サウジアラビアの q-PCR 試薬市場、タイプ別 (試薬と消耗品)、用途別 (臨床、研究、食品製品の安全性試験、法医学、その他)、エンドユーザー別 (病院、診断センター、製薬およびバイオ医薬品会社、研究および学術機関、臨床研究機関、獣医研究所、法医学研究所、その他)、流通チャネル別 (直接入札、小売販売、その他) - 2030 年までの業界動向と予測。
サウジアラビアの q-PCR 試薬市場の分析と洞察
慢性疾患や伝染性疾患に関する一般の認識の高まりにより、市場の需要が高まっています。主要な市場プレーヤーは、この重要な時期にさまざまな製品の発売と承認に注力しています。さらに、改良された高度な技術の増加も、市場の需要の高まりに貢献しています。
サウジアラビアのq-PCR試薬市場は、慢性疾患の増加により、2023年から2030年の予測期間に成長すると予想されています。政府やその他の団体による研究活動への多額の資金提供も、市場の成長を牽引すると予想されています。これに伴い、メーカーは市場に新製品を投入するための研究開発活動に取り組んでいます。ただし、製品の高コストが市場の成長を抑制すると予想されます。伝染病に対するより質の高いヘルスケアの需要の高まりと、個別化医療の採用の増加は、市場の成長を後押しすると予想されます。ただし、診断テストを実施する際に直面する運用上の障壁は、市場の成長を妨げると予想されます。
Data Bridge Market Researchは、サウジアラビアのq-PCR試薬市場は、2023年から2030年の予測期間中に8.3%のCAGRで成長し、2030年までに41,798.44千米ドルの価値に達すると予測していると分析しています。
|
レポートメトリック |
詳細 |
|
予測期間 |
2023年から2030年 |
|
基準年 |
2022 |
|
歴史的な年 |
2021 (2015~2020年にカスタマイズ可能) |
|
定量単位 |
収益(千米ドル) |
|
対象セグメント |
タイプ(試薬および消耗品)、用途(臨床、研究、食品安全試験、法医学、その他)、エンドユーザー(病院、診断センター、製薬およびバイオ医薬品会社、研究および学術機関、臨床研究機関、獣医研究所、法医学研究所、その他)、流通チャネル(直接入札、小売販売、その他) |
|
対象国 |
サウジアラビア |
|
対象となる市場プレーヤー |
F. Hoffmann-La Roche Ltd、Thermo Fisher Scientific Inc.、Agilent Technologies, Inc.、Bio-Rad Laboratories, Inc.、Quantabio、Sino Biological Inc.、QIAGEN、TAKARA HOLDINGS INC、Enzo Biochem Inc.、meridian BIOSCIENCE、Tonbo Biosciences(Cytek Biosciences の子会社)、Norgen Biotek Corp. |
市場の定義
q-PCR 試薬は、ポリメラーゼ連鎖反応 (PCR) を使用して核酸を同時に増幅および検出/定量するために使用されます。これらの試薬は、研究、診断、法医学などのさまざまな用途で使用されます。また、ウイルスの検出、遺伝子安定性テストなどにも使用されます。qPCR の主な利点は、テンプレート DNA (増幅ターゲット シーケンス) の初期コピー数を、広いダイナミック レンジにわたって正確かつ高感度に決定できることです。
サウジアラビアの q-PCR 試薬市場の動向
このセクションでは、市場の推進要因、利点、機会、制約、課題について理解します。これらについては、以下で詳しく説明します。
ドライバー
- 慢性疾患および伝染病の蔓延の増加
慢性疾患には心臓病、がん、糖尿病などがあり、これらは世界中の多くの国で死亡や障害の主な原因となっています。q-PCR 試薬は、ヒト免疫不全ウイルス(HIV) やマラリアなどの慢性疾患の診断に不可欠です。どちらの病気も非特異的な症状があります。q-PCR キットとミキサーには、非常に特異的なプライマー ペアを使用して HIV やその他の疾患を迅速かつ高感度で再現性の高いリアルタイム検出するために必要な PCR 試薬がすべて含まれています。
- 高齢者人口の増加
高齢者は慢性疾患や悪性疾患にかかりやすいです。非特異的抗体は高齢者に多く見られます。q-PCR 試薬による診断は高齢者にとって重要です。検査は非侵襲的で、感染性慢性疾患の抗原検出に役立つため、病気の広範な蔓延を防ぐために重要な早期診断を提供します。高齢者人口が増加しているため、q-PCR 試薬の必要性も増加します。
拘束
- 厳格な規則と規制
さまざまな主要市場プレーヤーによる試薬や製品の商業化は、国内で確立された規制枠組みの遵守によって促進されます。SFDA によるプライバシー ポリシーと規制の急速な発展が市場の成長を抑制しています。サウジアラビアで PCR 試薬を含む医療機器の登録と承認を規制する規制当局は、サウジ食品医薬品局として知られています。SFDA には、PCR 試薬の製造業者と販売業者が従わなければならない製品登録、ラベル表示、品質管理の規則があり、これが市場の成長を抑制しています。
機会
- 医療に対する政府の資金援助の増加
政府、連盟、および主要な市場プレーヤーは、発展途上国全体で機会を創出しています。プレーヤーと政府によるこれらの取り組みにより、ユーザーは低所得国や発展途上国向けの政策を活用できるようになり、早期に診断されれば予防できる病気について認識できるようになります。
医療費の増加に伴い、医療研究開発への投資も増加する可能性があります。遺伝子研究、病気の監視、診断の分野でより多くの研究が実施され、q-PCR 試薬の需要が増加する可能性があります。医療施設は、サービスを開発および強化するにつれて、提供する臨床検査の数と種類を増やす可能性があり、市場の成長の機会が生まれます。
チャレンジ
- 熟練労働力の不足
q-PCR 技術は継続的に進歩しており、製造業者は高度な q-PCR の導入に向けて継続的に研究開発を行っていますが、その操作には高度なスキルと認定を受けた専門家が必要です。試薬の取り扱いと PCR 反応の維持は簡単な作業ではありません。これは、実施された結果が正確で適切になるように適切な知識と経験を必要とする膨大なプロセスです。しかし、この専門的で熟練した労働力の不足により、q-PCR 試薬の市場は縮小しています。
熟練した認定専門家の要件は、市場にとって大きな課題です。q-PCR は複雑なプロセスであり、デバイスには高度な機能があり、患者に安全で効果的なサービスを提供するには熟練した専門家による操作が必要です。
最近の動向
- 2021年12月、サーモフィッシャーサイエンティフィック社は、バイオ医薬品およびバイオテクノロジー業界向けの臨床研究サービスを提供するサウジアラビアの大手プロバイダーであるPPD社の買収を完了したことを発表しました。この買収により、同社は製品ポートフォリオと売上を拡大することができました。
- 2021 年 9 月、アジレント テクノロジーズは、Visiopharm との世界規模の販売契約を締結したことを発表しました。これにより、アジレントは Visiopharm の CE-IVD マーク付き人工知能 (AI) 駆動型精密病理学ソフトウェアおよびその他の製品のポートフォリオを共同販売できるようになります。これにより、同社の製品範囲が拡大しました。
- バイオ・ラッド ラボラトリーズ社は2021年9月、サウジアラビアのマルチプレックス分子診断のリーダーであるSeegene社と、感染症分子診断製品の臨床開発および商品化に向けた提携を発表しました。これにより、同社の製品ポートフォリオが拡大しました。
- 2021 年 6 月、アジレント テクノロジーズは、バイオ医薬品業界のニーズを満たすために特別に開発された 3 つの InfinityLab Bio LC システムの発売を発表しました。これには、Agilent OpenLab および MassHunter ソフトウェアとシームレスに統合される機器、カラム、試薬、消耗品、およびバイオ医薬品ラボの効率を最大化する CrossLab サービスが含まれます。これにより、同社の売上と製品ポートフォリオが増加しました。
- 2020 年 7 月、サーモフィッシャーサイエンティフィック社は、90 分以内に高精度で一貫した結果を提供するように設計された初の完全統合型デジタル PCR (dPCR) システムである Applied Biosystems QuantStudio Absolute Q デジタル PCR システムの発売を発表しました。これにより、同社の収益と製品ポートフォリオが増加しました。
サウジアラビアのq-PCR試薬市場の範囲
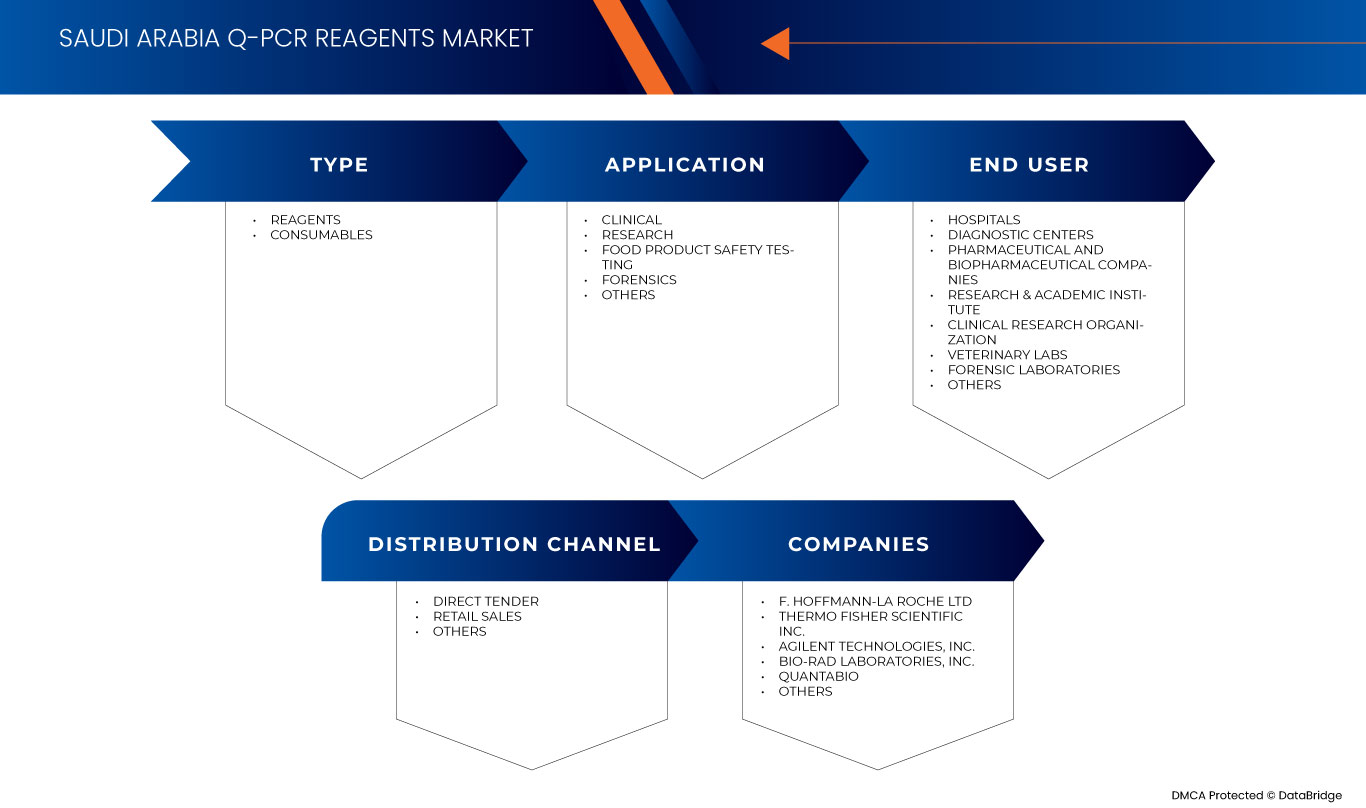
サウジアラビアの q-PCR 試薬市場は、タイプ、アプリケーション、エンド ユーザー、流通チャネルに基づいて 4 つの主要なセグメントに分割されています。セグメント間の成長は、ニッチな成長分野と市場へのアプローチ戦略を分析し、コア アプリケーション領域とターゲット市場の違いを判断するのに役立ちます。
タイプ
- 試薬
- 消耗品
タイプに基づいて、市場は試薬と消耗品に分類されます。
応用
- 臨床
- 研究
- 食品安全試験
- 法医学
- その他
アプリケーションに基づいて、市場は臨床、研究、食品安全性試験、法医学、その他に分類されます。
エンドユーザー
- 病院
- 診断センター
- 製薬・バイオ医薬品企業
- 研究・学術機関
- 臨床研究組織
- 獣医学研究所
- 法医学研究所
- その他
エンドユーザーに基づいて、市場は病院、診断センター、製薬およびバイオ医薬品会社、研究および学術機関、臨床研究機関、獣医研究所、法医学研究所、その他に分類されます。
流通チャネル
- 直接入札
- 小売販売
- その他
流通チャネルに基づいて、市場は直接入札、小売販売、その他に分類されます。
競争環境とサウジアラビアの q-PCR 試薬市場シェア分析
サウジアラビアの q-PCR 試薬市場の競争状況は、競合他社の詳細を提供します。詳細には、会社概要、会社の財務状況、収益、市場の可能性、研究開発への投資、新しい市場への取り組み、生産拠点と施設、会社の強みと弱み、製品の発売、製品試験パイプライン、製品の承認、特許、製品の幅と幅、アプリケーションの優位性、および技術ライフライン曲線が含まれます。提供されている上記のデータ ポイントは、サウジアラビアの q-PCR 試薬市場への会社の重点にのみ関連しています。
サウジアラビアのq-PCR試薬市場で活動している主要な市場プレーヤーには、F. Hoffmann-La Roche Ltd、Thermo Fisher Scientific Inc.、Agilent Technologies, Inc.、Bio-Rad Laboratories, Inc.、Quantabio、Sino Biological Inc.、QIAGEN、TAKARA HOLDINGS INC、Enzo Biochem Inc.、meridian BIOSCIENCE、Tonbo Biosciences(Cytek Biosciencesの子会社)、Norgen Biotek Corp.などがあります。
SKU-
世界初のマーケットインテリジェンスクラウドに関するレポートにオンラインでアクセスする
- インタラクティブなデータ分析ダッシュボード
- 成長の可能性が高い機会のための企業分析ダッシュボード
- カスタマイズとクエリのためのリサーチアナリストアクセス
- インタラクティブなダッシュボードによる競合分析
- 最新ニュース、更新情報、トレンド分析
- 包括的な競合追跡のためのベンチマーク分析のパワーを活用
目次
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF SAUDI ARABIA Q-PCR REAGENTS MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 PRODUCTS LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 MARKET END USER COVERAGE GRID
2.11 SECONDARY SOURCES
2.12 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHT
4.1 PESTEL ANALYSIS
4.2 PORTER’S FIVE FORCES
4.3 GLOBAL QPCR REAGENTS MARKET, PRICING ANALYSIS
5 COST ANALYSIS BREAKDOWN
6 HEALTHCARE ECONOMY
7 INDUSTRY INSIGHTS
8 INNOVATION TRACKER AND STRATEGIC ANALYSIS
9 TECHNOLOGY ROADMAP
10 VALUE CHAIN ANALYSIS
11 REGULATIONS
12 MARKET OVERVIEW
12.1 DRIVERS
12.1.1 INCREASING PREVALENCE OF CHRONIC AND COMMUNICABLE DISEASES
12.1.2 INCREASE IN GERIATRIC POPULATION
12.1.3 INCREASE IN EARLY DIAGNOSIS RATE
12.1.4 INCREASE IN ADVANCED TECHNOLOGY
12.2 RESTRAINTS
12.2.1 STRINGENT RULES AND REGULATIONS
12.2.2 HIGH COST OF PRODUCT
12.3 OPPORTUNITIES
12.3.1 RISE IN GOVERNMENT FUNDING FOR HEALTHCARE
12.3.2 RISE IN HEALTHCARE EXPENDITURE
12.4 CHALLENGES
12.4.1 LACK OF SKILLED WORKFORCE
12.4.2 OPERATIONAL BARRIERS FACED IN CONDUCTING DIAGNOSTIC TESTS
13 SAUDI ARABIA QPCR REAGENTS MARKET, BY TYPE
13.1 OVERVIEW
13.2 REAGENTS
13.2.1 MASTER MIX
13.2.1.1 DNA
13.2.1.2 RNA
13.2.2 PRIMERS AND DNTPS
13.2.3 ASSAY
13.2.4 NUCLEIC ACID GEL STAIN
13.2.5 DNASE BUFFER
13.2.6 OTHERS
13.2. CONSUMABLES
13.2.1. PIPETTE TIPS
13.2.2. TUBES
13.2.3. PLATES
13.2.3.1. STANDARD
13.2.3.2. CUSTOM
13.2.4. SEALING FOILS
13.2.5. TUBE STRIPS
13.2.6. OTHERS
14 SAUDI ARABIA QPCR REAGENTS MARKET, BY APPLICATION
14.2. OVERVIEW
14.3. CLINICAL
13.3 HUMAN DIAGNOSTICS
14.3.1.1. HOSPITALS
14.3.1.2. DIAGNOSTIC CENTERS
14.3.1.3. PATHOLOGY LABS
14.3.1.4. OTHERS
13.4 VETERINARY DIAGNOSTICS
14.3.1.5. VETERINARY HOSPITALS
14.3.1.6. DIAGNOSTIC CENTERS
14.3.1.7. VETERINARY LABORATORY
14.3.1.8. VETERINARY CLINICS
14.4. RESEARCH
14.4.1. GENE EXPRESSION ANALYSIS
14.4.1.1. RESEARCH & ACADEMIC INSTITUTES
14.4.1.2. PHARMACEUTICAL & BIOTECHNOLOGY COMPANIES
14.4.1.3. OTHERS
14.4.2. GENOTYPING
14.4.2.1. RESEARCH & ACADEMIC INSTITUTES
14.4.2.2. PHARMACEUTICAL & BIOTECHNOLOGY COMPANIES
14.4.2.3. OTHERS
14.4.3. MICROBE AND PATHOGEN DETECTION
14.4.3.1. RESEARCH & ACADEMIC INSTITUTES
14.4.3.2. PHARMACEUTICAL & BIOTECHNOLOGY COMPANIES
14.4.3.3. OTHERS
14.4.4. SINGLE NUCLEOTIDE POLYMORPHISM (SNP) DETECTION
14.4.4.1. RESEARCH & ACADEMIC INSTITUTES
14.4.4.2. PHARMACEUTICAL & BIOTECHNOLOGY COMPANIES
14.4.4.3. OTHERS
14.4.5. DRUG DEVELOPMENT
14.4.5.1. PHARMACEUTICAL & BIOTECHNOLOGY COMPANIES
14.4.5.2. CLINICAL RESEARCH ORGANIZATION
14.4.5.3. RESEARCH & ACADEMIC INSTITUTES
14.4.5.4. OTHERS
14.4.6. DRUG DISCOVERY
14.4.6.1. PHARMACEUTICAL & BIOTECHNOLOGY COMPANIES
14.4.6.2. CLINICAL RESEARCH ORGANIZATION
14.4.6.3. RESEARCH & ACADEMIC INSTITUTES
14.4.6.4. OTHERS
14.4.7. OTHERS
14.5. FOOD PRODUCT SAFETY TESTING
14.5.1. FOOD REGULATORY AGENCIES
14.5.2. FOOD TESTING LABORATORIES
14.5.3. FOOD COMPANIES
14.5.4. OTHERS
14.6. FORENSICS
14.6.1. PRIVATE FORENSIC LABORATORIES
14.6.2. PUBLIC FORENSIC LABORATORIES
14.7. OTHERS
15 SAUDI ARABIA QPCR REAGENTS MARKET, BY END USER
15.2. OVERVIEW
15.3. HOSPITALS
15.4. DIAGNOSTIC CENTERS
15.5. PHARMACEUTICAL AND BIOPHARMACEUTICAL COMPANIES
15.6. RESEARCH & ACADEMIC INSTITUTE
15.7. CLINICAL RESEARCH ORGANIZATION
15.8. VETERINARY LABS
15.9. FORENSIC LABORATORIES
15.10. OTHERS
16 SAUDI ARABIA QPCR REAGENTS MARKET, BY DISTRIBUTION CHANNEL
16.2. OVERVIEW
16.3. DIRECT TENDER
16.4. RETAIL SALES
16.5. OTHERS
17 SAUDI ARABIA QPCR REAGENTS MARKET: COMPANY LANDSCAPE
17.2. COMPANY SHARE ANALYSIS: SAUDI ARABIA
18 SWOT ANALYSIS
19 COMPANY PROFILE
19.2. F. HOFFMANN-LA ROCHE LTD
19.2.1. COMPANY SNAPSHOT
19.2.2. REVENUE ANALYSIS
19.2.3. PRODUCT PORTFOLIO
19.2.4. RECENT DEVELOPMENT
19.3. THERMO FISHER SCIENTIFIC INC.
19.3.1. COMPANY SNAPSHOT
19.3.2. REVENUE ANALYSIS
19.3.3. PRODUCT PORTFOLIO
19.3.4. RECENT DEVELOPMENTS
19.4. AGILENT TECHNOLOGIES, INC.
19.4.1. COMPANY SNAPSHOT
19.4.2. REVENUE ANALYSIS
19.4.3. PRODUCT PORTFOLIO
19.4.4. RECENT DEVELOPMENTS
19.4.4.1. DISTRIBUTION AGREEMENT
19.4.4.2. PRODUCT LAUNCH
19.5. BIO-RAD LABORATORIES, INC.
19.5.1. COMPANY SNAPSHOT
19.5.2. REVENUE ANALYSIS
19.5.3. PRODUCT PORTFOLIO
19.5.4. RECENT DEVELOPMENTS
19.6. QUANTABIO
19.6.1. COMPANY SNAPSHOT
19.6.2. PRODUCT PORTFOLIO
19.6.3. RECENT DEVELOPMENT
19.7. ENZO BIOCHEM INC
19.7.1. COMPANY SNAPSHOT
19.7.2. REVENUE ANALYSIS
19.7.3. PRODUCT PORTFOLIO
19.7.4. RECENT DEVELOPMENT
19.8. MERIDIAN BIOSCIENCE
19.8.1. COMPANY SNAPSHOT
19.8.2. PRODUCT PORTFOLIO
19.8.3. RECENT DEVELOPMENTS
19.9. NORGEN BIOTEK CORP
19.9.1. COMPANY SNAPSHOT
19.9.2. PRODUCT PORTFOLIO
19.9.3. RECENT DEVELOPMENT
19.10. QIAGEN
19.10.1. COMPANY SNAPSHOT
19.10.2. REVENUE ANALYSIS
19.10.3. PRODUCT PORTFOLIO
19.10.4. RECENT DEVELOPMENTS
19.11. SINO BIOLOGICAL INC.
19.11.1. COMPANY SNAPSHOT
19.11.2. PRODUCT PORTFOLIO
19.11.3. RECENT DEVELOPMENT
19.12. TAKARA HOLDINGS INC
19.12.1. COMPANY SNAPSHOT
19.12.2. REVENUE ANALYSIS
19.12.3. PRODUCT PORTFOLIO
19.12.4. RECENT DEVELOPMENTS
19.13. TONBO BIOSCIENCES (A SUBSIDIARY OF CYTEK BIOSCIENCES)
19.13.1. COMPANY SNAPSHOT
19.13.2. PRODUCT PORTFOLIO
19.13.3. RECENT DEVELOPMENT
20 QUESTIONNAIRE
21 RELATED REPORTS
表のリスト
TABLE 1 THE FOLLOWING ARE THE PRICES OF PRODUCTS (CONSUMABLES AND REAGENTS) OFFERED BY THE COMPANIES
TABLE 2 MEDICAL DEVICE CLASSIFICATION SYSTEM:
TABLE 3 APPROVAL PROCESS OF MEDICAL DEVICES:
TABLE 4 SAUDI ARABIA QPCR REAGENTS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 5 SAUDI ARABIA REAGENTS IN QPCR REAGENTS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 6 SAUDI ARABIA MASTER MIX IN QPCR REAGENTS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 7 SAUDI ARABIA CONSUMABLES IN QPCR REAGENTS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 8 SAUDI ARABIA PLATES IN QPCR REAGENTS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 9 SAUDI ARABIA QPCR REAGENTS MARKET, BY APPLICATION, 2021-2030 (USD THOUSAND)
TABLE 10 SAUDI ARABIA CLINICAL IN QPCR REAGENTS MARKET, BY APPLICATION, 2021-2030 (USD THOUSAND)
TABLE 11 SAUDI ARABIA HUMAN DIAGNOSTICS IN QPCR REAGENTS MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 12 SAUDI ARABIA VETERINARY DIAGNOSTICS IN QPCR REAGENTS MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 13 SAUDI ARABIA RESEARCH IN QPCR REAGENTS MARKET, BY APPLICATION, 2021-2030 (USD THOUSAND)
TABLE 14 SAUDI ARABIA GENE EXPRESSION ANALYSIS IN QPCR REAGENTS MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 15 SAUDI ARABIA GENOTYPING IN QPCR REAGENTS MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 16 SAUDI ARABIA MICROBE AND PATHOGEN DETECTION IN QPCR REAGENTS MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 17 SAUDI ARABIA SINGLE NUCLEOTIDE POLYMORPHISM (SNP) DETECTION IN QPCR REAGENTS MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 18 SAUDI ARABIA DRUG DEVELOPMENT IN QPCR REAGENTS MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 19 SAUDI ARABIA DRUG DISCOVERY IN QPCR REAGENTS MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 20 SAUDI ARABIA FOOD PRODUCT SAFETY TESTING IN QPCR REAGENTS MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 21 SAUDI ARABIA FORENSIC IN QPCR REAGENTS MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 22 SAUDI ARABIA QPCR REAGENTS MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 23 SAUDI ARABIA QPCR REAGENTS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
図表一覧
FIGURE 1 SAUDI ARABIA Q-PCR REAGENTS MARKET: SEGMENTATION
FIGURE 2 SAUDI ARABIA Q-PCR REAGENTS MARKET: DATA TRIANGULATION
FIGURE 3 SAUDI ARABIA Q-PCR REAGENTS MARKET: DROC ANALYSIS
FIGURE 4 SAUDI ARABIA Q-PCR REAGENTS MARKET: GLOBAL VS REGIONAL MARKET ANALYSIS
FIGURE 5 SAUDI ARABIA Q-PCR REAGENTS MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 SAUDI ARABIA Q-PCR REAGENTS MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 SAUDI ARABIA Q-PCR REAGENTS MARKET: DBMR MARKET POSITION GRID
FIGURE 8 SAUDI ARABIA Q-PCR REAGENTS MARKET: MARKET END USER GRID
FIGURE 9 SAUDI ARABIA Q-PCR REAGENTS MARKET: SEGMENTATION
FIGURE 10 INCREASING GERIATRIC POPULATION AND INCREASE IN EARLY DIAGNOSIS RATE IS EXPECTED TO DRIVE THE GROWTH OF THE SAUDI ARABIA Q-PCR REAGENTS MARKET IN THE FORECAST PERIOD OF 2023 TO 2030
FIGURE 11 TYPE SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE SAUDI ARABIA Q-PCR REAGENTS MARKET FROM 2023 TO 2030
FIGURE 12 DRIVERS, RESTRAINTS, OPPORTUNITIES AND CHALLENGES OF SAUDI ARABIA Q-PCR REGANETS MARKET
FIGURE 13 SAUDI ARABIA QPCR REAGENTS MARKET: BY TYPE, 2022
FIGURE 14 SAUDI ARABIA QPCR REAGENTS MARKET: BY APPLICATION, 2022
FIGURE 15 SAUDI ARABIA QPCR REAGENTS MARKET: BY END USER, 2022
FIGURE 16 SAUDI ARABIA QPCR REAGENTS MARKET: BY DISTRIBUTION CHANNEL, 2022
FIGURE 17 SAUDI ARABIA Q-PCR REAGENTS MARKET: COMPANY SHARE 2022 (%)

調査方法
データ収集と基準年分析は、大規模なサンプル サイズのデータ収集モジュールを使用して行われます。この段階では、さまざまなソースと戦略を通じて市場情報または関連データを取得します。過去に取得したすべてのデータを事前に調査および計画することも含まれます。また、さまざまな情報ソース間で見られる情報の不一致の調査も含まれます。市場データは、市場統計モデルと一貫性モデルを使用して分析および推定されます。また、市場シェア分析と主要トレンド分析は、市場レポートの主要な成功要因です。詳細については、アナリストへの電話をリクエストするか、お問い合わせをドロップダウンしてください。
DBMR 調査チームが使用する主要な調査方法は、データ マイニング、データ変数が市場に与える影響の分析、および一次 (業界の専門家) 検証を含むデータ三角測量です。データ モデルには、ベンダー ポジショニング グリッド、市場タイムライン分析、市場概要とガイド、企業ポジショニング グリッド、特許分析、価格分析、企業市場シェア分析、測定基準、グローバルと地域、ベンダー シェア分析が含まれます。調査方法について詳しくは、お問い合わせフォームから当社の業界専門家にご相談ください。
カスタマイズ可能
Data Bridge Market Research は、高度な形成的調査のリーダーです。当社は、既存および新規のお客様に、お客様の目標に合致し、それに適したデータと分析を提供することに誇りを持っています。レポートは、対象ブランドの価格動向分析、追加国の市場理解 (国のリストをお問い合わせください)、臨床試験結果データ、文献レビュー、リファービッシュ市場および製品ベース分析を含めるようにカスタマイズできます。対象競合他社の市場分析は、技術ベースの分析から市場ポートフォリオ戦略まで分析できます。必要な競合他社のデータを、必要な形式とデータ スタイルでいくつでも追加できます。当社のアナリスト チームは、粗い生の Excel ファイル ピボット テーブル (ファクト ブック) でデータを提供したり、レポートで利用可能なデータ セットからプレゼンテーションを作成するお手伝いをしたりすることもできます。