.21.3 RECENT DEVELOPMENTS
16.22 NELSON LABORATORIES, LLC- A SOTERA HEALTH COMPANY
16.22.1 COMPANY SNAPSHOT
16.22.2 REVENUE ANALYSIS
16.22.3 PRODUCT PORTFOLIO
16.22.4 RECENT DEVELOPMENTS
16.23 NSF.
16.23.1 COMPANY SNAPSHOT
16.23.2 PRODUCT PORTFOLIO
16.23.3 RECENT DEVELOPMENTS
16.24 PACE
16.24.1 COMPANY SNAPSHOT
16.24.2 PRODUCT PORTFOLIO
16.24.3 RECENT DEVELOPMENTS
16.25 Q LABORATORIES
16.25.1 COMPANY SNAPSHOT
16.25.2 PRODUCT PORTFOLIO
16.25.3 RECENT DEVELOPMENTS
16.26 TUV RHEINLAND
16.26.1 COMPANY SNAPSHOT
16.26.2 REVENUE ANALYSIS
16.26.3 PRODUCT PORTFOLIO
16.26.4 RECENT DEVELOPMENTS
16.27 UL LLC
16.27.1 COMPANY SNAPSHOT
16.27.2 PRODUCT PORTFOLIO
16.27.3 RECENT DEVELOPMENTS
17 QUESTIONNAIRE
18 RELATED REPORTS
표 목록
TABLE 1 FDA REGULATIONS BASED ON DEVICES TYPE
TABLE 2 PRICES OF ESSENTIAL MEDICAL DEVICES
TABLE 3 ASIA PACIFIC MEDICAL DEVICE TESTING MARKET, BY SERVICE TYPE, 2020-2029 (USD MILLION)
TABLE 4 ASIA PACIFIC TESTING SERVICES IN MEDICAL DEVICE TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 5 ASIA PACIFIC INSPECTION SERVICES IN MEDICAL DEVICE TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 6 ASIA PACIFIC CERTIFICATION SERVICES IN MEDICAL DEVICE TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 7 ASIA PACIFIC MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2020-2029 (USD MILLION)
TABLE 8 ASIA PACIFIC CHEMICAL/BIOLOGICAL TESTING IN MEDICAL DEVICE TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 9 ASIA PACIFIC MICROBIOLOGY AND STERILITY TESTING IN MEDICAL DEVICE TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 10 ASIA PACIFIC MICROBIOLOGY AND STERILITY TESTING IN MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2020-2029 (USD MILLION)
TABLE 11 ASIA PACIFIC PHYSICAL TESTING IN MEDICAL DEVICE TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 12 ASIA PACIFIC PHYSICAL TESTING IN MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2020-2029 (USD MILLION)
TABLE 13 ASIA PACIFIC CYBERSECURITY TESTING IN MEDICAL DEVICE TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 14 ASIA PACIFIC OTHERS IN MEDICAL DEVICE TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 15 ASIA PACIFIC MEDICAL DEVICE TESTING MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 16 ASIA PACIFIC PRECLINICAL IN MEDICAL DEVICE TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 17 ASIA PACIFIC CLINICAL IN MEDICAL DEVICE TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 18 ASIA PACIFIC MEDICAL DEVICE TESTING MARKET, BY SOURCING TYPE, 2020-2029 (USD MILLION)
TABLE 19 ASIA PACIFIC OUTSOURCED IN MEDICAL DEVICE TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 20 ASIA PACIFIC IN-HOUSE IN MEDICAL DEVICE TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 21 ASIA PACIFIC MEDICAL DEVICE TESTING MARKET, BY DEVICE CLASS, 2020-2029 (USD MILLION)
TABLE 22 ASIA PACIFIC CLASS I IN MEDICAL DEVICE TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 23 ASIA PACIFIC CLASS III IN MEDICAL DEVICE TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 24 ASIA PACIFIC CLASS II IN MEDICAL DEVICE TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 25 ASIA PACIFIC MEDICAL DEVICE TESTING MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 26 ASIA PACIFIC NON-ACTIVE MEDICAL DEVICE IN MEDICAL DEVICE TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 27 ASIA PACIFIC ORTHOPEDIC AND DENTAL MEDICAL DEVICE IN MEDICAL DEVICE TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 28 ASIA PACIFIC ACTIVE IMPLANT MEDICAL DEVICE IN MEDICAL DEVICE TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 29 ASIA PACIFIC VASCULAR MEDICAL DEVICE IN MEDICAL DEVICE TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 30 ASIA PACIFIC ACTIVE MEDICAL DEVICE IN MEDICAL DEVICE TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 31 ASIA PACIFIC IN-VITRO MEDICAL DEVICE IN MEDICAL DEVICE TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 32 ASIA PACIFIC OPTHALMIC MEDICAL DEVICE IN MEDICAL DEVICE TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 33 ASIA PACIFIC OTHERS IN MEDICAL DEVICE TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 34 ASIA-PACIFIC MEDICAL DEVICE TESTING MARKET, BY COUNTRY, 2020-2029 (USD MILLION)
TABLE 35 ASIA-PACIFIC MEDICAL DEVICE TESTING MARKET, BY SERVICE TYPE, 2020-2029 (USD MILLION)
TABLE 36 ASIA-PACIFIC MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2020-2029 (USD MILLION)
TABLE 37 ASIA-PACIFIC MICROBIOLOGY AND STERILITY TESTING IN MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2020-2029 (USD MILLION)
TABLE 38 ASIA-PACIFIC PHYSICAL TESTING IN MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2020-2029 (USD MILLION)
TABLE 39 ASIA-PACIFIC MEDICAL DEVICE TESTING MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 40 ASIA-PACIFIC MEDICAL DEVICE TESTING MARKET, BY SOURCING TYPE, 2020-2029 (USD MILLION)
TABLE 41 ASIA-PACIFIC MEDICAL DEVICE TESTING MARKET, BY DEVICE CLASS, 2020-2029 (USD MILLION)
TABLE 42 ASIA-PACIFIC MEDICAL DEVICE TESTING MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 43 CHINA MEDICAL DEVICE TESTING MARKET, BY SERVICE TYPE, 2020-2029 (USD MILLION)
TABLE 44 CHINA MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2020-2029 (USD MILLION)
TABLE 45 CHINA MICROBIOLOGY AND STERILITY TESTING IN MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2020-2029 (USD MILLION)
TABLE 46 CHINA PHYSICAL TESTING IN MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2020-2029 (USD MILLION)
TABLE 47 CHINA MEDICAL DEVICE TESTING MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 48 CHINA MEDICAL DEVICE TESTING MARKET, BY SOURCING TYPE, 2020-2029 (USD MILLION)
TABLE 49 CHINA MEDICAL DEVICE TESTING MARKET, BY DEVICE CLASS, 2020-2029 (USD MILLION)
TABLE 50 CHINA MEDICAL DEVICE TESTING MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 51 JAPAN MEDICAL DEVICE TESTING MARKET, BY SERVICE TYPE, 2020-2029 (USD MILLION)
TABLE 52 JAPAN MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2020-2029 (USD MILLION)
TABLE 53 JAPAN MICROBIOLOGY AND STERILITY TESTING IN MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2020-2029 (USD MILLION)
TABLE 54 JAPAN PHYSICAL TESTING IN MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2020-2029 (USD MILLION)
TABLE 55 JAPAN MEDICAL DEVICE TESTING MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 56 JAPAN MEDICAL DEVICE TESTING MARKET, BY SOURCING TYPE, 2020-2029 (USD MILLION)
TABLE 57 JAPAN MEDICAL DEVICE TESTING MARKET, BY DEVICE CLASS, 2020-2029 (USD MILLION)
TABLE 58 JAPAN MEDICAL DEVICE TESTING MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 59 INDIA MEDICAL DEVICE TESTING MARKET, BY SERVICE TYPE, 2020-2029 (USD MILLION)
TABLE 60 INDIA MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2020-2029 (USD MILLION)
TABLE 61 INDIA MICROBIOLOGY AND STERILITY TESTING IN MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2020-2029 (USD MILLION)
TABLE 62 INDIA PHYSICAL TESTING IN MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2020-2029 (USD MILLION)
TABLE 63 INDIA MEDICAL DEVICE TESTING MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 64 INDIA MEDICAL DEVICE TESTING MARKET, BY SOURCING TYPE, 2020-2029 (USD MILLION)
TABLE 65 INDIA MEDICAL DEVICE TESTING MARKET, BY DEVICE CLASS, 2020-2029 (USD MILLION)
TABLE 66 INDIA MEDICAL DEVICE TESTING MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 67 SOUTH KOREA MEDICAL DEVICE TESTING MARKET, BY SERVICE TYPE, 2020-2029 (USD MILLION)
TABLE 68 SOUTH KOREA MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2020-2029 (USD MILLION)
TABLE 69 SOUTH KOREA MICROBIOLOGY AND STERILITY TESTING IN MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2020-2029 (USD MILLION)
TABLE 70 SOUTH KOREA PHYSICAL TESTING IN MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2020-2029 (USD MILLION)
TABLE 71 SOUTH KOREA MEDICAL DEVICE TESTING MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 72 SOUTH KOREA MEDICAL DEVICE TESTING MARKET, BY SOURCING TYPE, 2020-2029 (USD MILLION)
TABLE 73 SOUTH KOREA MEDICAL DEVICE TESTING MARKET, BY DEVICE CLASS, 2020-2029 (USD MILLION)
TABLE 74 SOUTH KOREA MEDICAL DEVICE TESTING MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 75 AUSTRALIA MEDICAL DEVICE TESTING MARKET, BY SERVICE TYPE, 2020-2029 (USD MILLION)
TABLE 76 AUSTRALIA MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2020-2029 (USD MILLION)
TABLE 77 AUSTRALIA MICROBIOLOGY AND STERILITY TESTING IN MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2020-2029 (USD MILLION)
TABLE 78 AUSTRALIA PHYSICAL TESTING IN MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2020-2029 (USD MILLION)
TABLE 79 AUSTRALIA MEDICAL DEVICE TESTING MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 80 AUSTRALIA MEDICAL DEVICE TESTING MARKET, BY SOURCING TYPE, 2020-2029 (USD MILLION)
TABLE 81 AUSTRALIA MEDICAL DEVICE TESTING MARKET, BY DEVICE CLASS, 2020-2029 (USD MILLION)
TABLE 82 AUSTRALIA MEDICAL DEVICE TESTING MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 83 INDONESIA MEDICAL DEVICE TESTING MARKET, BY SERVICE TYPE, 2020-2029 (USD MILLION)
TABLE 84 INDONESIA MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2020-2029 (USD MILLION)
TABLE 85 INDONESIA MICROBIOLOGY AND STERILITY TESTING IN MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2020-2029 (USD MILLION)
TABLE 86 INDONESIA PHYSICAL TESTING IN MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2020-2029 (USD MILLION)
TABLE 87 INDONESIA MEDICAL DEVICE TESTING MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 88 INDONESIA MEDICAL DEVICE TESTING MARKET, BY SOURCING TYPE, 2020-2029 (USD MILLION)
TABLE 89 INDONESIA MEDICAL DEVICE TESTING MARKET, BY DEVICE CLASS, 2020-2029 (USD MILLION)
TABLE 90 INDONESIA MEDICAL DEVICE TESTING MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 91 THAILAND MEDICAL DEVICE TESTING MARKET, BY SERVICE TYPE, 2020-2029 (USD MILLION)
TABLE 92 THAILAND MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2020-2029 (USD MILLION)
TABLE 93 THAILAND MICROBIOLOGY AND STERILITY TESTING IN MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2020-2029 (USD MILLION)
TABLE 94 THAILAND PHYSICAL TESTING IN MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2020-2029 (USD MILLION)
TABLE 95 THAILAND MEDICAL DEVICE TESTING MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 96 THAILAND MEDICAL DEVICE TESTING MARKET, BY SOURCING TYPE, 2020-2029 (USD MILLION)
TABLE 97 THAILAND MEDICAL DEVICE TESTING MARKET, BY DEVICE CLASS, 2020-2029 (USD MILLION)
TABLE 98 THAILAND MEDICAL DEVICE TESTING MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 99 PHILIPPINES MEDICAL DEVICE TESTING MARKET, BY SERVICE TYPE, 2020-2029 (USD MILLION)
TABLE 100 PHILIPPINES MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2020-2029 (USD MILLION)
TABLE 101 PHILIPPINES MICROBIOLOGY AND STERILITY TESTING IN MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2020-2029 (USD MILLION)
TABLE 102 PHILIPPINES PHYSICAL TESTING IN MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2020-2029 (USD MILLION)
TABLE 103 PHILIPPINES MEDICAL DEVICE TESTING MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 104 PHILIPPINES MEDICAL DEVICE TESTING MARKET, BY SOURCING TYPE, 2020-2029 (USD MILLION)
TABLE 105 PHILIPPINES MEDICAL DEVICE TESTING MARKET, BY DEVICE CLASS, 2020-2029 (USD MILLION)
TABLE 106 PHILIPPINES MEDICAL DEVICE TESTING MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 107 MALAYSIA MEDICAL DEVICE TESTING MARKET, BY SERVICE TYPE, 2020-2029 (USD MILLION)
TABLE 108 MALAYSIA MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2020-2029 (USD MILLION)
TABLE 109 MALAYSIA MICROBIOLOGY AND STERILITY TESTING IN MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2020-2029 (USD MILLION)
TABLE 110 MALAYSIA PHYSICAL TESTING IN MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2020-2029 (USD MILLION)
TABLE 111 MALAYSIA MEDICAL DEVICE TESTING MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 112 MALAYSIA MEDICAL DEVICE TESTING MARKET, BY SOURCING TYPE, 2020-2029 (USD MILLION)
TABLE 113 MALAYSIA MEDICAL DEVICE TESTING MARKET, BY DEVICE CLASS, 2020-2029 (USD MILLION)
TABLE 114 MALAYSIA MEDICAL DEVICE TESTING MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 115 SINGAPORE MEDICAL DEVICE TESTING MARKET, BY SERVICE TYPE, 2020-2029 (USD MILLION)
TABLE 116 SINGAPORE MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2020-2029 (USD MILLION)
TABLE 117 SINGAPORE MICROBIOLOGY AND STERILITY TESTING IN MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2020-2029 (USD MILLION)
TABLE 118 SINGAPORE PHYSICAL TESTING IN MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2020-2029 (USD MILLION)
TABLE 119 SINGAPORE MEDICAL DEVICE TESTING MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 120 SINGAPORE MEDICAL DEVICE TESTING MARKET, BY SOURCING TYPE, 2020-2029 (USD MILLION)
TABLE 121 SINGAPORE MEDICAL DEVICE TESTING MARKET, BY DEVICE CLASS, 2020-2029 (USD MILLION)
TABLE 122 SINGAPORE MEDICAL DEVICE TESTING MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 123 REST OF ASIA-PACIFIC MEDICAL DEVICE TESTING MARKET, BY SERVICE TYPE, 2020-2029 (USD MILLION)
그림 목록
FIGURE 1 ASIA PACIFIC MEDICAL DEVICE TESTING MARKET: SEGMENTATION
FIGURE 2 ASIA PACIFIC MEDICAL DEVICE TESTING MARKET: DATA TRIANGULATION
FIGURE 3 ASIA PACIFIC MEDICAL DEVICE TESTING MARKET: DROC ANALYSIS
FIGURE 4 ASIA PACIFIC MEDICAL DEVICE TESTING MARKET: ASIA PACIFIC VS REGIONAL MARKET ANALYSIS
FIGURE 5 ASIA PACIFIC MEDICAL DEVICE TESTING MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 ASIA PACIFIC MEDICAL DEVICE TESTING MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 ASIA PACIFIC MEDICAL DEVICE TESTING MARKET: DBMR MARKET POSITION GRID
FIGURE 8 ASIA PACIFIC MEDICAL DEVICE TESTING MARKET: MARKET TESTING TYPE COVERAGE GRID
FIGURE 9 ASIA PACIFIC MEDICAL DEVICE TESTING MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 ASIA PACIFIC MEDICAL DEVICE TESTING MARKET: SEGMENTATION
FIGURE 11 INCREASING DEMAND FOR IN-VITRO TESTS AND DEVELOPMENT IN AI AND IOT IN VARIOUS MEDICAL DEVICES ARE EXPECTED TO DRIVE THE ASIA PACIFIC MEDICAL DEVICE TESTING MARKET IN THE FORECAST PERIOD OF 2022 TO 2029
FIGURE 12 TESTING SERVICES SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE ASIA PACIFIC MEDICAL DEVICE TESTING MARKET IN 2022 & 2029
FIGURE 13 NORTH AMERICA IS EXPECTED TO DOMINATE THE ASIA PACIFIC MEDICAL DEVICE TESTINGMARKET, AND ASIA-PACIFIC IS EXPECTED TO GROW WITH THE HIGHEST CAGR IN THE FORECAST PERIOD OF 2022 TO 2029
FIGURE 14 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF ASIA PACIFIC MEDICAL DEVICE TESTING MARKET
FIGURE 15 ASIA PACIFIC MEDICAL DEVICE TESTING MARKET: BY SERVICE TYPE, 2021
FIGURE 16 ASIA PACIFIC MEDICAL DEVICE TESTING MARKET: BY SERVICE TYPE, 2022-2029 (USD MILLION)
FIGURE 17 ASIA PACIFIC MEDICAL DEVICE TESTING MARKET: BY SERVICE TYPE, CAGR (2022-2029)
FIGURE 18 ASIA PACIFIC MEDICAL DEVICE TESTING MARKET: BY SERVICE TYPE, LIFELINE CURVE
FIGURE 19 ASIA PACIFIC MEDICAL DEVICE TESTING MARKET: BY TESTING TYPE, 2021
FIGURE 20 ASIA PACIFIC MEDICAL DEVICE TESTING MARKET: BY TESTING TYPE, 2022-2029 (USD MILLION)
FIGURE 21 ASIA PACIFIC MEDICAL DEVICE TESTING MARKET: BY TESTING TYPE, CAGR (2022-2029)
FIGURE 22 ASIA PACIFIC MEDICAL DEVICE TESTING MARKET: BY TESTING TYPE, LIFELINE CURVE
FIGURE 23 ASIA PACIFIC MEDICAL DEVICE TESTING MARKET: BY PHASE, 2021
FIGURE 24 ASIA PACIFIC MEDICAL DEVICE TESTING MARKET: BY PHASE, 2022-2029 (USD MILLION)
FIGURE 25 ASIA PACIFIC MEDICAL DEVICE TESTING MARKET: BY PHASE, CAGR (2022-2029)
FIGURE 26 ASIA PACIFIC MEDICAL DEVICE TESTING MARKET: BY PHASE, LIFELINE CURVE
FIGURE 27 ASIA PACIFIC MEDICAL DEVICE TESTING MARKET: BY SOURCING TYPE, 2021
FIGURE 28 ASIA PACIFIC MEDICAL DEVICE TESTING MARKET: BY SOURCING TYPE, 2022-2029 (USD MILLION)
FIGURE 29 ASIA PACIFIC MEDICAL DEVICE TESTING MARKET: BY SOURCING TYPE, CAGR (2022-2029)
FIGURE 30 ASIA PACIFIC MEDICAL DEVICE TESTING MARKET: BY SOURCING TYPE, LIFELINE CURVE
FIGURE 31 ASIA PACIFIC MEDICAL DEVICE TESTING MARKET: BY DEVICE CLASS, 2021
FIGURE 32 ASIA PACIFIC MEDICAL DEVICE TESTING MARKET: BY DEVICE CLASS, 2022-2029 (USD MILLION)
FIGURE 33 ASIA PACIFIC MEDICAL DEVICE TESTING MARKET: BY DEVICE CLASS, CAGR (2022-2029)
FIGURE 34 ASIA PACIFIC MEDICAL DEVICE TESTING MARKET: BY DEVICE CLASS, LIFELINE CURVE
FIGURE 35 ASIA PACIFIC MEDICAL DEVICE TESTING MARKET: BY PRODUCT, 2021
FIGURE 36 ASIA PACIFIC MEDICAL DEVICE TESTING MARKET: BY PRODUCT, 2022-2029 (USD MILLION)
FIGURE 37 ASIA PACIFIC MEDICAL DEVICE TESTING MARKET: BY PRODUCT, CAGR (2022-2029)
FIGURE 38 ASIA PACIFIC MEDICAL DEVICE TESTING MARKET: BY PRODUCT, LIFELINE CURVE
FIGURE 39 ASIA-PACIFIC MEDICAL DEVICE TESTING MARKET: SNAPSHOT (2021)
FIGURE 40 ASIA-PACIFIC MEDICAL DEVICE TESTING MARKET: BY COUNTRY (2021)
FIGURE 41 ASIA-PACIFIC MEDICAL DEVICE TESTING MARKET: BY COUNTRY (2022 & 2029)
FIGURE 42 ASIA-PACIFIC MEDICAL DEVICE TESTING MARKET: BY COUNTRY (2021 & 2029)
FIGURE 43 ASIA-PACIFIC MEDICAL DEVICE TESTING MARKET: BY SERVICE TYPE (2022-2029)
FIGURE 44 ASIA PACIFIC MEDICAL DEVICE TESTING MARKET: COMPANY SHARE 2021 (%)

연구 방법론
데이터 수집 및 기준 연도 분석은 대규모 샘플 크기의 데이터 수집 모듈을 사용하여 수행됩니다. 이 단계에는 다양한 소스와 전략을 통해 시장 정보 또는 관련 데이터를 얻는 것이 포함됩니다. 여기에는 과거에 수집한 모든 데이터를 미리 검토하고 계획하는 것이 포함됩니다. 또한 다양한 정보 소스에서 발견되는 정보 불일치를 검토하는 것도 포함됩니다. 시장 데이터는 시장 통계 및 일관된 모델을 사용하여 분석하고 추정합니다. 또한 시장 점유율 분석 및 주요 추세 분석은 시장 보고서의 주요 성공 요인입니다. 자세한 내용은 분석가에게 전화를 요청하거나 문의 사항을 드롭하세요.
DBMR 연구팀에서 사용하는 주요 연구 방법론은 데이터 마이닝, 시장에 대한 데이터 변수의 영향 분석 및 주요(산업 전문가) 검증을 포함하는 데이터 삼각 측량입니다. 데이터 모델에는 공급업체 포지셔닝 그리드, 시장 타임라인 분석, 시장 개요 및 가이드, 회사 포지셔닝 그리드, 특허 분석, 가격 분석, 회사 시장 점유율 분석, 측정 기준, 글로벌 대 지역 및 공급업체 점유율 분석이 포함됩니다. 연구 방법론에 대해 자세히 알아보려면 문의를 통해 업계 전문가에게 문의하세요.
사용자 정의 가능
Data Bridge Market Research는 고급 형성 연구 분야의 선두 주자입니다. 저희는 기존 및 신규 고객에게 목표에 맞는 데이터와 분석을 제공하는 데 자부심을 느낍니다. 보고서는 추가 국가에 대한 시장 이해(국가 목록 요청), 임상 시험 결과 데이터, 문헌 검토, 재생 시장 및 제품 기반 분석을 포함하도록 사용자 정의할 수 있습니다. 기술 기반 분석에서 시장 포트폴리오 전략에 이르기까지 타겟 경쟁업체의 시장 분석을 분석할 수 있습니다. 귀하가 원하는 형식과 데이터 스타일로 필요한 만큼 많은 경쟁자를 추가할 수 있습니다. 저희 분석가 팀은 또한 원시 엑셀 파일 피벗 테이블(팩트북)로 데이터를 제공하거나 보고서에서 사용 가능한 데이터 세트에서 프레젠테이션을 만드는 데 도움을 줄 수 있습니다.













