글로벌 유전성 암 검사 시장, 검사 유형(다중 패널 세트 및 단일 사이트 유전자 검사), 진단 유형(생체검사, 영상, 실험실 검사), 기술(시퀀싱, 중합효소 연쇄 반응 (PCR), 마이크로어레이), 질병 유형(유전성 유방암 및 난소암 증후군, 코우덴 증후군, 린치 증후군, 유전성 백혈병 및 혈액 악성 종양 증후군, 가족성 선종성 용종증(FAP), 리-프라우메니 증후군, 폰 히펠-린다우병, 다발 내분비선 종양(MEN) 증후군), 최종 사용자(병원, 진료소, 실험실, 영상의학 센터, 진단 센터, 기타), 유통 채널(직접 입찰, 소매 판매), 업계 동향 및 2029년까지의 예측.
유전성 암 검사 시장 시장 분석 및 통찰력
암은 세포의 기능을 제어하는 유전자의 특정 돌연변이로 인해 발생하는 유전적 질환으로, 특히 세포의 성장과 번식에 영향을 미칩니다. 유전된 유전자 돌연변이는 모든 암의 약 5-10%를 차지합니다. 연구자들은 특정 유전자의 돌연변이를 특정 암의 발병을 통해 사람들에게 영향을 미치는 50개 이상의 유전성 암 증후군과 연결했습니다. 또한 유방암 사례의 약 5-10%는 부모로부터 유전된 유전자 돌연변이와 관련이 있습니다. 따라서 암의 유병률이 증가함에 따라 유전성 암이 꾸준히 증가하고 있으며, 이로 인해 유전성 암 검사 시장이 성장하고 있습니다. 또한 비침습적 검사 방법에 대한 수요 증가와 더 나은 품질의 의료 및 조기 진단에 대한 수요 증가는 시장 성장의 주요 기회입니다. 더욱이 유전성 암 검사 중에 직면한 윤리적 문제와 시장 참여자 간의 경쟁 심화는 시장 성장의 주요 과제입니다.
그러나 엄격한 암 진단 규정과 검사에 따른 높은 비용은 시장 성장을 방해할 수 있습니다.
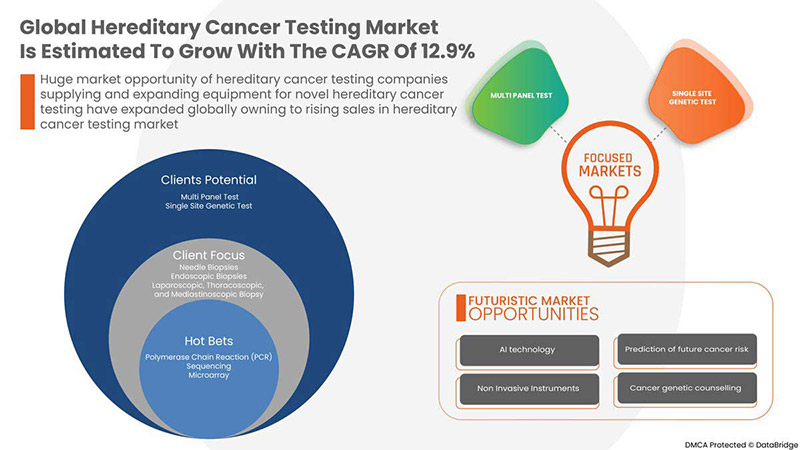
Data Bridge Market Research는 글로벌 유전성 암 검사 시장이 2029년까지 13,085.04백만 달러에 도달할 것으로 예상되며, 예측 기간 동안 CAGR은 12.9%라고 분석했습니다. 이 시장 보고서는 또한 가격 분석, 특허 분석 및 기술 발전에 대한 심층적인 내용을 다룹니다.
|
보고서 메트릭 |
세부 |
|
예측 기간 |
2022년부터 2029년까지 |
|
기준 연도 |
2021 |
|
역사적 연도 |
2020 (2019-2015까지 사용자 정의 가능) |
|
양적 단위 |
매출은 백만 달러, 볼륨은 단위, 가격은 달러로 표시 |
|
다루는 세그먼트 |
검사 유형(다중 패널 세트 및 단일 사이트 유전자 검사), 진단 유형(생체검사, 영상, 실험실 검사), 기술(시퀀싱, 중합효소 연쇄 반응(PCR), 마이크로어레이), 질병 유형(유전성 유방암 및 난소암 증후군, 코우덴 증후군, 린치 증후군, 유전성 백혈병 및 혈액 악성 종양 증후군, 가족성 선종성 용종증(FAP), 리-프라우메니 증후군, 폰 히펠-린다우병, 다발 내분비선 종양(MEN) 증후군), 최종 사용자(병원, 진료소, 실험실, 영상의학 센터, 진단 센터, 기타), 유통 채널(직접 입찰, 소매 판매)별. |
|
적용 국가 |
미국, 캐나다, 멕시코, 독일, 프랑스, 영국, 이탈리아, 스페인, 러시아, 터키, 벨기에, 네덜란드, 스위스, 기타 유럽 국가, 중국, 일본, 인도, 한국, 싱가포르, 태국, 말레이시아, 호주, 필리핀, 인도네시아, 기타 아시아 태평양 국가, 남아프리카, 사우디 아라비아, UAE, 이집트, 이스라엘, 기타 중동 및 아프리카 국가, 브라질, 아르헨티나, 기타 남미 국가. |
|
시장 참여자 포함 |
Invitae Corporation, Illumina, Inc., Natera, Inc., CENTOGENE NV, 4baseCare, Biocartis, Fulgent Genetics, Ambry Genetics, BioReference, PerkinElmer Inc., LifeLabs, Abbott, BIO-HELIX, Cepheid, Eurofins Scientific 등이 있습니다. |
글로벌 유전성 암 검사 시장 정의
유전성 암은 유전적 돌연변이로 인해 발생하는 모든 암입니다. 특정 유전자의 유해한 변이는 암 위험 증가와 관련이 있습니다. 유전자 검사를 통해 개인의 평생 암 발병 위험을 추정할 수 있습니다. 이는 유전자, 염색체 또는 단백질의 돌연변이를 찾아 수행할 수 있습니다. 유전자 검사는 여러 유형의 암에 대해 제공됩니다. 여기에는 유방암, 난소암, 대장암, 갑상선암, 전립선암, 췌장암, 피부암, 육종, 신장암 및 위암이 포함됩니다. 수많은 의학 연구에 따르면 일반적인 암의 5%~10%가 유전성으로 간주됩니다. 유전자 검사는 개인이 유해한 유전자 변이를 가지고 있는지 확인하기 위해 수행됩니다. 이러한 검사는 아직 암을 앓지 않은 가족 구성원이 암 감수성 대안이 있는 것으로 알려진 가족 구성원과 동일한 변이를 물려받았는지 확인하는 데에도 도움이 됩니다.
글로벌 유전성 암 검사 시장 역학
이 섹션에서는 시장 동인, 이점, 기회, 제약 및 과제를 이해하는 것을 다룹니다. 이 모든 내용은 아래에서 자세히 설명합니다.
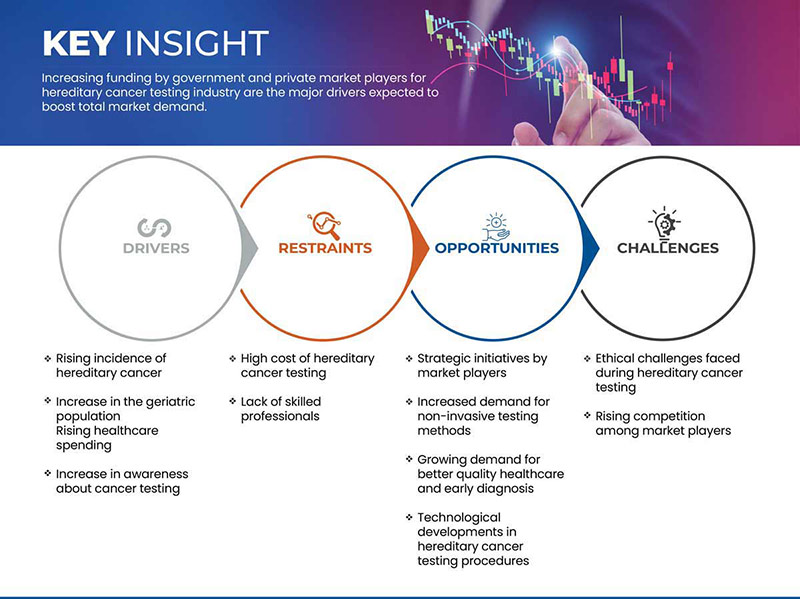
운전자
- 유전성 암 발병률 증가
암은 세포의 통제되지 않은 성장에서 발생합니다. 암은 세포의 성장과 분열을 제어하는 유전적 메시지(유전자)의 유해한 변화(돌연변이)로 인해 발생하며, 세포가 효과적으로 기능을 수행하지 못하게 합니다.
유전성 암의 경우, 개인은 한 부모로부터 돌연변이된 성장 조절 유전자의 사본을 물려받고 다른 부모로부터 동일한 유전자의 작동 사본을 물려받습니다. 돌연변이된 유전자는 "암 감수성 유전자"라고도 합니다. 이 암 감수성 유전자는 유전되므로 신체의 모든 세포에서 발견되지만, 유전자의 작동 사본은 각 세포가 제대로 기능하도록 유지합니다. 그러나 돌연변이로 인해 세포의 유전자의 기능적 사본이 손상되면 세포는 성장에 대한 통제력을 잃고 암이 될 수 있습니다. 따라서 암 유전자를 물려받은 개인은 평생 동안 특정 암을 발병할 가능성이 훨씬 더 높습니다.
따라서 유전성 암의 발생률이 증가함에 따라 유전성 암 검사에 대한 수요가 늘어나고 이는 글로벌 유전성 암 검사 시장 성장을 촉진하는 역할을 할 수 있습니다.
- 노령 인구 증가
암은 노령 환자의 질병이 될 수 있습니다. 전 세계적으로 노령 인구가 증가하고 있습니다. 노령 인구의 유전성 암 위험은 훨씬 더 큽니다. 노령 인구가 증가함에 따라 글로벌 유전성 암 검사 시장이 더 잘 공급될 수 있습니다. 글로벌 유전성 암 검사 시장의 수요가 급증할 것으로 예상되었습니다. 노령 인구는 인구 구조의 재분배를 일으키고 있으며 , 이는 의료의 미래에 영향을 미칠 것입니다. 의심할 여지 없이 암 위험은 나이가 들면서 기하급수적으로 증가합니다.
세계에서 가장 큰 완전한 가족 구조와 의학적으로 확인된 암 데이터베이스를 사용하여 유전성 암의 발병률과 관련 위험을 포함한 유전성 암은 친척이 없는 사람의 영향을 받은 부모나 형제 자매에게서 태어난 8~20세 인구에서 약 2배 더 높았습니다. 소장암, 고환암, 갑상선암 및 뼈암의 위험은 5~8배 더 높았습니다.
따라서 노령 인구의 암 발병률 증가는 글로벌 유전성 암 검사 시장 성장을 촉진하는 요인이 될 것으로 예상됩니다.
제지
- 유전성 암 검사의 높은 비용
유전성 암 검사는 기술적으로 매우 진보된 제품을 사용합니다. 이러한 제품의 개발에는 개발하는 플레이어의 엄격한 연구 및 개발이 필요합니다. 따라서 절차와 제품 비용이 높게 유지되어 검사 비용이 비례적으로 증가합니다. 검사 키트는 엄청나게 자원 집약적이고 고액의 의사, 운송 및 비싼 약물이 필요하기 때문에 비쌉니다.
- 또한, 암 검사에도 검사 절차가 사용되었습니다. 그러나 이러한 절차는 매우 비싸고 합병증과 더 나쁜 장기적 결과와 관련이 있을 수 있습니다.
따라서 첨단 방식과 기술 제품을 사용하여 암을 검사하는 데 드는 높은 비용은 글로벌 유전성 암 검사 시장 성장을 저해하는 주요 요인이 될 수 있습니다.
기회
-
시장 참여자의 전략적 이니셔티브
글로벌 유전성 암 검사 시장의 성장은 전략적 사업 아이디어에 대한 필요성을 증가시킵니다. 여기에는 파트너십, 사업 확장 및 기타 개발이 포함됩니다. 유전성 암 치료에 대한 수요의 급증은 진단 검사 방법에 대한 수요를 크게 증가시키고 있습니다. 계획된 전략을 통해 시장 참여자는 조직의 기능적 활동과 일치하여 설정된 목표를 달성할 수 있습니다. 이는 목표를 달성하기 위한 리소스 및 예산 요구 사항을 결정하는 데 있어 회사의 논의 및 의사 결정을 안내하여 운영 효율성을 높입니다.
제품 출시, 계약, 주요 시장 참여자의 사업 확장과 같은 이러한 전략적 이니셔티브는 시장 성장을 촉진하고 글로벌 유전성 암 검사 시장에 기회로 작용할 것으로 예상됩니다. 전략적 이니셔티브는 성장을 돕고 회사의 제품 포트폴리오를 개선하여 궁극적으로 더 많은 수익을 창출할 것으로 예상됩니다. 따라서 시장 참여자의 이러한 전략적 이니셔티브는 글로벌 유전성 암 검사 시장에서 성장의 기회로 작용할 것으로 예상됩니다.
도전
- 유전성 암 검사 중 직면한 윤리적 과제
유전성 암 유전자 검사 중에 중요한 윤리적 장애물 중 하나는 의료 전문가가 유전자 검사에 대한 기본 지식이 부족하고 가족성 질병 패턴을 해석하는 데 자신감이 없다는 것입니다. 의료 서비스 제공자의 과제는 환자의 의사 결정을 뒷받침할 충분한 정보와 제안할 수 있는 추론을 뒷받침할 증거를 제공하는 것입니다.
환불이 없으면 치료에 대한 경제적 장벽이 생깁니다. 유전성 암 위험 평가 및 상담 과정은 시간이 많이 걸리고, 이 서비스를 문서화하고 청구하는 가장 좋은 방법은 불분명합니다. 종양학자는 종종 제3자 지불자 간의 다양한 환불 정책으로 인해 유전자 검사에 대한 잠재적으로 불확실한 환불 환경을 탐색해야 합니다.
유전성 암에 대한 유전자 검사는 환자나 그 가족과 해결할 수 없는 윤리적 문제를 제기합니다. 윤리적, 문화적, 종교적 성격의 다양한 측면은 유전성 암 검사 행위에 대한 장벽이 되어서는 안 됩니다. 이 모든 것은 해결해야 할 문제입니다. 따라서 유전성 암 검사 중 윤리적 과제는 시장 성장에 도전할 것으로 예상됩니다.
COVID-19 이후 글로벌 유전성 암 검사 시장 에 미치는 영향
전 세계의 많은 산업이 지난 18개월 동안 불이익을 받았습니다. 이는 전 세계 시설이 따르고 있는 폐쇄 및 기타 제한과 같은 다양한 예방 조치로 인해 산업 및 공급망 프로세스가 큰 혼란을 겪고 있기 때문일 수 있습니다. 글로벌 유전성 암 검사 시장도 마찬가지입니다. 또한, 대부분의 사람들의 전반적인 재정이 붐으로 인해 심각한 영향을 받았기 때문에 사람들이 예산에서 불필요한 비용을 제외할 수 있는 기회가 더 많아지면서 소비 수요가 감소했습니다. 앞서 언급한 이러한 요인은 예측 기간 동안 글로벌 유전성 암 검사 시장의 수익 마진에 부담을 줄 것으로 예상할 수 있습니다.
제조업체들은 COVID-19 이후 회복하기 위해 다양한 전략적 결정을 내리고 있습니다. 업체들은 이식 진단 시장에 관련된 기술과 테스트 결과를 개선하기 위해 여러 R&D 활동과 제품 출시 및 전략적 파트너십을 진행하고 있습니다.
최근 개발 사항
- 2022년 7월, Helio Genomics와 사업 파트너인 Fulgent Genetics(FLGT)는 미국 의학 협회(AMA)가 HelioLiver에 대한 새로운 범주 I 현재 절차 용어(CPT) 독점 실험실 분석 코드를 발급하고 미국에서 간암에 대한 첨단 혁신적 감시 검사를 광범위하게 채택했다고 발표했습니다. 이를 통해 회사는 제품 포트폴리오를 확장할 수 있었습니다.
- 2022년 3월, Illumina, Inc.는 암 RNA 시퀀서인 체외 진단(IVD) 키트를 출시했습니다. 출시로 시퀀싱 제품 라인이 확장되었고, 이어서 시판 후 승인이 이어졌습니다. 선형적 시장 성장을 보여주는 것으로 묘사됩니다.
글로벌 유전성 암 검사 시장 범위
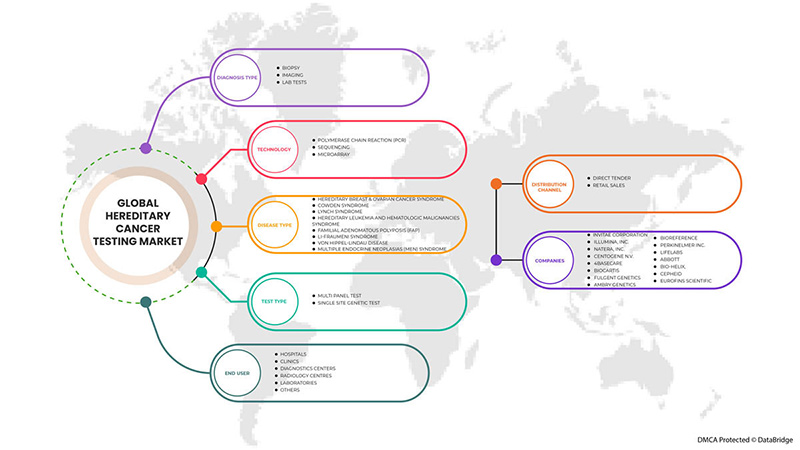
글로벌 유전성 암 검사 시장은 검사 유형, 진단 유형, 기술, 질병 유형, 최종 사용자 및 유통 채널로 세분화됩니다. 세그먼트 간의 성장은 틈새 성장 포켓과 시장에 접근하고 핵심 응용 분야와 타겟 시장의 차이점을 파악하기 위한 전략을 분석하는 데 도움이 됩니다.
테스트 유형별
- 다중 패널 테스트
- 단일 사이트 유전자 검사
검사 유형을 기준으로 볼 때, 글로벌 유전성 암 검사 시장은 다중 패널 검사와 단일 사이트 유전자 검사로 구분됩니다.
진단 유형별
- 생검
- 이미징
- 실험실 테스트
진단 유형을 기준으로 볼 때, 글로벌 유전성 암 검사 시장은 생검, 영상 검사, 실험실 검사로 구분됩니다.
기술에 의해
- 시퀀싱
- 중합효소 연쇄 반응(PCR)
- 마이크로어레이
기술을 기준으로 볼 때, 글로벌 유전성 암 검사 시장은 시퀀싱, 중합효소 연쇄 반응(PCR), 마이크로어레이로 구분됩니다.
질병 유형별
- 유전성 유방암 및 난소암 증후군
- 코우든 증후군
- 린치 증후군
- 유전성 백혈병 및 혈액 악성 종양 증후군
- 가족성 선종성 다발증(FAP)
- 리-프라우메니 증후군
- 폰 히펠-린다우병
- 다중 내분비 신생물(남성) 증후군
On the basis of disease type, the global hereditary cancer testing market is segmented into hereditary breast & ovarian cancer syndrome, cowden syndrome, lynch syndrome, hereditary leukemia and hematologic malignancies syndromes, familial adenomatous polyposis (FAP), li-fraumeni syndrome, vol-hippel lindau disease, multiple endocrine neoplasias (MEN) syndrome.
BY END USER
- HOSPITALS
- CLINICS
- LABORATORIES
- RADIOLOGY CENTERS
- DIAGNOSTIC CENTERS
- OTHERS
On the basis of end user, the global hereditary cancer testing market is segmented into hospitals, clinics, laboratories, radiology centers, diagnostic centers, and others.
BY DISTRIBUTION CHANNEL
- DIRECT TENDER
- RETAIL SALES
On the basis of distribution channel, the global hereditary cancer testing market is segmented into direct tender, retail sales.
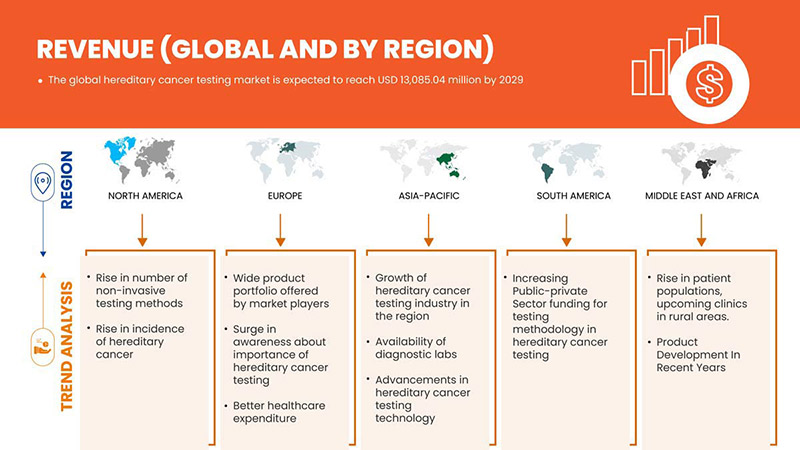
Global Hereditary Cancer Testing Market Regional Analysis/Insights
The global hereditary cancer testing market is analyzed and market size information is provided by country, test type, diagnosis type, technology, disease type, end user, and distribution channel.
The countries covered in this market report U.S., Canada, Mexico, Germany, France, U.K., Italy, Spain, Russia, Turkey, Belgium, Netherlands, Switzerland, and the rest of Europe, China, Japan, India, South Korea, Singapore, Thailand, Malaysia, Australia, Philippines, Indonesia, and the rest of Asia-Pacific, South Africa, Saudi Arabia, UAE, Egypt, Israel, and the rest of the Middle East and Africa, Brazil, Argentina, and the rest of South America.
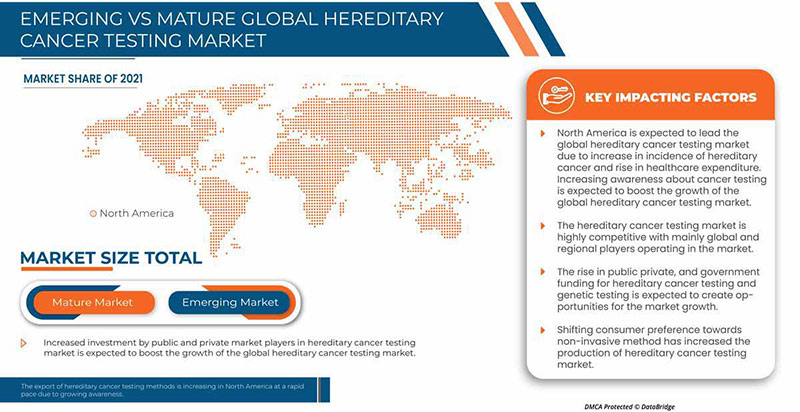
North America is dominating the market due to the increasing investment in R&D is expected to boost the market growth. The U.S. dominates North America region due to strong presence of key players Invitae Corporation, Illumina, Inc., Natera, Inc. and others. U.K. dominates Europe region due to the mass production of hereditary cancer tests and increasing demand from emerging markets and expansion of healthcare industries. China dominates Asia-Pacific region due to rise in cancer related diagnostic tests.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impact the current and future trends of the market. Data points such as new sales, replacement sales, country demographics, regulatory acts, and import-export tariffs are some of the major pointers used to forecast the market scenario for individual countries. Also, presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, and impact of sales channels are considered while providing forecast analysis of the country data.
Competitive Landscape and Global Hereditary Cancer Testing Market Share Analysis
글로벌 유전성 암 검사 시장 경쟁 구도는 경쟁자별 세부 정보를 제공합니다. 포함된 세부 정보에는 회사 개요, 회사 재무, 창출된 수익, 시장 잠재력, R&D 투자, 새로운 시장 이니셔티브, 생산 현장 및 시설, 회사의 강점과 약점, 제품 출시, 제품 시험 파이프라인, 제품 승인, 특허, 제품 폭 및 호흡, 응용 프로그램 우세, 기술 수명선 곡선이 있습니다. 위에 제공된 데이터 포인트는 회사의 글로벌 유전성 암 검사 시장에 대한 집중과만 관련이 있습니다.
글로벌 유전성 암 검사 시장의 주요 기업으로는 Invitae Corporation, Illumina, Inc., Natera, Inc., CENTOGENE NV, 4baseCare, Biocartis, Fulgent Genetics, Ambry Genetics, BioReference, PerkinElmer Inc., LifeLabs, Abbott, BIO-HELIX, Cepheid, Eurofins Scientific 등이 있습니다.
SKU-
세계 최초의 시장 정보 클라우드 보고서에 온라인으로 접속하세요
- 대화형 데이터 분석 대시보드
- 높은 성장 잠재력 기회를 위한 회사 분석 대시보드
- 사용자 정의 및 질의를 위한 리서치 분석가 액세스
- 대화형 대시보드를 통한 경쟁자 분석
- 최신 뉴스, 업데이트 및 추세 분석
- 포괄적인 경쟁자 추적을 위한 벤치마크 분석의 힘 활용
목차
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF THE GLOBAL HEREDITARY CANCER TESTING MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATIONS
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 DBMR TRIPOD DATA VALIDATION MODEL
2.5 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.6 MULTIVARIATE MODELLING
2.7 MARKET END USER COVERAGE GRID
2.8 SOURCE LIFELINE CURVE
2.9 DBMR MARKET POSITION GRID
2.1 VENDOR SHARE ANALYSIS
2.11 SECONDARY SOURCES
2.12 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHT
4.1 PESTEL ANALYSIS
4.2 PORTER’S FIVE FORCES
5 GLOBAL HEREDITARY CANCER TESTING MARKET: REGULATIONS
6 MARKET OVERVIEW
6.1 DRIVERS
6.1.1 RISING INCIDENCE OF HEREDITARY CANCER
6.1.2 INCREASE IN THE GERIATRIC POPULATION
6.1.3 RISING HEALTHCARE SPENDING
6.1.4 INCREASE IN AWARENESS ABOUT CANCER TESTING
6.2 RESTRAINTS
6.2.1 HIGH COST OF HEREDITARY CANCER TESTING
6.2.2 LACK OF SKILLED PROFESSIONALS
6.3 OPPORTUNITIES
6.3.1 STRATEGIC INITIATIVES BY MARKET PLAYERS
6.3.2 INCREASED DEMAND FOR NON-INVASIVE TESTING METHODS
6.3.3 GROWING DEMAND FOR BETTER QUALITY HEALTHCARE AND EARLY DIAGNOSIS
6.3.4 TECHNOLOGICAL DEVELOPMENTS IN HEREDITARY CANCER TESTING PROCEDURES
6.4 CHALLENGES
6.4.1 ETHICAL CHALLENGES FACED DURING HEREDITARY CANCER TESTING
6.4.2 RISING COMPETITION AMONG MARKET PLAYERS
7 GLOBAL HEREDITARY CANCER TESTING MARKET, BY TEST TYPE
7.1 OVERVIEW
7.2 MULTI PANEL TEST
7.3 SINGLE-SITE GENETIC TEST
8 GLOBAL HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE
8.1 OVERVIEW
8.2 BIOPSY
8.2.1 NEEDLE BIOPSIES
8.2.2 ENDOSCOPIC BIOPSIES
8.2.3 LAPAROSCOPIC, THORACOSCOPIC, AND MEDIASTINOSCOPIC BIOPSY
8.2.4 LAPAROTOMY AND THORACOTOMY
8.2.5 OTHERS
8.3 IMAGING
8.3.1 MAGNETIC RESONANCE IMAGING (MRI)
8.3.2 COMPUTED TOMOGRAPHY (CT) SCAN
8.3.3 POSITRON EMISSION TOMOGRAPHY (PET) SCAN
8.3.4 NUCLEAR SCAN
8.3.5 ULTRASOUND
8.3.6 X-RAYS
8.4 LAB TESTS
8.4.1 BLOOD
8.4.2 URINE
8.4.3 OTHERS
9 GLOBAL HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY
9.1 OVERVIEW
9.2 POLYMERASE CHAIN REACTION (PCR)
9.3 SEQUENCING
9.4 MICRO ARRAY
10 GLOBAL HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE
10.1 OVERVIEW
10.2 HEREDITARY BREAST & OVARIAN CANCER SYNDROME
10.3 COWDEN SYNDROME
10.4 LYNCH SYNDROME
10.5 HEREDITARY LEUKEMIA AND HEMATOLOGIC MALIGNANCIES SYNDROME
10.6 FAMILIAL ADENOMATOUS POLYPOSIS (FAP)
10.7 LI-FRAUMENI SYNDROME
10.8 VON HIPPEL-LINDAU DISEASE
10.9 MULTIPLE ENDOCRINE NEOPLASIAS (MEN) SYNDROME
11 GLOBAL HEREDITARY CANCER TESTING MARKET, BY END USER
11.1 OVERVIEW
11.2 HOSPITALS
11.3 CLINICS
11.4 DIAGNOSTIC CENTERS
11.5 RADIOLOGY CENTERS
11.6 LABORATORIES
11.7 OTHERS
12 GLOBAL HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL
12.1 OVERVIEW
12.2 DIRECT TENDER
12.3 RETAIL SALES
13 GLOBAL HEREDITARY CANCER TESTING MARKET, BY REGION
13.1 OVERVIEW
13.2 ASIA-PACIFIC
13.2.1 CHINA
13.2.2 JAPAN
13.2.3 SOUTH KOREA
13.2.4 INDIA
13.2.5 AUSTRALIA
13.2.6 SINGAPORE
13.2.7 THAILAND
13.2.8 MALAYSIA
13.2.9 INDONESIA
13.2.10 PHILIPPINES
13.2.11 REST OF ASIA-PACIFIC
13.3 NORTH AMERICA
13.3.1 U.S.
13.3.2 CANADA
13.3.3 MEXICO
13.4 EUROPE
13.4.1 GERMANY
13.4.2 FRANCE
13.4.3 U.K.
13.4.4 RUSSIA
13.4.5 ITALY
13.4.6 SPAIN
13.4.7 TURKEY
13.4.8 NETHERLANDS
13.4.9 SWITZERLAND
13.4.10 BELGIUM
13.4.11 REST OF EUROPE
13.5 SOUTH AMERICA
13.5.1 BRAZIL
13.5.2 ARGENTINA
13.5.3 REST OF SOUTH AMERICA
13.6 MIDDLE EAST AND AFRICA
13.6.1 SOUTH AFRICA
13.6.2 SAUDI ARABIA
13.6.3 U.A.E.
13.6.4 EGYPT
13.6.5 ISRAEL
13.6.6 REST OF THE MIDDLE EAST AND AFRICA
14 GLOBAL HEREDITARY CANCER TESTING MARKET: COMPANY LANDSCAPE
14.1 COMPANY SHARE ANALYSIS: GLOBAL
14.2 COMPANY SHARE ANALYSIS: NORTH AMERICA
14.3 COMPANY SHARE ANALYSIS: EUROPE
14.4 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
15 SWOT ANALYSIS
16 COMPANY PROFILE
16.1 ABBOTT
16.1.1 COMPANY SNAPSHOT
16.1.2 REVENUE ANALYSIS
16.1.3 COMPANY SHARE ANALYSIS
16.1.4 PRODUCT PORTFOLIO
16.1.5 RECENT DEVELOPMENTS
16.2 ILLUMINA, INC. (2021)
16.2.1 COMPANY SNAPSHOT
16.2.2 REVENUE ANALYSIS
16.2.3 COMPANY SHARE ANALYSIS
16.2.4 PRODUCT PORTFOLIO
16.2.5 RECENT DEVELOPMENTS
16.3 PERKINELMER INC. (2021)
16.3.1 COMPANY SNAPSHOT
16.3.2 REVENUE ANALYSIS
16.3.3 COMPANY SHARE ANALYSIS
16.3.4 PRODUCT PORTFOLIO
16.3.5 RECENT DEVELOPMENTS
16.4 LIFELABS GENETICS
16.4.1 COMPANY SNAPSHOT
16.4.2 COMPANY SHARE ANALYSIS
16.4.3 PRODUCT PORTFOLIO
16.4.4 RECENT DEVELOPMENTS
16.5 EUROFINS SCIENTIFIC (2021)
16.5.1 COMPANY SNAPSHOT
16.5.2 REVENUE ANALYSIS
16.5.3 COMPANY SHARE ANALYSIS
16.5.4 PRODUCT PORTFOLIO
16.5.5 RECENT DEVELOPMENT
16.6 AMBRY GENETICS
16.6.1 COMPANY SNAPSHOT
16.6.2 REVENUE ANALYSIS
16.6.3 PRODUCT PORTFOLIO
16.6.4 RECENT DEVELOPMENTS
16.7 BIOCARTIS
16.7.1 COMPANY SNAPSHOT
16.7.2 REVENUE ANALYSIS
16.7.3 PRODUCT PORTFOLIO
16.7.4 RECENT DEVELOPMENTS
16.8 BIO-HELIX
16.8.1 COMPANY SNAPSHOT
16.8.2 PRODUCT PORTFOLIO
16.8.3 RECENT DEVELOPMENTS
16.9 BIOREFERENCE (A SUBSIDIARY OF OPKO HEALTH, INC.) (2021)
16.9.1 COMPANY SNAPSHOT
16.9.2 REVENUE ANALYSIS
16.9.3 PRODUCT PORTFOLIO
16.9.4 RECENT DEVELOPMENT
16.1 CENTOGENE N.V. (2021)
16.10.1 COMPANY SNAPSHOT
16.10.2 REVENUE ANALYSIS
16.10.3 PRODUCT PORTFOLIO
16.10.4 RECENT DEVELOPMENT
16.11 CEPHEID
16.11.1 COMPANY SNAPSHOT
16.11.2 PRODUCT PORTFOLIO
16.11.3 RECENT DEVELOPMENT
16.12 FULGENT GENETICS
16.12.1 COMPANY SNAPSHOT
16.12.2 REVENUE ANALYSIS
16.12.3 PRODUCT PORTFOLIO
16.12.4 RECENT DEVELOPMENT
16.13 INVITAE CORPORATION
16.13.1 COMPANY SNAPSHOT
16.13.2 REVENUE ANALYSIS
16.13.3 PRODUCT PORTFOLIO
16.13.4 RECENT DEVELOPMENT
16.14 NATERA, INC. (2021)
16.14.1 COMPANY SNAPSHOT
16.14.2 REVENUE ANALYSIS
16.14.3 PRODUCT PORTFOLIO
16.14.4 RECENT DEVELOPMENTS
16.15 4BASECARE.
16.15.1 COMPANY SNAPSHOT
16.15.2 PRODUCT PORTFOLIO
16.15.3 RECENT DEVELOPMENTS
17 QUESTIONNAIRE
18 RELATED REPORTS
표 목록
TABLE 1 GLOBAL HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 2 GLOBAL MULTI PANEL TEST IN HEREDITARY CANCER TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 3 GLOBAL SINGLE-SITE GENETIC TEST IN HEREDITARY CANCER TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 4 GLOBAL HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 5 GLOBAL BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 6 GLOBAL BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 7 GLOBAL IMAGING IN HEREDITARY CANCER TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 8 GLOBAL IMAGING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 9 GLOBAL LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 10 GLOBAL LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 11 GLOBAL HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 12 GLOBAL POLYMERASE CHAIN REACTION (PCR) IN HEREDITARY CANCER TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 13 GLOBAL SEQUENCING IN HEREDITARY CANCER TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 14 GLOBAL MICROARRAY IN HEREDITARY CANCER TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 15 GLOBAL HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 16 GLOBAL HEREDITARY BREAST & OVARIAN CANCER SYNDROME IN HEREDITARY CANCER TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 17 GLOBAL COWDEN SYNDROME IN HEREDITARY CANCER TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 18 GLOBAL LYNCH SYNDROME IN HEREDITARY CANCER TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 19 GLOBAL HEREDITARY LEUKEMIA AND HEMATOLOGIC MALIGNANCIES SYNDROME IN HEREDITARY CANCER TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 20 GLOBAL FAMILIAL ADENOMATOUS POLYPOSIS (FAP) IN HEREDITARY CANCER TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 21 GLOBAL LI-FRAUMENI SYNDROME IN HEREDITARY CANCER TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 22 GLOBAL VON HIPPEL-LINDAU DISEASE IN HEREDITARY CANCER TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 23 GLOBAL MULTIPLE ENDOCRINE NEOPLASIAS (MEN) SYNDROME IN HEREDITARY CANCER TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 24 GLOBAL HEREDITARY CANCER TESTING MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 25 GLOBAL HOSPITALS CENTERS IN HEREDITARY CANCER TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 26 GLOBAL CLINICS IN HEREDITARY CANCER TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 27 GLOBAL DIAGNOSTIC CENTERS IN HEREDITARY CANCER TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 28 GLOBAL RADIOLOGY CENTERS IN HEREDITARY CANCER TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 29 GLOBAL LABORATORIES IN HEREDITARY CANCER TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 30 GLOBAL OTHERS IN HEREDITARY CANCER TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 31 GLOBAL HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 32 GLOBAL DIRECT TENDER IN HEREDITARY CANCER TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 33 GLOBAL RETAIL SALES IN HEREDITARY CANCER TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 34 GLOBAL HEREDITARY CANCER TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 35 ASIA-PACIFIC HEREDITARY CANCER TESTING MARKET, BY COUNTRY, 2020-2029 (USD MILLION)
TABLE 36 ASIA-PACIFIC HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 37 ASIA-PACIFIC HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 38 ASIA-PACIFIC BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 39 ASIA-PACIFIC IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 40 ASIA-PACIFIC LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 41 ASIA-PACIFIC HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 42 ASIA-PACIFIC HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 43 ASIA-PACIFIC HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 44 ASIA-PACIFIC HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 45 CHINA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 46 CHINA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 47 CHINA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 48 CHINA HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 49 CHINA BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 50 CHINA IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 51 CHINA LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 52 CHINA HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 53 CHINA HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 54 CHINA HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 55 CHINA HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 56 JAPAN HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 57 JAPAN HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 58 JAPAN HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 59 JAPAN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 60 JAPAN BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 61 JAPAN IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 62 JAPAN LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 63 JAPAN HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 64 JAPAN HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 65 JAPAN HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 66 JAPAN HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 67 SOUTH KOREA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 68 SOUTH KOREA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 69 SOUTH KOREA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 70 SOUTH KOREA HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 71 SOUTH KOREA BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 72 SOUTH KOREA IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 73 SOUTH KOREA LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 74 SOUTH KOREA HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 75 SOUTH KOREA HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 76 SOUTH KOREA HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 77 SOUTH KOREA HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 78 INDIA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 79 INDIA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 80 INDIA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 81 INDIA HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 82 INDIA BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 83 INDIA IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 84 INDIA LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 85 INDIA HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 86 INDIA HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 87 INDIA HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 88 INDIA HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 89 AUSTRALIA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 90 AUSTRALIA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 91 AUSTRALIA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 92 AUSTRALIA HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 93 AUSTRALIA BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 94 AUSTRALIA IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 95 AUSTRALIA LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 96 AUSTRALIA HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 97 AUSTRALIA HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 98 AUSTRALIA HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 99 AUSTRALIA HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 100 SINGAPORE HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 101 SINGAPORE HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 102 SINGAPORE HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 103 SINGAPORE HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 104 SINGAPORE BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 105 SINGAPORE IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 106 SINGAPORE LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 107 SINGAPORE HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 108 SINGAPORE HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 109 SINGAPORE HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 110 SINGAPORE HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 111 THAILAND HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 112 THAILAND HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 113 THAILAND HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 114 THAILAND HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 115 THAILAND BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 116 THAILAND IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 117 THAILAND LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 118 THAILAND HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 119 THAILAND HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 120 THAILAND HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 121 THAILAND HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 122 MALAYSIA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 123 MALAYSIA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 124 MALAYSIA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 125 MALAYSIA HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 126 MALAYSIA BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 127 MALAYSIA IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 128 MALAYSIA LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 129 MALAYSIA HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 130 MALAYSIA HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 131 MALAYSIA HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 132 MALAYSIA HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 133 INDONESIA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 134 INDONESIA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 135 INDONESIA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 136 INDONESIA HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 137 INDONESIA BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 138 INDONESIA IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 139 INDONESIA LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 140 INDONESIA HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 141 INDONESIA HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 142 INDONESIA HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 143 INDONESIA HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 144 PHILIPPINES HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 145 PHILIPPINES HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 146 PHILIPPINES HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 147 PHILIPPINES HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 148 PHILIPPINES BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 149 PHILIPPINES IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 150 PHILIPPINES LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 151 PHILIPPINES HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 152 PHILIPPINES HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 153 PHILIPPINES HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 154 PHILIPPINES HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 155 REST OF ASIA-PACIFIC HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 156 NORTH AMERICA HEREDITARY CANCER TESTING MARKET, BY COUNTRY, 2020-2029 (USD MILLION)
TABLE 157 NORTH AMERICA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 158 NORTH AMERICA HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 159 NORTH AMERICA BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 160 NORTH AMERICA IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 161 NORTH AMERICA LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 162 NORTH AMERICA HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 163 NORTH AMERICA HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 164 NORTH AMERICA HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 165 NORTH AMERICA HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 166 U.S. HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 167 U.S. HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 168 U.S. HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 169 U.S. HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 170 U.S. BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 171 U.S. IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 172 U.S. LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 173 U.S. HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 174 U.S. HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 175 U.S. HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 176 U.S. HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 177 CANADA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 178 CANADA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 179 CANADA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 180 CANADA HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 181 CANADA BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 182 CANADA IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 183 CANADA LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 184 CANADA HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 185 CANADA HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 186 CANADA HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 187 CANADA HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 188 MEXICO HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 189 MEXICO HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 190 MEXICO HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 191 MEXICO HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 192 MEXICO BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 193 MEXICO IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 194 MEXICO LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 195 MEXICO HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 196 MEXICO HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 197 MEXICO HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 198 MEXICO HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 199 EUROPE HEREDITARY CANCER TESTING MARKET, BY COUNTRY, 2020-2029 (USD MILLION)
TABLE 200 EUROPE HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 201 EUROPE HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 202 EUROPE BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 203 EUROPE IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 204 EUROPE LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 205 EUROPE HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 206 EUROPE HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 207 EUROPE HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 208 EUROPE HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 209 GERMANY HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 210 GERMANY HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 211 GERMANY HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 212 GERMANY HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 213 GERMANY BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 214 GERMANY IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 215 GERMANY LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 216 GERMANY HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 217 GERMANY HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 218 GERMANY HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 219 GERMANY HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 220 FRANCE HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 221 FRANCE HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 222 FRANCE HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 223 FRANCE HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 224 FRANCE BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 225 FRANCE IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 226 FRANCE LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 227 FRANCE HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 228 FRANCE HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 229 FRANCE HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 230 FRANCE HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 231 U.K. HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 232 U.K. HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 233 U.K. HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 234 U.K. HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 235 U.K. BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 236 U.K. IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 237 U.K. LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 238 U.K. HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 239 U.K. HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 240 U.K. HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 241 U.K. HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 242 RUSSIA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 243 RUSSIA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 244 RUSSIA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 245 RUSSIA HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 246 RUSSIA BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 247 RUSSIA IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 248 RUSSIA LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 249 RUSSIA HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 250 RUSSIA HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 251 RUSSIA HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 252 RUSSIA HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 253 ITALY HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 254 ITALY HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 255 ITALY HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 256 ITALY HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 257 ITALY BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 258 ITALY IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 259 ITALY LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 260 ITALY HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 261 ITALY HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 262 ITALY HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 263 ITALY HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 264 SPAIN HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 265 SPAIN HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 266 SPAIN HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 267 SPAIN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 268 SPAIN BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 269 SPAIN IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 270 SPAIN LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 271 SPAIN HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 272 SPAIN HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 273 SPAIN HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 274 SPAIN HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 275 TURKEY HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 276 TURKEY HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 277 TURKEY HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 278 TURKEY HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 279 TURKEY BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 280 TURKEY IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 281 TURKEY LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 282 TURKEY HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 283 TURKEY HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 284 TURKEY HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 285 TURKEY HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 286 NETHERLANDS HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 287 NETHERLANDS HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 288 NETHERLANDS HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 289 NETHERLANDS HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 290 NETHERLANDS BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 291 NETHERLANDS IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 292 NETHERLANDS LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 293 NETHERLANDS HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 294 NETHERLANDS HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 295 NETHERLANDS HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 296 NETHERLANDS HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 297 SWITZERLAND HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 298 SWITZERLAND HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 299 SWITZERLAND HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 300 SWITZERLAND HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 301 SWITZERLAND BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 302 SWITZERLAND IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 303 SWITZERLAND LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 304 SWITZERLAND HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 305 SWITZERLAND HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 306 SWITZERLAND HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 307 SWITZERLAND HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 308 BELGIUM HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 309 BELGIUM HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 310 BELGIUM HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 311 BELGIUM HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 312 BELGIUM BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 313 BELGIUM IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 314 BELGIUM LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 315 BELGIUM HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 316 BELGIUM HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 317 BELGIUM HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 318 BELGIUM HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 319 REST OF EUROPE HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 320 SOUTH AMERICA HEREDITARY CANCER TESTING MARKET, BY COUNTRY, 2020-2029 (USD MILLION)
TABLE 321 SOUTH AMERICA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 322 SOUTH AMERICA HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 323 SOUTH AMERICA BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 324 SOUTH AMERICA IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 325 SOUTH AMERICA LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 326 SOUTH AMERICA HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 327 SOUTH AMERICA HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 328 SOUTH AMERICA HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 329 SOUTH AMERICA HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 330 BRAZIL HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 331 BRAZIL HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 332 BRAZIL HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 333 BRAZIL HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 334 BRAZIL BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 335 BRAZIL IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 336 BRAZIL LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 337 BRAZIL HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 338 BRAZIL HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 339 BRAZIL HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 340 BRAZIL HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 341 ARGENTINA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 342 ARGENTINA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 343 ARGENTINA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 344 ARGENTINA HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 345 ARGENTINA BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 346 ARGENTINA IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 347 ARGENTINA LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 348 ARGENTINA HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 349 ARGENTINA HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 350 ARGENTINA HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 351 ARGENTINA HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 352 REST OF THE SOUTH AMERICA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 353 MIDDLE EAST AND AFRICA HEREDITARY CANCER TESTING MARKET, BY COUNTRY, 2020-2029 (USD MILLION)
TABLE 354 MIDDLE EAST AND AFRICA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 355 MIDDLE EAST AND AFRICA HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 356 MIDDLE EAST AND AFRICA BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 357 MIDDLE EAST AND AFRICA IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 358 MIDDLE EAST AND AFRICA LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 359 MIDDLE EAST AND AFRICA HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 360 MIDDLE EAST AND AFRICA HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 361 MIDDLE EAST AND AFRICA HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 362 MIDDLE EAST AND AFRICA HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 363 SOUTH AFRICA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 364 SOUTH AFRICA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 365 SOUTH AFRICA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 366 SOUTH AFRICA HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 367 SOUTH AFRICA BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 368 SOUTH AFRICA IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 369 SOUTH AFRICA LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 370 SOUTH AFRICA HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 371 SOUTH AFRICA HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 372 SOUTH AFRICA HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 373 SOUTH AFRICA HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 374 SAUDI ARABIA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 375 SAUDI ARABIA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 376 SAUDI ARABIA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 377 SAUDI ARABIA HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 378 SAUDI ARABIA BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 379 SAUDI ARABIA IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 380 SAUDI ARABIA LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 381 SAUDI ARABIA HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 382 SAUDI ARABIA HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 383 SAUDI ARABIA HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 384 SAUDI ARABIA HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 385 U.A.E. HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 386 U.A.E. HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 387 U.A.E. HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 388 U.A.E. HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 389 U.A.E. BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 390 U.A.E. IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 391 U.A.E. LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 392 U.A.E. HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 393 U.A.E. HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 394 U.A.E. HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 395 U.A.E. HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 396 EGYPT HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 397 EGYPT HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 398 EGYPT HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 399 EGYPT HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 400 EGYPT BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 401 EGYPT IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 402 EGYPT LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 403 EGYPT HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 404 EGYPT HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 405 EGYPT HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 406 EGYPT HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 407 ISRAEL HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 408 ISRAEL HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 409 ISRAEL HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 410 ISRAEL HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 411 ISRAEL BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 412 ISRAEL IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 413 ISRAEL LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 414 ISRAEL HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 415 ISRAEL HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 416 ISRAEL HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 417 ISRAEL HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 418 REST OF MIDDLE EAST AND AFRICA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
그림 목록
FIGURE 1 GLOBAL HEREDITARY CANCER TESTING MARKET: SEGMENTATION
FIGURE 2 GLOBAL HEREDITARY CANCER TESTING MARKET: DATA TRIANGULATION
FIGURE 3 GLOBAL HEREDITARY CANCER TESTING MARKET: DROC ANALYSIS
FIGURE 4 GLOBAL HEREDITARY CANCER TESTING MARKET: GLOBAL VS REGIONAL MARKET ANALYSIS
FIGURE 5 GLOBAL HEREDITARY CANCER TESTING MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 GLOBAL HEREDITARY CANCER TESTING MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 GLOBAL HEREDITARY CANCER TESTING MARKET: MARKET APPLICATION COVERAGE GRID
FIGURE 8 GLOBAL HEREDITARY CANCER TESTING MARKET: DBMR MARKET POSITION GRID
FIGURE 9 GLOBAL HEREDITARY CANCER TESTING MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 GLOBAL HEREDITARY CANCER TESTING MARKET: SEGMENTATION
FIGURE 11 NORTH AMERICA IS ANTICIPATED TO DOMINATE THE GLOBAL HEREDITARY CANCER TESTING MARKET, AND ASIA-PACIFIC IS ESTIMATED TO BE GROWING WITH THE HIGHEST CAGR IN THE FORECAST PERIOD
FIGURE 12 EXPANDING REPRODUCTIVE GENETIC HEALTH SPACE IS EXPECTED TO DRIVE THE GLOBAL HEREDITARY CANCER TESTING MARKET IN THE FORECAST PERIOD
FIGURE 13 MULTI PANEL TEST SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE GLOBAL HEREDITARY CANCER TESTING MARKET IN 2022 & 2029
FIGURE 14 NORTH AMERICA IS THE FASTEST-GROWING MARKET FOR HEREDITARY CANCER TESTING MANUFACTURERS IN THE FORECAST PERIOD
FIGURE 15 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF THE GLOBAL HEREDITARY CANCER TESTING MARKET
FIGURE 16 GLOBAL HEREDITARY CANCER TESTING MARKET: BY TEST TYPE, 2021
FIGURE 17 GLOBAL HEREDITARY CANCER TESTING MARKET: BY TEST TYPE, 2022-2029 (USD MILLION)
FIGURE 18 GLOBAL HEREDITARY CANCER TESTING MARKET: BY TEST TYPE, CAGR (2022-2029)
FIGURE 19 GLOBAL HEREDITARY CANCER TESTING MARKET: BY TEST TYPE, LIFELINE CURVE
FIGURE 20 GLOBAL HEREDITARY CANCER TESTING MARKET: BY DIAGNOSIS TYPE, 2021
FIGURE 21 GLOBAL HEREDITARY CANCER TESTING MARKET: BY DIAGNOSIS TYPE, 2022-2029 (USD MILLION)
FIGURE 22 GLOBAL HEREDITARY CANCER TESTING MARKET: BY DIAGNOSIS TYPE, CAGR (2022-2029)
FIGURE 23 GLOBAL HEREDITARY CANCER TESTING MARKET: BY DIAGNOSIS TYPE, LIFELINE CURVE
FIGURE 24 GLOBAL HEREDITARY CANCER TESTING MARKET : BY TECHNOLOGY, 2021
FIGURE 25 GLOBAL HEREDITARY CANCER TESTING MARKET : BY TECHNOLOGY, 2020-2029 (USD MILLION)
FIGURE 26 GLOBAL HEREDITARY CANCER TESTING MARKET : BY TECHNOLOGY, CAGR (2022-2029)
FIGURE 27 GLOBAL HEREDITARY CANCER TESTING MARKET : BY TECHNOLOGY, LIFELINE CURVE
FIGURE 28 GLOBAL HEREDITARY CANCER TESTING MARKET: BY DISEASE TYPE, 2021
FIGURE 29 GLOBAL HEREDITARY CANCER TESTING MARKET: BY DISEASE TYPE, 2022-2029 (USD MILLION)
FIGURE 30 GLOBAL HEREDITARY CANCER TESTING MARKET: BY DISEASE TYPE, CAGR (2022-2029)
FIGURE 31 GLOBAL HEREDITARY CANCER TESTING MARKET: BY DISEASE TYPE, LIFELINE CURVE
FIGURE 32 GLOBAL HEREDITARY CANCER TESTING MARKET : BY END USER, 2021
FIGURE 33 GLOBAL HEREDITARY CANCER TESTING MARKET : BY END USER, 2020-2029 (USD MILLION)
FIGURE 34 GLOBAL HEREDITARY CANCER TESTING MARKET : BY END USER, CAGR (2022-2029)
FIGURE 35 GLOBAL HEREDITARY CANCER TESTING MARKET : BY END USER, LIFELINE CURVE
FIGURE 36 GLOBAL HEREDITARY CANCER TESTING MARKET : BY DISTRIBUTION CHANNEL, 2021
FIGURE 37 GLOBAL HEREDITARY CANCER TESTING MARKET : BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
FIGURE 38 GLOBAL HEREDITARY CANCER TESTING MARKET : BY DISTRIBUTION CHANNEL, CAGR (2022-2029)
FIGURE 39 GLOBAL HEREDITARY CANCER TESTING MARKET : BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 40 GLOBAL HEREDITARY CANCER TESTING MARKET: SNAPSHOT (2021)
FIGURE 41 GLOBAL HEREDITARY CANCER TESTING MARKET: BY REGION (2021)
FIGURE 42 GLOBAL HEREDITARY CANCER TESTING MARKET: BY REGION (2022 & 2029)
FIGURE 43 GLOBAL HEREDITARY CANCER TESTING MARKET: BY REGION (2021 & 2029)
FIGURE 44 GLOBAL HEREDITARY CANCER TESTING MARKET: BY TEST TYPE (2022-2029)
FIGURE 45 ASIA-PACIFIC HEREDITARY CANCER TESTING MARKET: SNAPSHOT (2021)
FIGURE 46 ASIA-PACIFIC HEREDITARY CANCER TESTING MARKET: BY COUNTRY (2021)
FIGURE 47 ASIA-PACIFIC HEREDITARY CANCER TESTING MARKET: BY COUNTRY (2022 & 2029)
FIGURE 48 ASIA-PACIFIC HEREDITARY CANCER TESTING MARKET: BY COUNTRY (2021 & 2029)
FIGURE 49 ASIA-PACIFIC HEREDITARY CANCER TESTING MARKET: BY TEST TYPE (2022-2029)
FIGURE 50 NORTH AMERICA HEREDITARY CANCER TESTING MARKET: SNAPSHOT (2021)
FIGURE 51 NORTH AMERICA HEREDITARY CANCER TESTING MARKET: BY COUNTRY (2021)
FIGURE 52 NORTH AMERICA HEREDITARY CANCER TESTING MARKET: BY COUNTRY (2022 & 2029)
FIGURE 53 NORTH AMERICA HEREDITARY CANCER TESTING MARKET: BY COUNTRY (2021 & 2029)
FIGURE 54 NORTH AMERICA HEREDITARY CANCER TESTING MARKET: BY TEST TYPE (2022-2029)
FIGURE 55 EUROPE HEREDITARY CANCER TESTING MARKET: SNAPSHOT (2021)
FIGURE 56 EUROPE HEREDITARY CANCER TESTING MARKET: BY COUNTRY (2021)
FIGURE 57 EUROPE HEREDITARY CANCER TESTING MARKET: BY COUNTRY (2022 & 2029)
FIGURE 58 EUROPE HEREDITARY CANCER TESTING MARKET: BY COUNTRY (2021 & 2029)
FIGURE 59 EUROPE HEREDITARY CANCER TESTING MARKET: BY TEST TYPE (2022-2029)
FIGURE 60 SOUTH AMERICA HEREDITARY CANCER TESTING MARKET: SNAPSHOT (2021)
FIGURE 61 SOUTH AMERICA HEREDITARY CANCER TESTING MARKET: BY COUNTRY (2021)
FIGURE 62 SOUTH AMERICA HEREDITARY CANCER TESTING MARKET: BY COUNTRY (2022 & 2029)
FIGURE 63 SOUTH AMERICA HEREDITARY CANCER TESTING MARKET: BY COUNTRY (2021 & 2029)
FIGURE 64 SOUTH AMERICA HEREDITARY CANCER TESTING MARKET: BY TEST TYPE (2022-2029)
FIGURE 65 MIDDLE EAST & AFRICA HEREDITARY CANCER TESTING MARKET: SNAPSHOT (2021)
FIGURE 66 MIDDLE EAST & AFRICA HEREDITARY CANCER TESTING MARKET: BY COUNTRY (2021)
FIGURE 67 MIDDLE EAST & AFRICA HEREDITARY CANCER TESTING MARKET: BY COUNTRY (2022 & 2029)
FIGURE 68 MIDDLE EAST & AFRICA HEREDITARY CANCER TESTING MARKET: BY COUNTRY (2021 & 2029)
FIGURE 69 MIDDLE EAST & AFRICA HEREDITARY CANCER TESTING MARKET: BY TEST TYPE (2022-2029)
FIGURE 70 GLOBAL HEREDITARY CANCER TESTING MARKET: COMPANY SHARE 2021 (%)
FIGURE 71 NORTH AMERICA HEREDITARY CANCER TESTING MARKET: COMPANY SHARE 2021 (%)
FIGURE 72 EUROPE HEREDITARY CANCER TESTING MARKET: COMPANY SHARE 2021 (%)
FIGURE 73 ASIA-PACIFIC HEREDITARY CANCER TESTING MARKET: COMPANY SHARE 2021 (%)

연구 방법론
데이터 수집 및 기준 연도 분석은 대규모 샘플 크기의 데이터 수집 모듈을 사용하여 수행됩니다. 이 단계에는 다양한 소스와 전략을 통해 시장 정보 또는 관련 데이터를 얻는 것이 포함됩니다. 여기에는 과거에 수집한 모든 데이터를 미리 검토하고 계획하는 것이 포함됩니다. 또한 다양한 정보 소스에서 발견되는 정보 불일치를 검토하는 것도 포함됩니다. 시장 데이터는 시장 통계 및 일관된 모델을 사용하여 분석하고 추정합니다. 또한 시장 점유율 분석 및 주요 추세 분석은 시장 보고서의 주요 성공 요인입니다. 자세한 내용은 분석가에게 전화를 요청하거나 문의 사항을 드롭하세요.
DBMR 연구팀에서 사용하는 주요 연구 방법론은 데이터 마이닝, 시장에 대한 데이터 변수의 영향 분석 및 주요(산업 전문가) 검증을 포함하는 데이터 삼각 측량입니다. 데이터 모델에는 공급업체 포지셔닝 그리드, 시장 타임라인 분석, 시장 개요 및 가이드, 회사 포지셔닝 그리드, 특허 분석, 가격 분석, 회사 시장 점유율 분석, 측정 기준, 글로벌 대 지역 및 공급업체 점유율 분석이 포함됩니다. 연구 방법론에 대해 자세히 알아보려면 문의를 통해 업계 전문가에게 문의하세요.
사용자 정의 가능
Data Bridge Market Research는 고급 형성 연구 분야의 선두 주자입니다. 저희는 기존 및 신규 고객에게 목표에 맞는 데이터와 분석을 제공하는 데 자부심을 느낍니다. 보고서는 추가 국가에 대한 시장 이해(국가 목록 요청), 임상 시험 결과 데이터, 문헌 검토, 재생 시장 및 제품 기반 분석을 포함하도록 사용자 정의할 수 있습니다. 기술 기반 분석에서 시장 포트폴리오 전략에 이르기까지 타겟 경쟁업체의 시장 분석을 분석할 수 있습니다. 귀하가 원하는 형식과 데이터 스타일로 필요한 만큼 많은 경쟁자를 추가할 수 있습니다. 저희 분석가 팀은 또한 원시 엑셀 파일 피벗 테이블(팩트북)로 데이터를 제공하거나 보고서에서 사용 가능한 데이터 세트에서 프레젠테이션을 만드는 데 도움을 줄 수 있습니다.