北米非ホジキンリンパ腫診断市場規模、シェア、トレンド分析レポート
Market Size in USD Billion
CAGR :
% 
 USD
2.34 Billion
USD
4.69 Billion
2024
2032
USD
2.34 Billion
USD
4.69 Billion
2024
2032
| 2025 –2032 | |
| USD 2.34 Billion | |
| USD 4.69 Billion | |
|
|
|
|
北米における非ホジキンリンパ腫診断市場のセグメンテーション、検査タイプ別(画像診断、生検、免疫組織化学、バイオマーカー、 遺伝子検査、細胞遺伝学、腰椎穿刺、血液検査、細胞化学など)、がんのステージ別(ステージIV、ステージIII、ステージII、ステージI、ステージ0)、腫瘍の種類別(アグレッシブリンパ腫、インドレントリンパ腫)、製品別(機器ベースの製品、プラットフォームベースの製品、キットおよび試薬、その他の消耗品)、技術別(蛍光in situハイブリダイゼーション、次世代シーケンシング、蛍光免疫測定、比較ゲノムハイブリダイゼーション、免疫組織化学など)、用途別(スクリーニング、診断および予測、予後、研究)、エンドユーザー別(病院、診断センター、がん研究センター、学術機関、外来手術センターなど)、流通チャネル別(直接販売)入札、小売販売、その他 - 2032年までの業界動向と予測
北米の非ホジキンリンパ腫診断市場規模
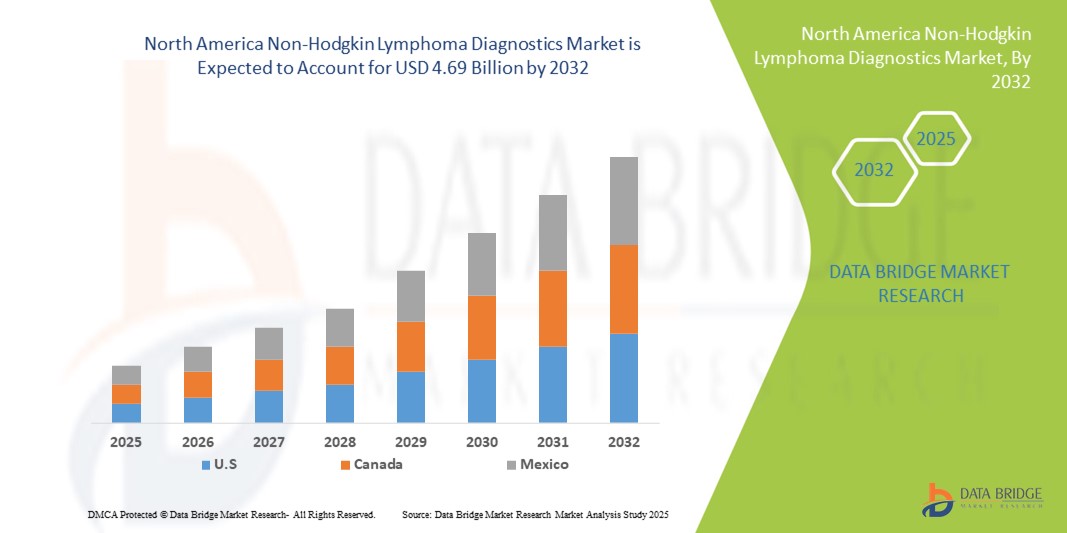
- 北米の非ホジキンリンパ腫診断市場規模は2024年に23億4000万米ドルと評価され、予測期間中に9.10%のCAGRで成長し、2032年には46億9000万米ドル に達すると予想されています 。
- 市場の成長は、主に非ホジキンリンパ腫(NHL)の世界的な罹患率の増加と、早期かつ正確な診断ソリューションに対する需要の高まりによって牽引されています。フローサイトメトリー、免疫組織化学、分子プロファイリングといった高度な診断技術は、疾患の検出と患者の転帰の改善に重要な役割を果たしています。
- さらに、医療従事者と患者の間で個別化治療計画と精密医療に関する意識が高まり、革新的な診断ソリューションの導入が促進されています。検査室の自動化、ハイスループット検査、臨床意思決定支援システムとの統合における継続的な進歩は、NHL診断の効率と精度を向上させています。
北米における非ホジキンリンパ腫診断市場分析
- 非ホジキンリンパ腫診断市場は、がんの早期発見に対する意識の高まり、診断ツールの技術的進歩、精密医療アプローチの採用の増加により、急速な成長を遂げています。
- 非ホジキンリンパ腫診断の需要の高まりは、主に医療費の増加、血液がんの発生率の上昇、AIを活用した次世代診断技術の導入の増加によって促進されている。
- 米国は、2025年に78.6%という最大の収益シェアで非ホジキンリンパ腫診断市場を支配しました。その特徴は、高い医療費、高度な医療インフラ、そして主要な業界プレーヤーの強力な存在です。AIを活用したスクリーニングと精密診断に重点を置く既存企業とスタートアップ企業の両方によるイノベーションによって、診断設備が大幅に増加しています。
- カナダは、早期診断の意識の高まり、高度な診断検査の利用可能性の向上、医療へのアクセスの拡大により、予測期間中に非ホジキンリンパ腫診断市場で最も急速に成長する国になると予想されています。
- 非ホジキンリンパ腫診断市場において、2025年にはアグレッシブリンパ腫セグメントが57.2%のシェアを占め、市場を席巻しました。これは、急速に進行する症例における迅速かつ正確な診断の緊急性によるものです。アグレッシブリンパ腫では、迅速な治療決定を導くために、集中的なモニタリングと正確な分子特性評価が求められます。
レポートの範囲と非ホジキンリンパ腫診断市場のセグメンテーション
|
属性 |
非ホジキンリンパ腫診断における主要市場洞察 |
|
対象セグメント |
|
|
対象国 |
北米
|
|
主要な市場プレーヤー |
|
|
市場機会 |
|
|
付加価値データ情報セット |
データブリッジマーケットリサーチがまとめた市場レポートには、市場価値、成長率、セグメンテーション、地理的範囲、主要プレーヤーなどの市場シナリオに関する洞察に加えて、専門家による詳細な分析、価格設定分析、ブランドシェア分析、消費者調査、人口統計分析、サプライチェーン分析、バリューチェーン分析、原材料/消耗品の概要、ベンダー選択基準、PESTLE分析、ポーター分析、規制の枠組みも含まれています。 |
北米における非ホジキンリンパ腫診断市場の動向
非ホジキンリンパ腫診断の進歩
- 米国の非ホジキンリンパ腫診断市場における重要な傾向として、フローサイトメトリー、免疫組織化学、次世代シーケンシング(NGS)などの高度な診断技術の導入が増加しており、これにより精度が向上し、病気の早期発見が可能になっている。
- 例えば、最新のフローサイトメトリープラットフォームは、リンパ腫のサブタイプを迅速に特定・分類できるため、臨床医はより正確な治療計画を策定できます。同様に、NGSベースの診断は包括的な遺伝子プロファイリングを提供し、個別化治療アプローチをサポートします。
- 自動化された検査システムと高度な診断ワークフローの統合により、処理時間の短縮、人的ミスの削減、検査室全体の効率向上が可能になります。
- これらの技術の進歩により、患者データの集中管理が容易になり、医療提供者は病気の進行を追跡し、治療への反応を監視し、合理化された診断プロセスを通じて情報に基づいた意思決定を行うことができます。
- より正確で迅速かつ信頼性の高い診断への流れは、非ホジキンリンパ腫治療における臨床実践と患者管理を根本的に変革しています。その結果、企業は革新的な診断ソリューションへの投資、検査の可用性の拡大、そして医療従事者へのアクセス向上に取り組んでいます。
- 米国では、医療システムが早期発見、正確な分類、患者の転帰改善のための個別化された治療アプローチに重点を置いているため、高度な非ホジキンリンパ腫診断の需要が急速に高まっています。
北米における非ホジキンリンパ腫診断市場の動向
ドライバ
意識の高まりと早期発見の取り組みによるニーズの高まり
- 血液がんの罹患率の増加と早期発見のメリットに対する認識の高まりが、非ホジキンリンパ腫診断の需要の高まりの大きな要因となっている。
- 例えば、2024年4月には、大手診断企業が、非ホジキンリンパ腫の早期発見と正確な分類の向上を目的とした、高度なフローサイトメトリーと次世代シーケンシングプラットフォームを導入しました。主要企業によるこうしたイノベーションは、予測期間中の市場成長を牽引すると予想されます。
- 患者と医療提供者が早期診断と個別化された治療計画の重要性をより深く認識するにつれ、高度な診断ソリューションはより高い精度、迅速な結果、包括的な疾患プロファイリングを提供し、従来の診断方法を大幅に向上させています。
- さらに、個別化医療と精密腫瘍学への重点が高まっているため、臨床ワークフロー内でシームレスな検査、監視、報告を可能にする統合診断プラットフォームの採用が増加しています。
- 迅速な検査結果の利便性、低侵襲検査オプション、そして専門的な診断へのアクセス性の向上は、病院、診断ラボ、専門クリニックにおける非ホジキンリンパ腫診断の導入を促進する重要な要素です。集中化・自動化された検査ソリューションへのトレンドは、市場の成長をさらに促進しています。
抑制/挑戦
高額な費用とアクセスの制限に関する懸念
- フローサイトメトリーやNGSプラットフォームを含む高度な非ホジキンリンパ腫診断システムの初期費用が高いため、特に小規模クリニックや予算重視の医療提供者にとって、より広範な市場浸透に大きな課題が生じています。
- 例えば、自動化や試薬効率の革新により、一部の診断検査はより手頃な価格になったが、マルチパラメータ分析や迅速な遺伝子プロファイリングなどのプレミアム機能は、多くの場合、より高いコストを伴うため、リソースが限られた環境では導入が制限される可能性がある。
- 費用対効果の高いソリューション、医療機関との提携、そしてより広範な保険適用を通じてこれらの課題に対処することは、市場へのアクセス拡大に不可欠です。企業は、多様な規模の医療施設に対応するために、拡張性とモジュール性を兼ね備えた診断プラットフォームの提供を増やしています。
- 技術の進歩と競争により価格は徐々に低下しているものの、高度な診断にかかるコストが高いと認識されていることから、特に小規模な病院や新興の医療市場では、普及が妨げられている。
- 手頃な価格の改善、早期発見の利点に関する患者教育、診断サービスの利用可能性の向上を通じてこれらの課題を克服することが、持続的な市場成長にとって不可欠となる。
北米における非ホジキンリンパ腫診断市場の展望
市場は、検査の種類、がんのステージ、腫瘍の種類、製品、技術、用途、エンドユーザー、流通チャネルに基づいて分類されています。
- テストの種類別
検査タイプに基づいて、北米の非ホジキンリンパ腫診断市場は、イメージング、生検、免疫組織化学、バイオマーカー、遺伝子検査、細胞遺伝学、腰椎穿刺、血液検査、細胞化学、その他に分類されています。生検セグメントは、高い診断精度とリンパ腫サブタイプの決定的な組織病理学的確認を提供する能力に牽引され、2025年には38.5%という最大の収益シェアで市場を支配しました。病院や診断センターは、その信頼性と確立された臨床的信頼のために、生検手順を広く採用しています。自動化された組織処理や高解像度イメージングなどの技術の進歩により、結果の精度とワークフローの効率がさらに向上しています。生検検査は、個別化された治療計画の指針となり、治療反応をモニタリングする上で重要な役割を果たします。分子プロファイリングと免疫組織化学との統合により、精密腫瘍学におけるその有用性が強化されます。このセグメントの優位性は、主要な病院や専門のがんセンターで一貫して採用されていることに支えられており、非ホジキンリンパ腫の診断における推奨標準となっています。
バイオマーカー分野は、早期発見と治療モニタリングの需要増加に支えられ、2025年から2032年にかけて年平均成長率(CAGR)22.0%と最も高い成長率を示すと予想されています。バイオマーカー検査は、患者の正確な層別化を可能にし、個別化された治療アプローチを導きます。分子アッセイ機能とハイスループット検査プラットフォームの拡張は、病院と専門診断ラボの両方での導入を加速させます。バイオマーカーの重要性に対する臨床医の認識と規制当局の承認は、市場の成長をさらに後押しします。これらの検査は、次世代シーケンシングやその他の分子診断との統合も促進します。研究イニシアチブと臨床試験においてバイオマーカー検査がますます取り入れられるようになり、さらなる導入を促進しています。アッセイの感度と特異性の継続的な革新は、市場の急速な拡大を保証します。
- がんのステージ別
北米の非ホジキンリンパ腫診断市場は、がんのステージに基づいて、ステージIV、ステージIII、ステージII、ステージI、ステージ0に分類されています。ステージIIセグメントは、中間ステージと診断された患者に対する標準化された診断プロトコルに支えられ、2025年には35.8%のシェアで市場を支配しました。ステージIIの検査は、臨床医が治療の強度を最適化し、副作用を最小限に抑えるのに役立ちます。このステージの診断手順は、病気の進行を監視し、併用療法を計画するために、病院や外来センターで広く採用されています。ステージIIの診断は、分子生物学的研究や画像診断とうまく統合され、診断の信頼性を向上させます。この段階での早期介入は、患者の予後を大幅に改善します。このセグメントは、患者数が多く、ワークフローが確立されていることから恩恵を受けており、リーダーシップを強化しています。臨床医は、即時の治療と長期的な病気の管理の両方を導くためにステージIIの診断に依存しています。
ステージIVセグメントは、進行期患者の包括的なモニタリングニーズに牽引され、2025年から2032年にかけて年平均成長率(CAGR)19.5%と最も高い成長率を示すと予想されています。ステージIVの診断には、画像診断、分子プロファイリング、遺伝子解析など、複雑で多様な検査手法が求められます。病院や専門がんセンターでは、疾患の進行と治療反応を追跡するために、これらの高精度技術の導入がますます進んでいます。後期診断の発生率の上昇と個別化治療戦略への需要も、この成長をさらに加速させています。技術の進歩と高度な診断へのアクセス性の向上が、急速な導入を後押ししています。ステージIV検査は、腫瘍学ケアにおいて依然として重要な焦点領域です。
- 腫瘍の種類別
北米の非ホジキンリンパ腫診断市場は、腫瘍の種類に基づいて、攻撃的リンパ腫と低悪性度リンパ腫に分類されます。攻撃的リンパ腫セグメントは、進行の速い症例における迅速かつ正確な診断の緊急のニーズにより、2025年には57.2%のシェアで市場を支配しました。攻撃的リンパ腫には、迅速な治療決定を導くための集中的なモニタリングと正確な分子特性評価が必要です。病院やがんセンターは、これらの高リスク患者を優先し、高度な検査方法の採用を確実にしています。攻撃的なサブタイプを迅速に特定することで、腫瘍医は集中的な治療をカスタマイズし、治療結果を監視できます。北米では攻撃的リンパ腫が多く、臨床的な緊急性と相まって、安定した需要を促進しています。確立された診断ワークフローと分子プロファイリングとの統合により、このセグメントの優位性が強化されています。攻撃的リンパ腫の検出に焦点を当てた研究イニシアチブにより、市場でのリーダーシップがさらに強化されています。
低悪性度リンパ腫セグメントは、長期モニタリングと低侵襲検査の需要増加に牽引され、2025年から2032年にかけて17.8%という最も高いCAGR(年平均成長率)を達成すると予想されています。低悪性度リンパ腫の診断では、病状の経時的な進行を追跡するために、バイオマーカー検査と遺伝子検査が重視されています。病院、外来診療所、専門検査室では、早期発見と個別化医療のためにこれらの診断法を導入するケースが増えています。このセグメントは、継続的な患者モニタリングの重要性に対する臨床医の意識の高まりから恩恵を受けています。分子生物学的検査および免疫組織化学的検査における技術革新は、診断精度の向上を支えています。低悪性度リンパ腫を対象とした研究調査と臨床試験も、成長をさらに促進しています。精密腫瘍学プログラムにおける導入の増加も、このセグメントの市場拡大を加速させています。
- 製品別
製品ベースで、北米の非ホジキンリンパ腫診断市場は、機器ベース製品、プラットフォームベース製品、キットおよび試薬、その他の消耗品に分類されます。キットおよび試薬セグメントは、標準化されたすぐに使用できるソリューションに対する病院や診断ラボにおける継続的な需要に牽引され、2025年には42.0%のシェアで市場を支配しました。キットは、処理時間を短縮し、オペレーターのばらつきを最小限に抑え、複数の検査施設で一貫した結果を保証します。生検、分子生物学的検査、バイオマーカー検査など、幅広い診断アプリケーションをサポートしています。病院や研究機関は、日常的な診断と臨床研究の両方で高品質のキットに依存しています。分子診断と免疫組織化学の採用増加も、需要をさらに押し上げています。キットと試薬は、保管の容易さ、信頼性、および複数の機器との互換性を提供し、市場の優位性を強化します。確立されたサプライヤーからの入手可能性は、大規模な医療施設の調達効率を高めます。
プラットフォームベース製品セグメントは、統合システムが複数の診断機能を単一の自動化ワークフローに統合することで、2025年から2032年にかけて20.2%という最も高いCAGRを達成すると予想されています。プラットフォームソリューションは、検査プロセスを合理化し、手作業による介入を減らし、ハイスループット検査をサポートします。病院や診断センターでは、拡張性、効率性、使いやすさからプラットフォームへの関心が高まっています。分子診断モジュールとイメージングモジュールとの統合により、単一システムからの包括的な分析が可能になります。このセグメントは、自動化、ソフトウェア分析、モジュール設計における革新の恩恵を受けています。学術機関や研究機関での導入拡大も、成長をさらに加速させています。プラットフォームベースの診断は、マルチテスト接続もサポートし、検査室全体の生産性を向上させます。
- テクノロジー別
北米の非ホジキンリンパ腫診断市場は、技術に基づいて、蛍光in situハイブリダイゼーション(FISH)、次世代シーケンシング(NGS)、蛍光免疫測定、比較ゲノムハイブリダイゼーション、免疫組織化学、その他に分類されています。次世代シーケンシング(NGS)セグメントは、包括的な遺伝子プロファイリング機能と精密腫瘍学における重要な役割により、2025年には40.5%のシェアで市場を支配しました。NGSは複数の変異を同時に検出することを可能にし、患者の層別化と治療法の選択を容易にします。病院やがんセンターは、その高い感度、拡張性、およびバイオマーカー研究との互換性のために、NGSを広く採用しています。生検および分子診断との統合は、臨床的有用性を高めます。進行中の研究と臨床試験により、ハイスループットNGSプラットフォームの需要が高まっています。このセグメントは、規制当局の承認と、日常的な腫瘍学ワークフローにおける採用の増加によってさらに支えられています。
蛍光in situハイブリダイゼーション(FISH)分野は、遺伝子異常や染色体転座を迅速に検出する能力に牽引され、2025年から2032年にかけて18.7%という最も高いCAGR(年平均成長率)を達成すると予想されています。FISHは早期診断、予後評価、そして治療計画に不可欠です。病院、診断ラボ、専門研究センターでの導入が拡大しています。プローブ設計と画像化システムの技術向上により、精度とターンアラウンドタイムが向上しています。FISH検査は、他の分子診断法を補完し、包括的な患者評価を可能にします。造血悪性腫瘍における診断価値への認識の高まりが市場の成長を牽引しています。臨床応用の拡大も、急速な導入を後押ししています。
- アプリケーション別
北米の非ホジキンリンパ腫診断市場は、用途別にスクリーニング、診断・予測、予後、研究に分類されます。診断・予測セグメントは、正確な疾患分類と治療ガイダンスへのニーズの高まりを背景に、2025年には北米の非ホジキンリンパ腫診断市場において45.3%のシェアを占め、市場を牽引しました。診断・予測検査は、分子、遺伝子、画像データを統合し、臨床医に疾患の進行と患者の予後に関する包括的な知見を提供します。病院や専門診断センターは、治療計画と臨床意思決定を支援するために、これらの検査に大きく依存しています。このセグメントは、個別化医療プログラムとの統合による恩恵を受けており、個々の患者に合わせた治療戦略を可能にします。予測バイオマーカーの継続的な研究と臨床検証は、このセグメントの市場優位性をさらに強化しています。確立された臨床的有用性と腫瘍学センターにおける広範な導入により、このセグメントはリーダーシップを維持しています。治療プロトコルの複雑化と高リスク患者の早期発見の必要性が、このセグメントの北米における強力な地位をさらに強固なものにしています。
スクリーニング分野は、早期発見と予防医療への意識の高まりを背景に、2025年から2032年にかけて19.0%という最も高いCAGRを達成すると予想されています。スクリーニング検査はタイムリーな診断を促進し、医師が病気の進行前に治療を開始するのに役立ちます。外来診断センターや病院では、高リスク集団に対するスクリーニングプログラムの導入が進んでいます。バイオマーカーアッセイ、画像技術、低侵襲技術の進歩は、検査の精度と患者の快適性を向上させます。早期発見を支援する政府の取り組みや保険適用プログラムも、この導入をさらに促進しています。技術革新は、処理時間と運用コストを削減し、より広範な展開を可能にします。この分野は、医師と患者を対象とした教育キャンペーンの増加からも恩恵を受けています。臨床および研究の両方の環境におけるスクリーニングプログラムの拡大は、予測期間中の市場成長を大幅に加速すると予想されます。
- エンドユーザー別
エンドユーザーに基づいて、北米の非ホジキンリンパ腫診断市場は、病院、診断センター、がん研究センター、学術機関、外来手術センター、その他に分類されています。病院セグメントは、複雑な診断ワークフローを管理する能力、高度な機器へのアクセス、専門スタッフの利用可能性により、2025年に50.0%のシェアで市場を支配しました。病院は、初期検査から治療モニタリングまで統合的なサービスを提供しており、非ホジキンリンパ腫患者のケアの継続性を確保しています。学際的な腫瘍学チームは、病院ベースの診断に依存して治療決定を導き、患者の反応を監視しています。病院はまた、大量の患者を効率的に処理するために、ハイスループットプラットフォームと分子検査機能に投資しています。このセグメントは、確立された調達チャネルと長期的なサプライヤー関係の恩恵を受けています。さらに、病院は臨床試験や研究イニシアチブに参加しており、セグメントのリーダーシップをさらに強化しています。集中化されたインフラストラクチャ、規制遵守、高度なラボネットワークにより、北米市場における優位性が強化されます。
診断センターセグメントは、迅速で費用対効果の高い診断を提供する専門外来検査施設の拡大に牽引され、2025年から2032年にかけて21.5%という最も高いCAGRを達成すると予想されています。診断センターは、入院せずに便利でタイムリーな検査を求める患者に柔軟性を提供します。これらのセンターでは、バイオマーカーベースおよび分子診断検査の採用が増えており、成長を支えています。自動化と高度な検査機器への投資は、効率性と結果の精度を向上させます。病院や学術機関との提携は、サービス提供の拡大に役立ちます。患者啓発キャンペーンや医療提供者による紹介プログラムも、採用をさらに促進します。規制当局のサポートと保険適用の改善は、利用を促進します。北米全域で予防医療と早期発見への取り組みがますます重視されていることも、診断センターの成長を後押ししています。
- 流通チャネル別
北米の非ホジキンリンパ腫診断市場は、流通チャネルに基づいて、直接入札、小売販売、その他に分類されます。直接入札セグメントは、病院、政府機関、大規模診断ネットワークによる大量調達に支えられ、2025年には北米の非ホジキンリンパ腫診断市場の46.8%のシェアを占めました。直接入札契約は、長期契約、予測可能な供給、コスト効率を可能にし、診断サービスの継続性を保証します。病院やがんセンターは、高品質の機器、試薬、消耗品を確保するために直接入札に依存しています。これらの契約には、技術サポート、トレーニング、メンテナンスも含まれており、エンドユーザーの信頼性を高めています。サプライヤーは、安定した収益源と主要顧客とのより強固な関係から利益を得ます。このセグメントの優位性は、統合診断プラットフォームと高度な検査システムの採用の増加によって強化されています。効率的なサプライチェーン管理と合理化された調達プロセスは、北米における直接入札流通のリーダーシップをさらに強化します。
小売販売セグメントは、商業販売業者を通じてすぐに使用できる診断キット、試薬、消耗品の入手しやすさの向上に牽引され、2025年から2032年にかけて18.9%という最も高いCAGRを達成すると予想されています。小売チャネルは、バルク入札契約に参加できない可能性のある小規模な研究室、学術機関、外来診療所へのアクセスを提供します。入手しやすさ、競争力のある価格設定、製品の多様性により、小売流通はエンドユーザーにとって魅力的な選択肢となっています。パッケージング、保存期間の延長、事前検証済みキットにおける技術革新は、採用を促進します。小規模な医療施設を対象とした啓発キャンペーンも成長をさらに促進します。小売チャネルはまた、新しい診断技術や試薬の迅速な導入をサポートし、市場範囲を拡大します。拡大するオンラインおよびオフラインの流通ネットワークは、北米全域でのアクセス性を高め、このセグメントの急速な拡大に貢献しています。
北米の非ホジキンリンパ腫診断市場の地域分析
- 北米は、医療費の高騰、高度な医療インフラ、そして大手診断サービスプロバイダーの強力な存在により、2025年に最大の収益シェアで非ホジキンリンパ腫診断市場を支配した。
- この地域は、AIを活用したスクリーニング、精密診断、革新的な検査プラットフォームの普及により、早期発見と個別化された患者管理が可能になり、恩恵を受けています。
- 継続的な研究開発、既存企業と新興企業間の協力の増加、リンパ腫検査に関する意識の高まりが市場の成長をさらに支えています。
米国における非ホジキンリンパ腫診断市場の洞察
米国の非ホジキンリンパ腫診断市場は、高度な分子生物学的、遺伝学的、免疫組織化学に基づく診断ソリューションの急速な導入に支えられ、2025年には北米で最大のシェア78.6%を獲得しました。米国の医療費支出の高さ、充実した医療インフラ、そして早期がん発見への強い関心は、精密診断の広範な導入を支えています。AIを活用したツールやハイスループット検査プラットフォームなど、既存企業とスタートアップ企業の両方によるイノベーションが、大幅な成長を牽引しています。病院、研究センター、臨床検査室における高度な診断ワークフローの統合は、この地域における米国の主要市場としての地位をさらに強化しています。
カナダにおける非ホジキンリンパ腫診断市場の洞察
カナダの非ホジキンリンパ腫診断市場 は、予測期間中、非ホジキンリンパ腫診断市場の中で最も急速に成長する国になると予想されており、がんの早期発見に対する意識の高まり、高度な診断検査へのアクセス拡大、そして医療近代化に対する政府の支援により、堅調な年平均成長率(CAGR)で拡大すると予測されています。病院、診断センター、研究機関では、次世代シーケンシング、バイオマーカー検査、分子診断の導入が増加しています。医療インフラへの投資に加え、早期スクリーニングと個別化された患者ケアの改善に向けた取り組みが、カナダにおける市場の急速な拡大を牽引しています。
北米における非ホジキンリンパ腫診断市場シェア
非ホジキンリンパ腫診断業界は、主に、以下を含む定評のある企業によって牽引されています。
- キヤノンメディカルシステムズ株式会社(日本)
- Koninklijke Philips NV (オランダ)
- シーメンス ヘルスケア AG(ドイツ)
- ダナハーコーポレーション(米国)
- バイオ・ラッド・ラボラトリーズ社(米国)
- ゼネラル・エレクトリック・カンパニー(米国)
- シスメックス株式会社(日本)
- グレイル(米国)
- F. ホフマン・ラ・ロシュ社(スイス)
- 東軟集団(中国)
- アジレント・テクノロジーズ社(米国)
- ネオジェノミクス・ラボラトリーズ(米国)
- ホロジック社(米国)
- インテグレーテッドDNAテクノロジーズ社(米国)
- CENTOGENE NV(ドイツ)
- メリットメディカルシステムズ(米国)
- ラボコープ・ジェネティクス社(米国)
- パーキンエルマー(米国)
- キアゲン(米国)
- GeneDx LLC(米国)
北米の非ホジキンリンパ腫診断市場の最新動向
- ロシュ社は2025年5月、再発性または難治性のびまん性大細胞型B細胞リンパ腫(DLBCL)に対するコロンビ(グロフィタマブ)療法の適応拡大を発表しました。2年間の追跡調査データでは、コロンビ®とゲムシタビンおよびオキサリプラチン(GemOx)の併用療法を受けた患者は、標準的なリツキシマブとGemOxの併用療法と比較して、全生存率が40%改善したことが示されました。この進展は、革新的な治療法を通じてNHL患者の治療選択肢を拡大するというロシュ社のコミットメントを裏付けるものです。
- 2025年8月、Foresight Diagnosticsは、PhasED-Seq技術に関してRoche Molecular SystemsおよびRoche Sequencing Systemsとライセンス契約を締結しました。この提携は、Foresightの特許取得済みシーケンシング技術を活用し、非ホジキンリンパ腫の診断能力を向上させることを目的としています。また、この契約により、両社間の過去の訴訟も解決され、腫瘍学における診断精度の向上に向けた戦略的パートナーシップが実現しました。
SKU-
世界初のマーケットインテリジェンスクラウドに関するレポートにオンラインでアクセスする
- インタラクティブなデータ分析ダッシュボード
- 成長の可能性が高い機会のための企業分析ダッシュボード
- カスタマイズとクエリのためのリサーチアナリストアクセス
- インタラクティブなダッシュボードによる競合分析
- 最新ニュース、更新情報、トレンド分析
- 包括的な競合追跡のためのベンチマーク分析のパワーを活用
調査方法
データ収集と基準年分析は、大規模なサンプル サイズのデータ収集モジュールを使用して行われます。この段階では、さまざまなソースと戦略を通じて市場情報または関連データを取得します。過去に取得したすべてのデータを事前に調査および計画することも含まれます。また、さまざまな情報ソース間で見られる情報の不一致の調査も含まれます。市場データは、市場統計モデルと一貫性モデルを使用して分析および推定されます。また、市場シェア分析と主要トレンド分析は、市場レポートの主要な成功要因です。詳細については、アナリストへの電話をリクエストするか、お問い合わせをドロップダウンしてください。
DBMR 調査チームが使用する主要な調査方法は、データ マイニング、データ変数が市場に与える影響の分析、および一次 (業界の専門家) 検証を含むデータ三角測量です。データ モデルには、ベンダー ポジショニング グリッド、市場タイムライン分析、市場概要とガイド、企業ポジショニング グリッド、特許分析、価格分析、企業市場シェア分析、測定基準、グローバルと地域、ベンダー シェア分析が含まれます。調査方法について詳しくは、お問い合わせフォームから当社の業界専門家にご相談ください。
カスタマイズ可能
Data Bridge Market Research は、高度な形成的調査のリーダーです。当社は、既存および新規のお客様に、お客様の目標に合致し、それに適したデータと分析を提供することに誇りを持っています。レポートは、対象ブランドの価格動向分析、追加国の市場理解 (国のリストをお問い合わせください)、臨床試験結果データ、文献レビュー、リファービッシュ市場および製品ベース分析を含めるようにカスタマイズできます。対象競合他社の市場分析は、技術ベースの分析から市場ポートフォリオ戦略まで分析できます。必要な競合他社のデータを、必要な形式とデータ スタイルでいくつでも追加できます。当社のアナリスト チームは、粗い生の Excel ファイル ピボット テーブル (ファクト ブック) でデータを提供したり、レポートで利用可能なデータ セットからプレゼンテーションを作成するお手伝いをしたりすることもできます。