北米の膵臓がん診断市場、検査タイプ別(画像検査、生検、血液検査、ゲノム検査など)、がんのステージ(ステージ0、ステージI、ステージII、ステージIII、ステージIV)、腫瘍タイプ別(外分泌腫瘍および神経内分泌腫瘍)、製品(機器ベースの製品、プラットフォームベースの製品、キットおよび試薬、その他の消耗品)、技術(蛍光in situハイブリダイゼーション、次世代シーケンシング、蛍光免疫測定法、比較ゲノムハイブリダイゼーション、免疫組織化学、その他)、用途別(スクリーニング、診断および予測、予後、研究)、エンドユーザー別(病院、診断センター、がん研究センター、学術機関、外来手術センター、その他)、流通チャネル別(直接入札、小売販売、その他)、業界動向および2030年までの予測。
北米の膵臓がん診断市場の分析と洞察
膵臓がんの罹患率の増加と、これらの疾患に対する診断用製品の必要性の高まりにより、市場の需要が高まっています。製品の容易な供給と迅速な製造施設のための技術の進歩も、市場の成長に貢献しています。主要な市場プレーヤーは、この重要な時期に製品の発売と製品承認に重点を置いています。さらに、政府と規制当局は、急増する患者のために製品承認によって市場プレーヤーをサポートしています。
北米の膵臓がん診断市場は、市場プレーヤーの増加と高度なサービスの利用可能性により、予測年度に成長すると予想されています。これに伴い、メーカーは市場で新しいサービスを開始するための研究開発活動に取り組んでいます。白血病の診断と開発の分野での研究の増加は、市場の成長をさらに後押しすると予想されます。しかし、白血病のスクリーニング技術の難しさは、予測期間中の北米の膵臓がん診断市場の成長を妨げると予想されます。がんの診断と治療に対する医療費の増加は、市場に治療を強化する機会を与えると予想されます。定期的な健康診断、今後の診断センター、膵臓がんの診断方法の進歩、技術開発に関する認識の向上は、市場の成長を後押しすると予想されます。しかし、検査の高コストと、がん診断製品および機器の承認と商品化に関する厳格な規制と基準は、市場の成長を困難にすると予想されます。
老年人口の増加、市場関係者と政府による戦略的取り組み、医療費の急増により、市場には治療を強化する機会が生まれています。しかし、熟練した専門家の不足と厳格な規制枠組みが市場の成長にとっての大きな課題となっています。ただし、機器と治療の高コストにより、北米の膵臓がん診断市場の成長が抑制されると予想されます。
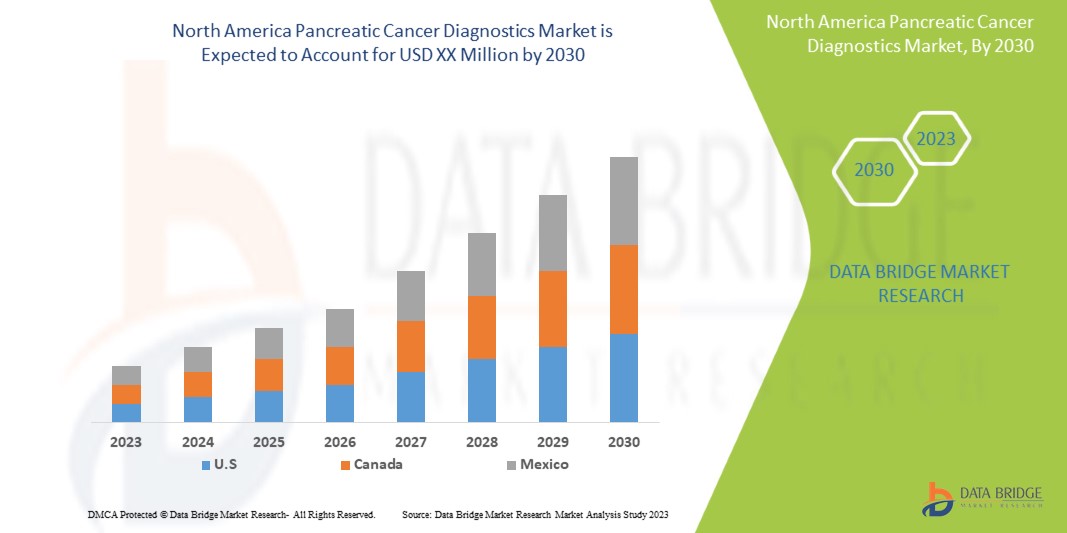
北米の膵臓がん診断市場は支援的であり、病気を減らして個人の回復とパフォーマンスを向上させることを目指しています。Data Bridge Market Research は、北米の膵臓がん診断市場は 2023 年から 2030 年の予測期間中に 7.7% の CAGR で成長すると分析しています。
|
レポートメトリック |
詳細 |
|
予測期間 |
2023年から2030年 |
|
基準年 |
2022 |
|
歴史的な年 |
2021 (2020~2016年にカスタマイズ可能) |
|
定量単位 |
売上高は百万米ドル、価格は米ドル |
|
対象セグメント |
検査タイプ(画像検査、生検、血液検査、ゲノム検査、その他)、がんのステージ(ステージ 0、ステージ I、ステージ II、ステージ III、ステージ IV)、腫瘍の種類(外分泌腫瘍および神経内分泌腫瘍)、製品(機器ベースの製品、プラットフォームベースの製品、キットおよび試薬、その他の消耗品)、技術(蛍光 in situ ハイブリダイゼーション、次世代シーケンシング、蛍光免疫測定法、比較ゲノムハイブリダイゼーション、免疫組織化学、その他)、用途(スクリーニング、診断および予測、予後、研究)、エンドユーザー(病院、診断センター、がん研究センター、学術機関、外来手術センター、その他)、流通チャネル(直接入札、小売販売、その他)別。 |
|
対象国 |
米国、カナダ、メキシコ。 |
|
対象となる市場プレーヤー |
Siemens Healthcare Private Limited、Koninklijke Philips NV、富士フイルム株式会社、Grail、Laboratory Corporation of America Holdings、DiaSource、Abbott、Agilent Technologies, Inc.、Lee Biosolutions, Inc、MP BIOMEDICALS、Setia Scientific Solution、Boditech Med Inc.、AccuBioTech Co., Ltd.、Thermo Fisher Scientific、Creative Biolabs、Myriad Genetics, Inc.、BD、CANON MEDICAL SYSTEMS CORPORATION、QIAGEN、Meridian Life Science, Inc.、CTK Biotech, Inc.、その他 |
市場の定義
膵臓がんは致命的であり、膵臓がんの診断プロセスにも安全性の問題があり、費用対効果が高くありません。北米で治療に最も費用がかかる疾患の 1 つはがんです。がん患者は入院し、手術、放射線治療、全身療法など、腫瘍に対するさまざまな治療を受ける場合があります。がん患者の健康保険料は、現在、過去よりも高額になっています。さらに、自己負担額、控除額、共同保険料も上昇しています。膵臓がんの診断には、超音波、生検手順、血液検査が含まれます。膵臓がんは、世界中で主要な死亡原因の 1 つであり、この病気の罹患率は驚くべき速度で増加しています。
北米の膵臓がん診断市場の動向
このセクションでは、市場の推進要因、機会、制約、課題について理解します。これらはすべて、以下で詳しく説明します。
ドライバー
- 膵臓がんの罹患率が上昇
このタイプのがんは、あらゆる年齢層の人に発症する可能性があります。膵臓がんは、その幅広い兆候や症状にもかかわらず、非特異的であり、他のより広範な病状に関連している可能性があるため、診断が難しい場合があります。膵臓がんは、女性では8番目に多いがんで、男性では10番目に多いがんです。膵臓がんの発生率は、毎年約1%増加しています。発生頻度は低いです。女性よりも男性にわずかに多く見られますが、男女ともに膵臓がんになる平均生涯リスクは平均で約0.5%です。これらの症状には、腹痛、食欲不振または意図しない体重減少、皮膚や白目の黄色化(黄疸)、色の薄い便、色の濃い尿、皮膚のかゆみなどがあります。これは、成人および小児で診断される8番目に多いタイプのがんですが、ほとんどの症例は成人に発生します。どの年齢でも診断される可能性がありますが、45 歳未満で診断されることはまれです。診断される平均年齢は 68 歳です。
さまざまなリスク要因により、北米では膵臓がんの発症率が上昇しており、重大な社会経済的問題となっています。これは、北米の膵臓がん診断市場の推進力となることが期待されています。
- 膵臓診断における新たな技術的進歩
膵臓がんは、最も治癒しやすい初期段階で発見されることはほとんどありません。これは、他の臓器に転移するまで症状が現れないことが多いためです。専門家は、顕微鏡で細胞画像を調べ、注釈でラベルを付けることで、がん細胞と非がん細胞を手動で診断する必要があります。しかし、この手作業による顕微鏡検査は時間がかかり、誤った診断を下す可能性があります。その後、コンピューター ソフトウェアを使用して、誤った薬を処方するリスクが軽減されました。膵臓疾患の壊滅的な影響を阻止するには、自動で信頼性の高い分類システムの作成が不可欠になりました。複数のセグメンテーション手法が、既存の膵臓がん分類アルゴリズムの基礎を構成しました。
機会
- がんの診断と治療にかかる医療費の増加
Across the globe, research and development activities are escalating owing to the public health expenditure with economic performances. Whereas the healthcare industry ranks second among all industries when it comes to the amount spent on healthcare. Rising healthcare expenditure can result in better provision of research and development opportunities. It is anticipated to increase the demand for pancreatic cancer diagnostics. Increasing the healthcare expenditure for pancreatic cancer treatment also helps the patient take hassle-free advanced diagnostics and treatment for fast recovery. The spending on healthcare is made up of the combination of out-of-pocket payments (people paying for their care), government expenditure, and sources. It also includes health insurance and activities by non-governmental organizations. This increasing healthcare expenditure for cancer treatment is an opportunity for the market's demand.
Restraint/Challenge
- Late diagnosis and poor prognosis of pancreatic cancer
Late diagnosis of disease is due to the increasing pancreatic cancer tumors which do not respond as well to commonly used cancer therapies as other, less lethal types of cancer. But there are treatment options, including surgery, chemotherapy and radiation. There are different types of pancreatic cancer. Most pancreatic cancers are the exocrine type. This means that they start in cells that produce pancreatic digestive juices. About 30 percent of patients are smokers, and 5 percent have a history of pancreatitis, an inflammation of the pancreas, which can be caused by stones or heavy alcohol intake.
Post COVID-19 Impact on North America Pancreatic Cancer Diagnostics Market
COVID-19 has negatively affected the growth of the market as patients suffering for pancreatic cancer postponed their surgery due to rapid surge in covid-19 cases across geographies. Additionally, people having pancreatic cancer were at risk of becoming severely ill. The fear of corona virus infection affected the growth of the pancreatic cancer diagnostic market amid pandemic.
Recent Developments
- In December 2022, FUJIFILM Holdings America Corporation announced the company has purchase agreement asset with Inspirata, Inc. to acquire digital pathology business to expand robust enterprise imaging offering. This results in enabling the integration of pathology images and data into a healthcare organization’s electronic health record system to streamline care delivery for oncology patients.
- In August 2020, Siemens Healthcare GmbH announced that it has entered in to an agreement with Varian Medical Systems, Inc. With this acquisition, Siemens Healthcare has helped in developing advanced solutions to treat against cancer and strengthen their position in healthcare industry.
North America Pancreatic Cancer Diagnostics Market Scope
北米の膵臓がん診断市場は、検査の種類、がんの段階、腫瘍の種類、製品、用途、技術、エンドユーザー、流通チャネルに基づいて、8 つの主要なセグメントに分類されています。セグメント間の成長は、ニッチな成長分野と市場にアプローチするための戦略を分析し、コア アプリケーション領域とターゲット市場の違いを決定するのに役立ちます。
テストの種類
- 画像検査
- 生検
- 血液検査
- ゲノム検査
- その他
検査の種類に基づいて、北米の膵臓がん診断市場は、画像検査、生検、血液検査、ゲノム検査などに分類されます。
がんのステージ
- ステージ0
- ステージI
- ステージ II
- ステージIII
- ステージIV
がんのステージに基づいて、北米の膵臓がん診断市場は、ステージ 0、ステージ I、ステージ II、ステージ III、ステージ IV に分類されます。
腫瘍の種類
- 外分泌腫瘍
- 神経内分泌腫瘍
腫瘍の種類に基づいて、北米の膵臓がん診断市場は外分泌腫瘍と神経内分泌腫瘍に分類されます。
製品
- 計測機器ベースの製品
- プラットフォームベースの製品
- キットと試薬
- その他の消耗品
製品に基づいて、北米の膵臓がん診断市場は、機器ベースの製品、プラットフォームベースの製品、キットと試薬、およびその他の消耗品に分類されます。
応用
- スクリーニング
- 診断と予測
- 予後
- 研究
用途に基づいて、北米の膵臓がん診断市場は、スクリーニング、診断および予測、予後、および研究に分類されます。
テクノロジー
- 蛍光in situハイブリダイゼーション
- 次世代シーケンシング
- 蛍光免疫測定法
- 比較ゲノムハイブリダイゼーション
- 免疫組織化学
- その他
技術に基づいて、北米の膵臓がん診断市場は、蛍光 in situ ハイブリダイゼーション、次世代シーケンシング、蛍光免疫測定、比較ゲノムハイブリダイゼーション、免疫組織化学などに分類されます。
エンドユーザー
- 病院
- 診断センター
- がん研究センター
- 学術機関
- 外来手術センター
- その他
エンドユーザーに基づいて、北米の膵臓がん診断市場は、病院、診断センター、がん研究センター、学術機関、外来手術センターなどに分類されます。
流通チャネル
- 直接入札
- 小売販売
- その他
流通チャネルに基づいて、北米の膵臓がん診断市場は、直接入札、小売販売、その他に分類されます。
北米の膵臓がん診断市場の国別分析/洞察
北米の膵臓がん診断市場が分析され、上記の国、検査タイプ、がんのステージ、腫瘍の種類、製品、アプリケーション、テクノロジー、エンドユーザー、流通チャネル別に市場規模の洞察と傾向が提供されます。
- 2023年には、膵臓がんの有病率と発症率の上昇、および膵臓がん診断に関する認知度の高まりにより、米国の膵臓がん診断市場は成長すると予想されています。これらは、同国の市場の成長を後押しすると予想される主な要因です。
レポートの国別セクションでは、市場の現在および将来の動向に影響を与える個別の市場影響要因と市場規制の変更も提供しています。下流および上流のバリュー チェーン分析、技術動向、ポーターの 5 つの力の分析、ケース スタディなどのデータ ポイントは、個々の国の市場シナリオを予測するために使用される指標の一部です。また、ブランドの存在と可用性、地元および国内ブランドとの競争が激しいか少ないために直面する課題、国内関税と貿易ルートの影響を考慮しながら、国別データの予測分析を提供します。
競争環境と北米の膵臓がん診断市場シェア分析
北米の膵臓がん診断市場の競争状況は、競合他社の詳細を提供します。含まれる詳細には、会社概要、会社の財務状況、収益、市場の可能性、研究開発への投資、新しい市場への取り組み、プレゼンス、生産拠点と施設、生産能力、会社の強みと弱み、製品の発売、製品の幅と広さ、アプリケーションの優位性などがあります。提供されている上記のデータ ポイントは、北米の膵臓がん診断市場への会社の重点にのみ関連しています。
市場で活動している主要企業としては、Siemens Healthcare Private Limited、Koninklijke Philips NV、富士フイルム株式会社、Grail、Laboratory Corporation of America Holdings、DiaSource、Abbott、Agilent Technologies, Inc.、Lee Biosolutions, Inc、MP BIOMEDICALS、Setia Scientific Solution、Boditech Med Inc.、AccuBioTech Co., Ltd.、Thermo Fisher Scientific、Creative Biolabs、Myriad Genetics, Inc.、BD、CANON MEDICAL SYSTEMS CORPORATION、QIAGEN、Meridian Life Science, Inc.、CTK Biotech, Inc. などがあります。
SKU-
世界初のマーケットインテリジェンスクラウドに関するレポートにオンラインでアクセスする
- インタラクティブなデータ分析ダッシュボード
- 成長の可能性が高い機会のための企業分析ダッシュボード
- カスタマイズとクエリのためのリサーチアナリストアクセス
- インタラクティブなダッシュボードによる競合分析
- 最新ニュース、更新情報、トレンド分析
- 包括的な競合追跡のためのベンチマーク分析のパワーを活用
目次
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF THE NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATIONS
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 DBMR TRIPOD DATA VALIDATION MODEL
2.5 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.6 MULTIVARIATE MODELLING
2.7 MARKET END USER COVERAGE GRID
2.8 PRODUCT LIFELINE CURVE
2.9 DBMR MARKET POSITION GRID
2.1 VENDOR SHARE ANALYSIS
2.11 SECONDARY SOURCES
2.12 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL ANALYSIS
4.2 PORTER’S FIVE FORCES MODEL
5 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, INDUSTRY INSIGHTS
6 EPIDEMIOLOGY
7 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, REGULATIONS
8 MARKET OVERVIEW
8.1 DRIVERS
8.1.1 GROW IN PREVALENCE OF PANCREATIC CANCER
8.1.2 NOVEL TECHNOLOGICAL ADVANCEMENTS IN PANCREATIC DIAGNOSTICS
8.1.3 RISING PREFERENCE FOR PREVENTIVE HEALTH CHECK-UPS
8.1.4 INCREASE IN AWARENESS REGARDING PANCREATIC CANCER
8.2 RESTRAINTS
8.2.1 STRICT REGULATIONS AND STANDARDS FOR THE APPROVAL AND COMMERCIALIZATION OF PANCREATIC CANCER DIAGNOSTIC PRODUCTS
8.2.2 LATE DIAGNOSIS AND POOR PROGNOSIS OF PANCREATIC CANCER
8.3 OPPORTUNITIES
8.3.1 INCREASE IN DIAGNOSTIC PRODUCTS FOR PANCREATIC CANCER
8.3.2 RISE IN HEALTHCARE EXPENDITURE FOR CANCER DIAGNOSIS AND TREATMENT
8.3.3 GOVERNMENT INITIATIVES TOWARD PANCREATIC CANCER DIAGNOSTICS
8.4 CHALLENGES
8.4.1 INCREASED COST, SAFETY, AND CONVENIENCE ISSUES
8.4.2 LACK OF SKILLED AND CERTIFIED PROFESSIONALS
9 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE
9.1 OVERVIEW
9.2 IMAGING TEST
9.2.1 COMPUTED TOMOGRAPHY (CT) SCAN
9.2.2 MAGNETIC RESONANCE IMAGING (MRI)
9.2.2.1 MR CHOLANGIOPANCREATOGRAPHY
9.2.2.2 MR ANGIOGRAPHY (MRA)
9.2.3 ULTRASOUND
9.2.3.1 ABDOMINAL ULTRASOUND
9.2.3.2 ENDOSCOPIC ULTRASOUND (EUS)
9.2.4 CHOLANGIOPANCREATOGRAPHY
9.2.4.1 MAGNETIC RESONANCE CHOLANGIOPANCREATOGRAPHY (MRCP)
9.2.4.2 ENDOSCOPIC RETROGRADE CHOLANGIOPANCREATOGRAPHY (ERCP)
9.2.4.3 PRECUTANEOUS TRANSHEPTIC CHOLANGIOPANCREATOGRAPHY (PTC)
9.2.5 POSITRON EMISSION TOMOHRAPHY (PET)
9.2.6 OTHERS
9.3 BIOPSY
9.3.1 CT-GUIDED NEEDLE BIOPSY
9.3.2 FINE NEEDLE ASPIRATION (FNA)
9.3.3 CORE NEEDLE BIOPSY
9.3.4 OTHERS
9.4 BLOOD TEST
9.4.1 LIVER FUNCTION TEST
9.4.2 TUMOR MARKER
9.4.2.1 CA 19-9 BIOMARKER TEST
9.4.2.2 CARCINOEMBROYNIC ANTIGEN (CEA) TEST
9.4.2.3 CA 50 MARKER TEST
9.4.2.4 OTHERS
9.4.3 OTHERS
9.5 GENOMIC TEST
9.6 OTHERS
10 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY CANCER STAGES
10.1 OVERVIEW
10.2 STAGE IV
10.3 STAGE III
10.4 STAGE II
10.4.1 STAGE IIA
10.4.2 STAGE IIB
10.5 STAGE I
10.5.1 STAGE IA
10.5.2 STAGE IB
10.6 STAGE 0
11 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE
11.1 OVERVIEW
11.2 EXOCRINE TUMORS
11.2.1 INSTRUMENT-BASED PRODUCTS
11.2.2 PLATFORM-BASED PRODUCTS
11.2.3 KITS AND REAGENTS
11.2.4 OTHER CONSUMABLES
11.3 NEUROENDOCRINE TUMORS
11.3.1 INSTRUMENT-BASED PRODUCTS
11.3.2 PLATFORM-BASED PRODUCTS
11.3.3 KITS AND REAGENTS
11.3.4 OTHER CONSUMABLES
12 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT
12.1 OVERVIEW
12.2 INSTRUMENT-BASED PRODUCTS
12.2.1 IMAGING
12.2.2 BIOPSY
12.3 PLATFORM-BASED PRODUCTS
12.3.1 NEXT-GENERATION SEQUENCING
12.3.2 MICROARRAYS
12.3.3 PCR
12.3.4 OTHERS
12.4 KITS AND REAGENTS
12.4.1 CA19-9 PANCREATIC CANCER TEST KITS
12.4.1.1 ELISA TEST KITS
12.4.1.2 CASETTE TEST KITS
12.4.1.3 OTHERS
12.4.2 CEA PANCREATIC CANCER TEST KITS
12.4.2.1 ELISA TEST KITS
12.4.2.2 CASETTE TEST KITS
12.4.2.3 OTHERS
12.5 OTHER CONSUMABLES
13 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY TECHNOLOGY
13.1 OVERVIEW
13.2 FLUORESCENT IN SITU HYBRIDIZATION
13.3 NEXT GENERATION SEQUENCING
13.4 FLUORIMMUNOASSAY
13.5 COMPARATIVE GENOMIC HYBRIDIZATION
13.6 IMMUNOHISTOCHEMICAL
13.7 OTHERS
14 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY APPLICATION
14.1 OVERVIEW
14.2 SCREENING
14.2.1 INSTRUMENT-BASED PRODUCTS
14.2.2 PLATFORM-BASED PRODUCTS
14.2.3 KITS AND REAGENTS
14.2.4 OTHER CONSUMABLES
14.3 DIAGNOSTIC AND PREDICTIVE
14.3.1 INSTRUMENT-BASED PRODUCTS
14.3.2 PLATFORM-BASED PRODUCTS
14.3.3 KITS AND REAGENTS
14.3.4 OTHER CONSUMABLES
14.4 PROGNOSTIC
14.4.1 INSTRUMENT-BASED PRODUCTS
14.4.2 PLATFORM-BASED PRODUCTS
14.4.3 KITS AND REAGENTS
14.4.4 OTHER CONSUMABLES
14.5 RESEARCH
14.5.1 INSTRUMENT-BASED PRODUCTS
14.5.2 PLATFORM-BASED PRODUCTS
14.5.3 KITS AND REAGENTS
14.5.4 OTHER CONSUMABLES
15 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY END USER
15.1 OVERVIEW
15.2 HOSPITALS
15.3 DIAGNOSTIC CENTERS
15.4 CANCER RESEARCH CENTERS
15.5 ACADEMIC INSTITUTES
15.6 AMBULATORY SURGICAL CENTERS
15.7 OTHERS
16 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL
16.1 OVERVIEW
16.2 DIRECT TENDER
16.3 RETAIL SALES
16.4 OTHERS
17 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION
17.1 NORTH AMERICA
17.1.1 U.S.
17.1.2 CANADA
17.1.3 MEXICO
18 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: COMPANY LANDSCAPE
18.1 COMPANY SHARE ANALYSIS: NORTH AMERICA
19 SWOT ANALYSIS
20 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET
20.1 CANON MEDICAL SYSTEMS CORPORATION
20.1.1 COMPANY SNAPSHOT
20.1.2 REVENUE ANALYSIS
20.1.3 COMPANY SHARE ANALYSIS
20.1.4 PRODUCT PORTFOLIO
20.1.5 RECENT DEVELOPMENT
20.2 KONINKLIJKE PHILIPS N.V.
20.2.1 COMPANY SNAPSHOT
20.2.2 REVENUE ANALYSIS
20.2.3 COMPANY SHARE ANALYSIS
20.2.4 PRODUCT PORTFOLIO
20.2.5 RECENT DEVELOPMENTS
20.3 SIEMENS HEALTHCARE GMBH
20.3.1 COMPANY SNAPSHOT
20.3.2 REVENUE ANALYSIS
20.3.3 COMPANY SHARE ANALYSIS
20.3.4 PRODUCT PORTFOLIO
20.3.5 RECENT DEVELOPMENT
20.4 GRAIL
20.4.1 COMPANY PROFILE
20.4.2 COMPANY SHARE ANALYSIS
20.4.3 PRODUCT PORTFOLIO
20.4.4 RECENT DEVELOPMENT
20.5 MYRIAD GENETICS, INC.
20.5.1 COMPANY SNAPSHOT
20.5.2 REVENUE ANALYSIS
20.5.3 COMPANY SHARE ANALYSIS
20.5.4 PRODUCT PORTFOLIO
20.5.5 RECENT DEVELOPMENT
20.6 BD
20.6.1 COMPANY SNAPSHOT
20.6.2 REVENUE ANALYSIS
20.6.3 PRODUCT PORTFOLIO
20.6.4 RECENT DEVELOPMENT
20.7 BODITECH MED INC.
20.7.1 COMPANY PROFILE
20.7.2 PRODUCT PORTFOLIO
20.7.3 RECENT DEVELOPMENT
20.8 ABBOTT (2022)
20.8.1 COMPANY SNAPSHOT
20.8.2 REVENUE ANALYSIS
20.8.3 PRODUCT PORTFOLIO
20.8.4 RECENT DEVELOPMENT
20.9 FUJIFILM HOLDINGS AMERICA CORPORATION
20.9.1 COMPANY SNAPSHOT
20.9.2 REVENUE ANALYSIS
20.9.3 PRODUCT PORTFOLIO
20.9.4 RECENT DEVELOPMENT
20.1 ACCUBIOTECH CO., LTD.
20.10.1 COMPANY PROFILE
20.10.2 PRODUCT PORTFOLIO
20.10.3 RECENT DEVELOPMENTS
20.11 AGILENT TECHNOLOGIES, INC.
20.11.1 COMPANY PROFILE
20.11.2 REVENUE ANALYSIS
20.11.3 PRODUCT PORTFOLIO
20.11.4 RECENT DEVELOPMENT
20.12 CREATIVE BIOLABS.
20.12.1 COMPANY PROFILE
20.12.2 PRODUCT PORTFOLIO
20.12.3 RECENT DEVELOPMENT
20.13 CTK BIOTECH, INC.
20.13.1 COMPANY PROFILE
20.13.2 PRODUCT PORTFOLIO
20.13.3 RECENT DEVELOPMENT
20.14 DIASOURCE
20.14.1 COMPANY SNAPSHOT
20.14.2 PRODUCT PORTFOLIO
20.14.3 RECENT DEVELOPMENT
20.15 LABORATORY CORPORATION OF AMERICA HOLDINGS
20.15.1 COMPANY SNAPSHOT
20.15.2 REVENUE ANALYSIS
20.15.3 PRODUCT PORTFOLIO
20.15.4 RECENT DEVELOPMENTS
20.16 LEE BIOSCIENCE
20.16.1 COMPANY SNAPSHOT
20.16.2 PRODUCT PORTFOLIO
20.16.3 RECENT DEVELOPMENT
20.17 MERIDIAN BIOSCIENCE INC.
20.17.1 COMPANY PROFILE
20.17.2 PRODUCT PORTFOLIO
20.17.3 RECENT DEVELOPMENT
20.18 MP BIOMEDICALS.
20.18.1 COMPANY PROFILE
20.18.2 PRODUCT PORTFOLIO
20.18.3 RECENT DEVELOPMENTS
20.19 QIAGEN
20.19.1 COMPANY SNAPSHOT
20.19.2 REVENUE ANALYSIS
20.19.3 PRODUCT PORTFOLIO
20.19.4 RECENT DEVELOPMENT
20.2 SETIA SCIENTIFIC SOLUTION
20.20.1 COMPANY PROFILE
20.20.2 PRODUCT PORTFOLIO
20.20.3 RECENT DEVELOPMENTS
20.21 THERMO FISHER SCIENTIFIC INC.
20.21.1 COMPANY SNAPSHOT
20.21.2 REVENUE ANALYSIS
20.21.3 PRODUCT PORTFOLIO
20.21.4 RECENT DEVELOPMENT
21 QUESTIONNAIRE
22 RELATED REPORTS
表のリスト
TABLE 1 APPROVED DIAGNOSTICS OF PANCREATIC CANCER
TABLE 2 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 3 NORTH AMERICA IMAGING TEST IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 4 NORTH AMERICA IMAGING TEST IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 5 NORTH AMERICA MAGNETIC RESONANCE IMAGING (MRI) IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 6 NORTH AMERICA ULTRASOUND IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 7 NORTH AMERICA CHOLANGIOPANCREATOGRAPHY IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 8 NORTH AMERICA BIOPSY IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 9 NORTH AMERICA BIOPSY IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 10 NORTH AMERICA BLOOD TEST IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 11 NORTH AMERICA BLOOD TEST IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 12 NORTH AMERICA TUMOR MARKER IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 13 NORTH AMERICA GENOMIC TEST IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 14 NORTH AMERICA OTHERS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 15 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY CANCER STAGES, 2021-2030 (USD MILLION)
TABLE 16 NORTH AMERICA STAGE IV IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 17 NORTH AMERICA STAGE III IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 18 NORTH AMERICA STAGE II IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 19 NORTH AMERICA STAGE II IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 20 NORTH AMERICA STAGE I IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 21 NORTH AMERICA STAGE I IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 22 NORTH AMERICA STAGE 0 IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 23 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 24 NORTH AMERICA EXOCRINE TUMORS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 25 NORTH AMERICA EXOCRINE TUMORS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 26 NORTH AMERICA NEUROENDOCRINE TUMORS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 27 NORTH AMERICA NEUROENDOCRINE TUMORS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 28 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 29 NORTH AMERICA INSTRUMENT-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 30 NORTH AMERICA INSTRUMENT-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 31 NORTH AMERICA PLATFORM-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 32 NORTH AMERICA PLATFORM-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 33 NORTH AMERICA KITS AND REAGENTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 34 NORTH AMERICA KITS AND REAGENTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 35 NORTH AMERICA CA19-9 PANCREATIC CANCER TEST KITS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 36 NORTH AMERICA CEA PANCREATIC CANCER TEST KITS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 37 NORTH AMERICA OTHER CONSUMABLES IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 38 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 39 NORTH AMERICA FLUORESCENT IN SITU HYBRIDIZATION IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 40 NORTH AMERICA NEXT GENERATION SEQUENCING IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 41 NORTH AMERICA FLUORIMMUNOASSAY IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 42 NORTH AMERICA COMPARATIVE GENOMIC HYBRIDIZATION IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 43 NORTH AMERICA IMMUNOHISTOCHEMICAL IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 44 NORTH AMERICA OTHERS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 45 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 46 NORTH AMERICA SCREENING IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 47 NORTH AMERICA SCREENING IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 48 NORTH AMERICA DIAGNOSTIC AND PREDICTIVE IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 49 NORTH AMERICA DIAGNOSTIC AND PREDICTIVE IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 50 NORTH AMERICA PROGNOSTIC IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 51 NORTH AMERICA PROGNOSTIC IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 52 NORTH AMERICA RESEARCH IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 53 NORTH AMERICA RESEARCH IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 54 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 55 NORTH AMERICA HOSPITALS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 56 NORTH AMERICA DIAGNOSTIC CENTERS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 57 NORTH AMERICA CANCER RESEARCH CENTERS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 58 NORTH AMERICA ACADEMIC INSTITUTES IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 59 NORTH AMERICA AMBULATORY SURGICAL CENTERS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 60 NORTH AMERICA OTHERS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 61 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 62 NORTH AMERICA DIRECT TENDER IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 63 NORTH AMERICA RETAIL SALES IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 64 NORTH AMERICA OTHERS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 65 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY COUNTRY, 2021-2030 (USD MILLION)
TABLE 66 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 67 NORTH AMERICA IMAGING TEST IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 68 NORTH AMERICA MAGNETIC RESONANCE IMAGING (MRI) IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 69 NORTH AMERICA ULTRASOUND IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 70 NORTH AMERICA CHOLANGIOPANCREATOGRAPHY IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 71 NORTH AMERICA BLOOD TEST IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 72 NORTH AMERICA TUMOR MARKER IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 73 NORTH AMERICA BIOPSY IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 74 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 75 NORTH AMERICA STAGE I IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 76 NORTH AMERICA STAGE II IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 77 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 78 NORTH AMERICA EXOCRINE TUMORS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 79 NORTH AMERICA NEUROENDOCRINE TUMORS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 80 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 81 NORTH AMERICA INSTRUMENT-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 82 NORTH AMERICA INSTRUMENT-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 83 NORTH AMERICA INSTRUMENT-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 84 NORTH AMERICA PLATFORM-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 85 NORTH AMERICA PLATFORM-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 86 NORTH AMERICA PLATFORM-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 87 NORTH AMERICA KITS AND REAGENTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 88 NORTH AMERICA CA19-9 PANCREATIC CANCER TEST KITS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 89 NORTH AMERICA CEA PANCREATIC CANCER TEST KITS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 90 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 91 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 92 NORTH AMERICA SCREENING IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 93 NORTH AMERICA DIAGNOSTIC AND PREDICTIVE IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 94 NORTH AMERICA PROGNOSTIC IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 95 NORTH AMERICA RESEARCH IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 96 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 97 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 98 U.S. PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 99 U.S. IMAGING TEST IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 100 U.S. MAGNETIC RESONANCE IMAGING (MRI) IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 101 U.S. ULTRASOUND IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 102 U.S. CHOLANGIOPANCREATOGRAPHY IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 103 U.S. BLOOD TEST IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 104 U.S. TUMOR MARKER IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 105 U.S. BIOPSY IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 106 U.S. PANCREATIC CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 107 U.S. STAGE I IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 108 U.S. STAGE II IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 109 U.S. PANCREATIC CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 110 U.S. EXOCRINE TUMORS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 111 U.S. NEUROENDOCRINE TUMORS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 112 U.S. PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 113 U.S. INSTRUMENT-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 114 U.S. INSTRUMENT-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 115 U.S. INSTRUMENT-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 116 U.S. PLATFORM-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 117 U.S. PLATFORM-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 118 U.S. PLATFORM-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 119 U.S. KITS AND REAGENTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 120 U.S. CA19-9 PANCREATIC CANCER TEST KITS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 121 U.S. CEA PANCREATIC CANCER TEST KITS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 122 U.S. PANCREATIC CANCER DIAGNOSTICS MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 123 U.S. PANCREATIC CANCER DIAGNOSTICS MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 124 U.S. SCREENING IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 125 U.S. DIAGNOSTIC AND PREDICTIVE IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 126 U.S. PROGNOSTIC IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 127 U.S. RESEARCH IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 128 U.S. PANCREATIC CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 129 U.S. PANCREATIC CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 130 CANADA PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 131 CANADA IMAGING TEST IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 132 CANADA MAGNETIC RESONANCE IMAGING (MRI) IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 133 CANADA ULTRASOUND IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 134 CANADA CHOLANGIOPANCREATOGRAPHY IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 135 CANADA BLOOD TEST IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 136 CANADA TUMOR MARKER IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 137 CANADA BIOPSY IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 138 CANADA PANCREATIC CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 139 CANADA STAGE I IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 140 CANADA STAGE II IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 141 CANADA PANCREATIC CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 142 CANADA EXOCRINE TUMORS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 143 CANADA NEUROENDOCRINE TUMORS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 144 CANADA PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 145 CANADA INSTRUMENT-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 146 CANADA INSTRUMENT-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 147 CANADA INSTRUMENT-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 148 CANADA PLATFORM-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 149 CANADA PLATFORM-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 150 CANADA PLATFORM-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 151 CANADA KITS AND REAGENTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 152 CANADA CA19-9 PANCREATIC CANCER TEST KITS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 153 CANADA CEA PANCREATIC CANCER TEST KITS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 154 CANADA PANCREATIC CANCER DIAGNOSTICS MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 155 CANADA PANCREATIC CANCER DIAGNOSTICS MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 156 CANADA SCREENING IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 157 CANADA DIAGNOSTIC AND PREDICTIVE IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 158 CANADA PROGNOSTIC IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 159 CANADA RESEARCH IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 160 CANADA PANCREATIC CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 161 CANADA PANCREATIC CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 162 MEXICO PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 163 MEXICO IMAGING TEST IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 164 MEXICO MAGNETIC RESONANCE IMAGING (MRI) IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 165 MEXICO ULTRASOUND IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 166 MEXICO CHOLANGIOPANCREATOGRAPHY IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 167 MEXICO BLOOD TEST IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 168 MEXICO TUMOR MARKER IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 169 MEXICO BIOPSY IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 170 MEXICO PANCREATIC CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 171 MEXICO STAGE I IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 172 MEXICO STAGE II IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 173 MEXICO PANCREATIC CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 174 MEXICO EXOCRINE TUMORS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 175 MEXICO NEUROENDOCRINE TUMORS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 176 MEXICO PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 177 MEXICO INSTRUMENT-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 178 MEXICO INSTRUMENT-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 179 MEXICO INSTRUMENT-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 180 MEXICO PLATFORM-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 181 MEXICO PLATFORM-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 182 MEXICO PLATFORM-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 183 MEXICO KITS AND REAGENTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 184 MEXICO CA19-9 PANCREATIC CANCER TEST KITS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 185 MEXICO CEA PANCREATIC CANCER TEST KITS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 186 MEXICO PANCREATIC CANCER DIAGNOSTICS MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 187 MEXICO PANCREATIC CANCER DIAGNOSTICS MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 188 MEXICO SCREENING IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 189 MEXICO DIAGNOSTIC AND PREDICTIVE IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 190 MEXICO PROGNOSTIC IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 191 MEXICO RESEARCH IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 192 MEXICO PANCREATIC CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 193 MEXICO PANCREATIC CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
図表一覧
FIGURE 1 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: SEGMENTATION
FIGURE 2 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: DATA TRIANGULATION
FIGURE 3 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: DROC ANALYSIS
FIGURE 4 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: NORTH AMERICA VS REGIONAL MARKET ANALYSIS
FIGURE 5 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: MARKET END USER COVERAGE GRID
FIGURE 8 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: DBMR MARKET POSITION GRID
FIGURE 9 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: SEGMENTATION
FIGURE 11 GROWING AWARENESS OF PANCREATIC CANCER AND INCREASING HEALTHCARE EXPENDITURE IS EXPECTED TO DRIVE THE GROWTH OF THE NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET FROM 2023 TO 2030
FIGURE 12 IMAGING TEST SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET IN 2023 & 2030
FIGURE 13 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF THE NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET
FIGURE 14 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY TEST TYPE, 2022
FIGURE 15 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY TEST TYPE, 2023-2030 (USD MILLION)
FIGURE 16 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY TEST TYPE, CAGR (2023-2030)
FIGURE 17 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY TEST TYPE, LIFELINE CURVE
FIGURE 18 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY CANCER STAGES, 2022
FIGURE 19 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY CANCER STAGES, 2023-2030 (USD MILLION)
FIGURE 20 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY CANCER STAGES, CAGR (2023-2030)
FIGURE 21 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY CANCER STAGES, LIFELINE CURVE
FIGURE 22 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY TUMOR TYPE, 2022
FIGURE 23 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY TUMOR TYPE, 2023-2030 (USD MILLION)
FIGURE 24 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY TUMOR TYPE, CAGR (2023-2030)
FIGURE 25 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY TUMOR TYPE, LIFELINE CURVE
FIGURE 26 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY PRODUCT, 2022
FIGURE 27 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY PRODUCT, 2023-2030 (USD MILLION)
FIGURE 28 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY PRODUCT, CAGR (2023-2030)
FIGURE 29 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY PRODUCT, LIFELINE CURVE
FIGURE 30 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY TECHNOLOGY, 2022
FIGURE 31 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY TECHNOLOGY, 2023-2030 (USD MILLION)
FIGURE 32 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY TECHNOLOGY, CAGR (2023-2030)
FIGURE 33 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY TECHNOLOGY, LIFELINE CURVE
FIGURE 34 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY APPLICATION, 2022
FIGURE 35 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY APPLICATION, 2023-2030 (USD MILLION)
FIGURE 36 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY APPLICATION, CAGR (2023-2030)
FIGURE 37 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY APPLICATION, LIFELINE CURVE
FIGURE 38 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY END USER, 2022
FIGURE 39 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY END USER, 2023-2030 (USD MILLION)
FIGURE 40 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY END USER, CAGR (2023-2030)
FIGURE 41 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY END USER, LIFELINE CURVE
FIGURE 42 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY DISTRIBUTION CHANNEL, 2022
FIGURE 43 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY DISTRIBUTION CHANNEL, 2023-2030 (USD MILLION)
FIGURE 44 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY DISTRIBUTION CHANNEL, CAGR (2023-2030)
FIGURE 45 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 46 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: SNAPSHOT (2022)
FIGURE 47 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY COUNTRY (2022)
FIGURE 48 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY COUNTRY (2023 & 2030)
FIGURE 49 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY COUNTRY (2022 & 2030)
FIGURE 50 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: CATEGORY (2023-2030)
FIGURE 51 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: COMPANY SHARE 2022 (%)

調査方法
データ収集と基準年分析は、大規模なサンプル サイズのデータ収集モジュールを使用して行われます。この段階では、さまざまなソースと戦略を通じて市場情報または関連データを取得します。過去に取得したすべてのデータを事前に調査および計画することも含まれます。また、さまざまな情報ソース間で見られる情報の不一致の調査も含まれます。市場データは、市場統計モデルと一貫性モデルを使用して分析および推定されます。また、市場シェア分析と主要トレンド分析は、市場レポートの主要な成功要因です。詳細については、アナリストへの電話をリクエストするか、お問い合わせをドロップダウンしてください。
DBMR 調査チームが使用する主要な調査方法は、データ マイニング、データ変数が市場に与える影響の分析、および一次 (業界の専門家) 検証を含むデータ三角測量です。データ モデルには、ベンダー ポジショニング グリッド、市場タイムライン分析、市場概要とガイド、企業ポジショニング グリッド、特許分析、価格分析、企業市場シェア分析、測定基準、グローバルと地域、ベンダー シェア分析が含まれます。調査方法について詳しくは、お問い合わせフォームから当社の業界専門家にご相談ください。
カスタマイズ可能
Data Bridge Market Research は、高度な形成的調査のリーダーです。当社は、既存および新規のお客様に、お客様の目標に合致し、それに適したデータと分析を提供することに誇りを持っています。レポートは、対象ブランドの価格動向分析、追加国の市場理解 (国のリストをお問い合わせください)、臨床試験結果データ、文献レビュー、リファービッシュ市場および製品ベース分析を含めるようにカスタマイズできます。対象競合他社の市場分析は、技術ベースの分析から市場ポートフォリオ戦略まで分析できます。必要な競合他社のデータを、必要な形式とデータ スタイルでいくつでも追加できます。当社のアナリスト チームは、粗い生の Excel ファイル ピボット テーブル (ファクト ブック) でデータを提供したり、レポートで利用可能なデータ セットからプレゼンテーションを作成するお手伝いをしたりすることもできます。