北米腎臓がん診断市場、検査タイプ別(イメージング、バイオマーカー検査、血液検査、生検、遺伝子検査、その他)、がんのステージ別(ステージI、ステージII、ステージIII、ステージIV)、腫瘍タイプ別(腎細胞がん、明細胞腎細胞がん、非明細胞腎細胞がん)、製品別(プラットフォームベースの製品、機器ベースの製品、キットおよび試薬、その他の消耗品)、技術別(蛍光in situハイブリダイゼーション、次世代シーケンシング、蛍光免疫測定法、比較ゲノムハイブリダイゼーション、免疫組織化学、その他)、用途別(スクリーニング、診断および予測、予後、研究)、エンドユーザー別(病院、診断センター、がん研究センター、学術機関、外来手術センター、その他)、流通チャネル別(直接入札、小売販売、その他)、業界動向および2030年までの予測。
北米腎臓がん診断市場の分析と規模
腎臓がんは、片方または両方の腎臓の健康な細胞が変化し、制御不能に増殖して皮質腫瘍と呼ばれる塊を形成することで始まります。腫瘍は悪性、無症状、良性の場合があります。悪性腫瘍は癌であり、増殖して体の他の部位に転移する可能性があります。無症状腫瘍も癌ですが、このタイプの腫瘍が体の他の部位に転移することはほとんどありません。良性腫瘍は、腫瘍が増殖することはあっても転移しないことを意味します。
北米における腎臓がんに対する意識の高まりにより、市場の需要が高まっています。より良い医療サービスを求める医療費の増加も、市場の成長に貢献しています。主要な市場プレーヤーは、この重要な時期にさまざまなサービスの立ち上げと承認に重点を置いています。さらに、腎臓がんの診断手順の改善の増加も、腎臓がんの診断検査の需要の増加に貢献しています。
北米の腎臓がん診断市場は、市場プレーヤーの増加と高度なサービスの利用可能性により、予測年度に成長すると予想されています。これに伴い、メーカーは市場で新しいサービスを開始するための研究開発活動に取り組んでいます。腎臓診断と開発の分野での研究の増加により、市場の成長がさらに促進されると予想されます。ただし、画像検査による高放射線被曝による組織損傷は、予測期間中の北米の腎臓がん診断市場の成長を妨げると予想されます。
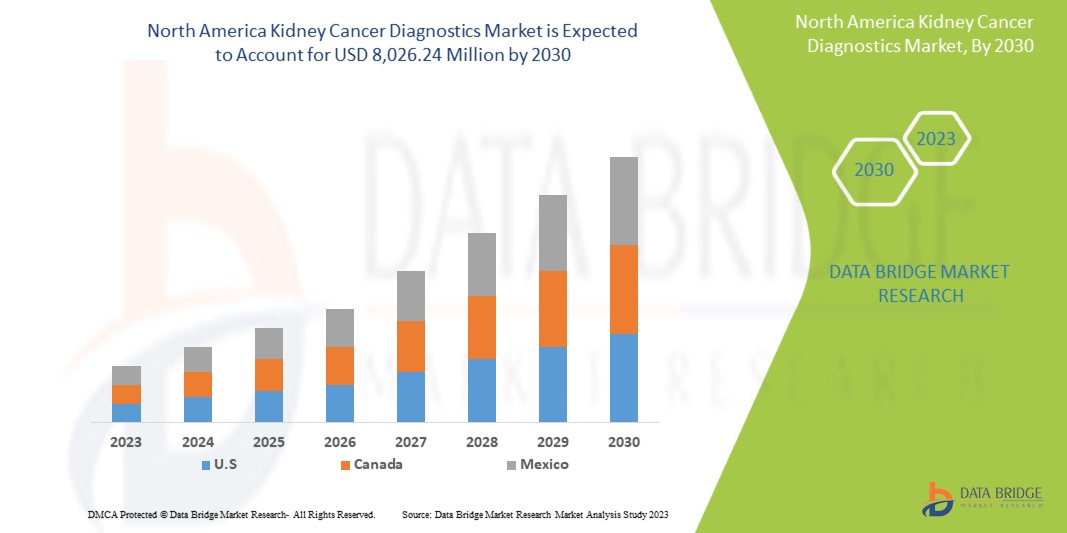
Data Bridge Market Research の分析によると、腎臓がん診断市場は予測期間中に 6.7% の CAGR で成長し、2030 年までに 80 億 2,624 万米ドルに達する見込みです。スマート デバイスの需要が高まっているため、イメージングが市場で最大のテスト タイプ セグメントを占めており、医療費の増加によりスマート医療デバイスの需要が加速しています。
|
レポートメトリック |
詳細 |
|
予測期間 |
2023年から2030年 |
|
基準年 |
2022 |
|
歴史的な年 |
2021 (2020~2016年にカスタマイズ可能) |
|
定量単位 |
売上高(百万米ドル)、販売数量(個数)、価格(米ドル) |
|
対象セグメント |
検査タイプ(イメージング、バイオマーカー検査、血液検査、生検、遺伝子検査、その他)、がんのステージ(ステージ I、ステージ II、ステージ III、ステージ IV)、腫瘍の種類(腎細胞がん、淡明細胞腎細胞がん、非淡明細胞腎細胞がん)、製品(プラットフォームベースの製品、機器ベースの製品、キットおよび試薬、その他の消耗品)、テクノロジー(蛍光 in situ ハイブリダイゼーション、次世代シーケンシング、蛍光免疫測定、比較ゲノムハイブリダイゼーション、免疫組織化学、その他)、用途(スクリーニング、診断および予測、予後、研究)、エンドユーザー(病院、診断センター、がん研究センター、学術機関、外来手術センター、その他)、流通チャネル(直接入札、小売販売、その他)別。 |
|
対象国 |
米国、カナダ、メキシコ |
|
対象となる市場プレーヤー |
Siemens Healthcare GmbH、Koninklijke Philips NV、富士フイルム株式会社、Grail、Laboratory Corporation of America Holdings、Thermo Fisher Scientific、Myriad Genetics、Inc.、CANON MEDICAL SYSTEMS CORPORATION、QIAGEN、Illumina Inc.、Ambry Genetics、Invitae Corporation、General Electric Company、Centogene NV、GenPath、Creative Diagnostics、GeneDx LLC、Blueprint Genetics Oy、BioVendor R&D、CD Genomics、BD など |
市場の定義
腎臓がんは、一般的に腎がんとして知られ、腎臓細胞が悪性(がん性)腫瘍に成長し、制御不能に増殖する病気です。最も罹患率の高いがんのトップ 10 に腎臓がんがあります。腎臓がんは致命的であり、診断プロセスにも安全性の問題があり、費用対効果が高くありません。がん患者は入院し、手術、放射線療法、全身療法などのさまざまな治療を受ける場合があります。腎臓の腫瘍の約 40% は、小さな局所的な腫瘤です。局所的とは、元の位置から広がっていない腫瘍を指します。腎臓の腫瘤は、通常の臨床検査では検出できません。腎臓がんの診断には、生検手順、血液検査、および画像検査が含まれます。免疫療法、放射線療法などの高度な腎臓がん治療が推奨されます。最先端の方法により、凍結療法(がん細胞を凍結する)や高周波アブレーションなどの非外科的手順が、軽度の腎臓腫瘍(がん細胞を加熱する)の治療に使用されることがあります。
腎臓がんは、その兆候や症状が多岐にわたるにもかかわらず、非特異的であり、他のより広範な病状と関連している可能性があるため、診断が難しい場合があります。毎年、43,000 人以上の男性と 25,000 人以上の女性が腎臓がんおよび腎盂がんと診断され、9,000 人の男性と 5,000 人の女性がこの病気で亡くなっています。しかし、腎臓がん診断製品の承認と商品化に関する厳格な規制と基準により、市場の成長が抑制されると予想されています。
北米腎臓がん診断市場の動向
このセクションでは、市場の推進要因、利点、機会、制約、課題について理解します。これらについては、以下で詳しく説明します。
ドライバー
- 腎臓がんの罹患率増加
このタイプのがんは、あらゆる年齢層の人に発症する可能性があります。腎臓がんは、その幅広い兆候や症状にもかかわらず、非特異的であり、他のより広範な病状に関連している可能性があるため、診断が難しい場合があります。腎臓がんは、通常、初期段階では兆候や症状がありません。時間が経つにつれて、ピンク色、赤色、またはコーラ色の尿中の血液、治らない背中や脇の痛み、食欲不振、原因不明の体重減少、疲労、発熱などの兆候や症状が現れることがあります。成人では、腎臓がんが最も一般的なタイプのがんです。幼児は、ウィルムス腫瘍と呼ばれるタイプの腎臓がんを発症する可能性が高くなります。腎臓がん(腎臓がんまたは腎細胞腺がんとも呼ばれる)は、世界で14番目に多いがんです。男性では9番目、女性では14番目です。2020年には、30,000件を超える新しい腎臓がんの症例が診断されました。
喫煙、肥満、高血圧、腎臓がんの家族歴などのさまざまなリスク要因により、腎臓がんの患者は北米で増加しており、重大な社会経済的問題になっています。したがって、腎臓がんの患者数の増加により、腎臓がん診断製品の需要が高まり、北米の腎臓がん診断市場の推進力となっています。
- 腎臓がんの診断手順の増加
腎臓がんの診断に使用される技術には、超音波、コンピューター断層撮影 (CT) スキャン、磁気共鳴画像 (MRI)、場合によっては陽電子放出断層撮影 (PET) スキャンなどがあります。成長速度が遅い腎臓がんの治療には、モニタリングが必要になる場合があります。悪性腫瘍に対する化学療法は、放射線療法や幹細胞移植と組み合わせられることがあります。がん発生率の上昇は、診断製品の承認増加の要因となっています。
このように、診断用製品の承認数の増加により、腎臓がんの診断治療市場における高効率製品の数が増加しており、これが北米の腎臓がん診断市場の成長の原動力となることが期待されています。
機会
-
予防健康診断の好感度上昇
Preventive health check-up is preventive actions performed for the initial detection of kidney cancer disease. Also, a rising preference for preventive health check-ups provides a safeguard against likely exposure to any disease in the future.
Awareness to promote screening is the most important component of kidney cancer prevention. The check-up is comprised of the identification of cancer and examinations of risk factors to limit loss at an early stage.
Preventive kidney cancer check-up is performed with the help of various diagnostic test, which includes biopsy, immunohistochemistry, cancer screening, MRI and among others.
People are relatively more prone to kidney cancer diseases. Therefore, they require going through regular check-ups to facilitate physicians develop an understanding of diseases and to provide better treatment to a patient who is suffering from cancer, for the reason rising preference for preventive health check-ups is expected to act as a driver for the growth of North America kidney cancer diagnostics market.
Restraint/Challenge
- Strict Regulations And Standards For The Approval And Commercialization Of Kidney Cancer Diagnostic Products
The stringent regulations for the commercialization of any product in the market are proving to be a big challenge for the manufacturers of cancer diagnostic products North America that have regulations and a different body for the regulatory procedures.
Manufacturers first have to check the CE mark approval for their product's commercialization into the North America market. The stringent regulatory policies are expected to hinder the development of the cancer diagnostic market. The regulatory requirement for approvals of marketing or CE certification and application of laws and regulations could lead to major business changes or paying penalties, including the potential loss of business licenses. The resources and costs required to comply with these laws, rules, and regulations are high.
The regulatory requirement for marketing approvals, declaration of conformity, and the time required for regulatory review may vary for different products. The company which fails to get regulatory approval harms the business because without getting approval or failing to get CE to mark approval on the products, manufacturers are not able to launch their product in the North America market, and for this reason, strict regulations and standards for the approval and commercialization of kidney cancer diagnostic products are expected to act as a restrain for the North America kidney cancer diagnostics market.
Recent Developments
- In November 2022, Koninklijke Philips N.V. announced the North America launch of a next-generation compact portable ultrasound solution at the Radiological Society of North America (RSNA) annual meeting to bring the diagnostic quality associated with premium cart-based ultrasound systems to more patients. It is portable and versatile with good image quality or performance. It is Compatible with Philips ultrasound systems Affiniti and EPIQ transducer. This has helped the company to expand its product portfolio.
- In October 2022, General Electric Company had collaborated with several research institutes like the University of Cambridge Hospitals, Sophia Genetics, and earlier with Optellum to use imaging data in collaboration with Artificial intelligence. This will help to reduce the diagnosing time of several cancers and help to provide personalized care to patients. This has helped the company to widen its horizons in cancer diagnostics.
- In July 2022, Canon Medical Systems USA Inc. announced the completion of the acquisition of NXC Imaging, a medical imaging equipment distributor and service provider located in Minnesota, U.S. This results in expanding the service outreach in the North America market.
North America Kidney Cancer Diagnostics Market Scope
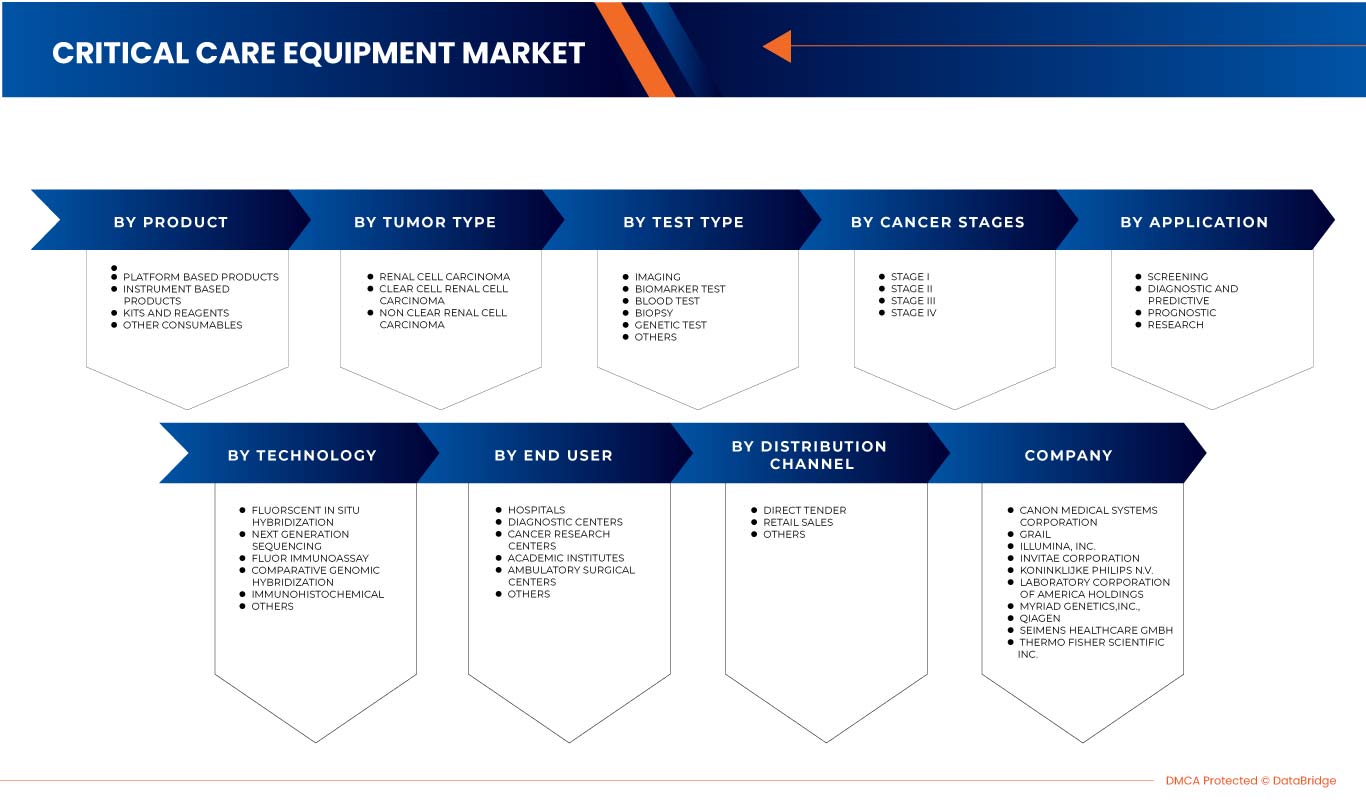
The North America kidney cancer diagnostics market is segmented into eight notable segments based on test type, cancer stage, tumor type, product, application, technology, end user and distribution channel. The growth among segments helps you analyze niche pockets of growth and strategies to approach the market and determine your core application areas and the difference in your target markets.
Test Type
- IMAGING TEST
- BIOMARKER TEST
- BLOOD TEST
- BIOPSY
- GENETIC TEST
- OTHERS
On the basis of test type, the North America kidney cancer diagnostics market is segmented into imaging, biomarker test, blood test, biopsy, genetic tests, and others.
Cancer Stage
- STAGE I
- STAGE II
- STAGE III
- STAGE IV
On the basis of cancer stage, the North America kidney cancer diagnostics market is segmented into stage I, stage II, stage III and stage IV.
Tumor Type
- RENAL CELL CARCINOMA
- CLEAR CELL RENAL CELL CARCINOMA
- NON-CLEAR CELL RENAL CELL CARCINOMA
On the basis of tumor type, the North America kidney cancer diagnostics market is segmented into renal cell carcinoma, Clear cell renal cell carcinoma, and non-clear cell renal cell carcinoma.
Product
- PLATFORM BASED PRODUCTS
- INSTRUMENT BASED PRODUCTS
- KITS AND REAGENTS
- OTHER CONSUMABLES
On the basis of product, the North America kidney cancer diagnostics market is segmented into instrument-based products, platform-based products, kits and reagents, and other consumables.
Technology
- FLUORESCENT IN SITU HYBRIDIZATION
- NEXT GENERATION SEQUENCING
- FLUOROIMMUNOASSAY
- COMPARATIVE GENOMIC HYBRIDIZATION
- IMMUNOHISTOCHEMICAL
- OTHERS
On the basis of technology, the North America kidney cancer diagnostics market is segmented into fluorescent in situ hybridization, next-generation sequencing, fluorimmunoassay, comparative genomic hybridization, immunohistochemical, and others.
Application
- SCREENING
- DIAGNOSTIC AND PREDICTIVE
- PROGNOSTIC
- RESEARCH
On the basis of application, the North America kidney cancer diagnostics market is segmented into screening, diagnostic and predictive, prognostic, and research.
End User
- HOSPITALS
- CANCER RESEARCH CENTERS
- ACADEMIC INSTITUTES
- DIAGNOSTIC CENTERS
- AMBULATORY SURGICAL CENTERS
- OTHERS
On the basis of end users, the North America kidney cancer diagnostics market is segmented into hospitals, diagnostic centers, cancer research centers, academic institutes, ambulatory surgical centers, and others.
Distribution Channel
- DIRECT TENDERS
- RETAIL SALES
- OTHERS
On the basis of distribution channel, the North America kidney cancer diagnostics market is segmented into direct tender, retail sales and others.
North America Kidney Cancer Diagnostics Market Regional Analysis/Insights
The North America kidney cancer diagnostics market is analyzed and market size information is provided based on country, test type, cancer stage, tumor type, product, application, technology, end user and distribution channel.
The countries covered in this market report are the U.S., Canada, and Mexico.
North America is dominating due to the presence of key market players in the largest consumer market with high GDP. The U.S. is expected to grow due to the rise in technological advancement in the healthcare industry.
The country section of the report also provides individual market-impacting factors and changes in regulation in the market domestically that impact the current and future trends of the market. Data points such as new sales, replacement sales, country demographics, regulatory acts, and import-export tariffs are some of the major pointers used to forecast the market scenario for individual countries. Also, the presence and availability of North America brands and their challenges faced due to large or scarce competition from local and domestic brands, and the impact of sales channels are considered while providing forecast analysis of the country data.
Competitive Landscape and North America Kidney Cancer Diagnostics Market Share Analysis
The kidney cancer diagnostics market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in R&D, new market initiatives, production sites and facilities, company strengths and weaknesses, product launch, product approvals, product width and breadth, application dominance, and product type lifeline curve. The above data points provided are only related to the company’s focus on the kidney cancer diagnostics market.
この市場で活動している主要企業としては、Siemens Healthcare GmbH、Koninklijke Philips NV、富士フイルム株式会社、Grail、Laboratory Corporation of America Holdings、Thermo Fisher Scientific、Myriad Genetics、Inc.、CANON MEDICAL SYSTEMS CORPORATION、QIAGEN、Illumina Inc.、Ambry Genetics、Invitae Corporation、General Electric Company、Centogene NV、GenPath、Creative Diagnostics、GeneDx LLC、Blueprint Genetics Oy、BioVendor R&D、CD Genomics、BD などが挙げられます。
SKU-
世界初のマーケットインテリジェンスクラウドに関するレポートにオンラインでアクセスする
- インタラクティブなデータ分析ダッシュボード
- 成長の可能性が高い機会のための企業分析ダッシュボード
- カスタマイズとクエリのためのリサーチアナリストアクセス
- インタラクティブなダッシュボードによる競合分析
- 最新ニュース、更新情報、トレンド分析
- 包括的な競合追跡のためのベンチマーク分析のパワーを活用
目次
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF THE NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATIONS
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 DBMR TRIPOD DATA VALIDATION MODEL
2.5 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.6 MULTIVARIATE MODELLING
2.7 MARKET APPLICATION COVERAGE GRID
2.8 TEST TYPE LIFELINE CURVE
2.9 DBMR MARKET POSITION GRID
2.1 VENDOR SHARE ANALYSIS
2.11 SECONDARY SOURCES
2.12 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL ANALYSIS
4.2 PORTER'S 5 FORCES
4.3 EPIDEMIOLOGY
4.3.1 KIDNEY CANCER INCIDENCES, 2020, BY BOTH SEXES
4.3.2 KIDNEY CANCER MORTALITY, 2020, BY BOTH SEXES
5 INDUSTRY INSIGHTS
6 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET, REGULATIONS
7 MARKET OVERVIEW
7.1 DRIVERS
7.1.1 GROWING PREVALENCE OF KIDNEY CANCER
7.1.2 INCREASE IN DIAGNOSTIC PROCEDURES FOR KIDNEY CANCER
7.1.3 RISE IN HEALTHCARE EXPENDITURE FOR CANCER DIAGNOSIS AND TREATMENT
7.1.4 RISING AWARENESS TOWARDS KIDNEY CANCER
7.2 RESTRAINTS
7.2.1 STRICT REGULATIONS AND STANDARDS FOR THE APPROVAL AND COMMERCIALIZATION OF KIDNEY CANCER DIAGNOSTIC PRODUCTS
7.2.2 TISSUE DAMAGE DUE TO HIGH RADIATION EXPOSURE FROM IMAGING TESTS
7.3 OPPORTUNITIES
7.3.1 RISING PREFERENCE FOR PREVENTIVE HEALTH CHECK-UPS
7.3.2 GOVERNMENT INITIATIVES TOWARD KIDNEY CANCER DIAGNOSTICS
7.3.3 GROWING DEMAND FOR BETTER QUALITY HEALTHCARE
7.3.4 INCREASED DEMAND FOR NON-INVASIVE TESTING METHODS
7.4 CHALLENGES
7.4.1 LACK OF SKILLED AND CERTIFIED PROFESSIONALS
7.4.2 HIGH COST OF DIAGNOSTICS PROCEDURE FOR KIDNEY CANCERS
8 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE
8.1 OVERVIEW
8.2 IMAGING
8.2.1 COMPUTED TOMOGRAPHY
8.2.2 ULTRASOUND
8.2.3 MAGNETIC RESONANCE IMAGING (MRI)
8.2.4 ANGIOGRAPHY
8.2.5 X-RAY
8.2.6 OTHERS
8.3 BLOOD TEST
8.4 BIOPSY
8.4.1 FINE NEEDLE ASPIRATION
8.4.2 NEEDLE CORE BIOPSY
8.5 BIOMARKER TEST
8.5.1 AQUAPORIN 1 (AQP1)
8.5.2 PERILIPIN (PLIN2)
8.5.3 N-METHYLTRANSFERASE (NMNT)
8.5.4 L-PLASTIN (LCP-1)
8.5.5 NM23A
8.6 GENETIC TEST
8.7 OTHERS
9 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET, BY CANCER STAGE
9.1 OVERVIEW
9.2 STAGE I
9.3 STAGE II
9.4 STAGE III
9.5 STAGE IV
10 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE
10.1 OVERVIEW
10.2 RENAL CELL CARCINOMA
10.2.1 IMAGING
10.2.2 BLOOD TEST
10.2.3 BIOPSY
10.2.4 BIOMARKER TEST
10.2.5 GENETIC TEST
10.2.6 OTHERS
10.3 CLEAR CELL RENAL CELL CARCINOMA
10.3.1 IMAGING
10.3.2 BLOOD TEST
10.3.3 BIOPSY
10.3.4 BIOMARKER TEST
10.3.5 GENETIC TEST
10.3.6 OTHERS
10.4 NON CLEAR CELL RENAL CELL CARCINOMA
10.4.1 PAPILLARY RENAL CELL CARCINOMA
10.4.1.1 IMAGING
10.4.1.2 BLOOD TEST
10.4.1.3 BIOPSY
10.4.1.4 BIOMARKER TEST
10.4.1.5 GENETIC TEST
10.4.1.6 OTHERS
10.4.2 CHROMOPHOBE RENAL CELL CARCINOMA
10.4.2.1 IMAGING
10.4.2.2 BLOOD TEST
10.4.2.3 BIOPSY
10.4.2.4 BIOMARKER TEST
10.4.2.5 GENETIC TEST
10.4.2.6 OTHERS
11 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT
11.1 OVERVIEW
11.2 INSTRUMENT BASED PRODUCTS
11.2.1 IMAGING
11.2.2 BIOPSY
11.3 PLATFORM BASED PRODUCTS
11.3.1 NEXT GENERATION SEQUENCING
11.3.2 MICROARRAYS
11.3.3 PCR
11.3.4 OTHERS
11.4 KITS AND REAGENTS
11.4.1 RENAL CANCER PANELS
11.4.2 RENAL CANCER ANTIBODIES
11.5 OTHER CONSUMABLES
12 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET, BY TECHNOLOGY
12.1 OVERVIEW
12.2 FLUORESCENT IN SITU HYBRIDIZATION
12.3 NEXT GENERATION SEQUENCING
12.4 FLUORIMMUNOASSAY
12.5 COMPARATIVE GENOMIC HYBRIDIZATION
12.6 IMMUNOHISTOCHEMICAL
12.7 OTHERS
13 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET, BY APPLICATION
13.1 OVERVIEW
13.2 SCREENING
13.2.1 INSTRUMENT BASED PRODUCTS
13.2.2 PLATFORM BASED PRODUCTS
13.2.3 KITS AND REAGENTS
13.2.4 OTHER CONSUMABLES
13.3 DIAGNOSTIC AND PREDICTIVE
13.3.1 INSTRUMENT BASED PRODUCTS
13.3.2 PLATFORM BASED PRODUCTS
13.3.3 KITS AND REAGENTS
13.3.4 OTHER CONSUMABLES
13.4 PROGNOSTIC
13.4.1 INSTRUMENT BASED PRODUCTS
13.4.2 PLATFORM BASED PRODUCTS
13.4.3 KITS AND REAGENTS
13.4.4 OTHER CONSUMABLES
13.5 RESEARCH
13.5.1 INSTRUMENT BASED PRODUCTS
13.5.2 PLATFORM BASED PRODUCTS
13.5.3 KITS AND REAGENTS
13.5.4 OTHER CONSUMABLES
14 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET, BY END USER
14.1 OVERVIEW
14.2 HOSPITALS
14.3 DIAGNOSTIC CENTERS
14.4 CANCER RESEARCH CENTERS
14.5 ACADEMIC INSTITUTES
14.6 AMBULATORY SURGICAL CENTERS
14.7 OTHERS
15 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL
15.1 OVERVIEW
15.2 DIRECT TENDER
15.3 RETAIL SALES
15.4 OTHERS
16 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION
16.1 NORTH AMERICA
16.1.1 U.S.
16.1.2 CANADA
16.1.3 MEXICO
17 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET: COMPANY LANDSCAPE
17.1 COMPANY SHARE ANALYSIS: NORTH AMERICA
18 SWOT ANALYSIS
19 COMPANY PROFILE
19.1 CANON MEDICAL SYSTEMS CORPORATION
19.1.1 COMPANY SNAPSHOT
19.1.2 REVENUE ANALYSIS
19.1.3 COMPANY PROFILE
19.1.4 PRODUCT PORTFOLIO
19.1.5 RECENT DEVELOPMENT
19.2 KONINKLIJKE PHILIPS N.V.
19.2.1 COMPANY SNAPSHOT
19.2.2 REVENUE ANALYSIS
19.2.3 COMPANY PROFILE
19.2.4 PRODUCT PORTFOLIO
19.2.5 RECENT DEVELOPMENTS
19.3 GENERAL ELECTRIC COMPANY
19.3.1 COMPANY SNAPSHOT
19.3.2 REVENUE ANALYSIS
19.3.3 COMPANY PROFILE
19.3.4 PRODUCT PORTFOLIO
19.3.5 RECENT DEVELOPMENTS
19.4 SIEMENS HEALTHCARE GMBH
19.4.1 COMPANY SNAPSHOT
19.4.2 REVENUE ANALYSIS
19.4.3 COMPANY PROFILE
19.4.4 PRODUCT PORTFOLIO
19.4.5 RECENT DEVELOPMENT
19.5 GRAIL
19.5.1 COMPANY SNAPSHOT
19.5.2 PRODUCT PORTFOLIO
19.5.3 RECENT DEVELOPMENTS
19.6 AMBRY GENETICS
19.6.1 COMPANY SNAPSHOT
19.6.2 PRODUCT PORTFOLIO
19.6.3 RECENT DEVELOPMENT
19.7 BIOVENDOR R&D
19.7.1 COMPANY SNAPSHOT
19.7.2 PRODUCT PORTFOLIO
19.7.3 RECENT DEVELOPMENT
19.8 BLUEPRINT GENETICS OY.
19.8.1 COMPANY SNAPSHOT
19.8.2 PRODUCT PORTFOLIO
19.8.3 RECENT DEVELOPMENT
19.9 CD GENOMICS
19.9.1 COMPANY SNAPSHOT
19.9.2 PRODUCT PORTFOLIO
19.9.3 RECENT DEVELOPMENT
19.1 CENTOGENE N.V.
19.10.1 COMPANY SNAPSHOT
19.10.2 REVENUE ANALYSIS
19.10.3 PRODUCT PORTFOLIO
19.10.4 RECENT DEVELOPMENT
19.11 CREATIVE DIAGNOSTICS
19.11.1 COMPANY SNAPSHOT
19.11.2 PRODUCT PORTFOLIO
19.11.3 RECENT DEVELOPMENT
19.12 FUJIFILM CORPORATION
19.12.1 COMPANY SNAPSHOT
19.12.2 REVENUE ANALYSIS
19.12.3 PRODUCT PORTFOLIO
19.12.4 RECENT DEVELOPMENTS
19.13 GENEDX, LLC
19.13.1 COMPANY SNAPSHOT
19.13.2 REVENUE ANALYSIS
19.13.3 PRODUCT PORTFOLIO
19.13.4 RECENT DEVELOPMENT
19.14 GENPATH, A DIVISION OF BIOREFERENCE LABORATORIES, AN OPKO HEALTH INC. COMPANY
19.14.1 COMPANY SNAPSHOT
19.14.2 PRODUCT PORTFOLIO
19.14.3 RECENT DEVELOPMENT
19.15 ILLUMINA, INC.
19.15.1 COMPANY SNAPSHOT
19.15.2 REVENUE ANALYSIS
19.15.3 PRODUCT PORTFOLIO
19.15.4 RECENT DEVELOPMENT
19.16 INVITAE CORPORATION
19.16.1 COMPANY SNAPSHOT
19.16.2 REVENUE ANALYSIS
19.16.3 PRODUCT PORTFOLIO
19.16.4 RECENT DEVELOPMENT
19.17 LABORATORY CORPORATION OF AMERICA HOLDINGS
19.17.1 COMPANY SNAPSHOT
19.17.2 REVENUE ANALYSIS
19.17.3 PRODUCT PORTFOLIO
19.17.4 RECENT DEVELOPMENTS
19.18 MYRIAD GENETICS, INC.
19.18.1 COMPANY SNAPSHOT
19.18.2 REVENUE ANALYSIS
19.18.3 PRODUCT PORTFOLIO
19.18.4 RECENT DEVELOPMENT
19.19 THERMO FISHER SCIENTIFIC INC.
19.19.1 COMPANY SNAPSHOT
19.19.2 REVENUE ANALYSIS
19.19.3 PRODUCT PORTFOLIO
19.19.4 RECENT DEVELOPMENT
19.2 QIAGEN
19.20.1 COMPANY SNAPSHOT
19.20.2 REVENUE ANALYSIS
19.20.3 PRODUCT PORTFOLIO
19.20.4 RECENT DEVELOPMENT
20 QUESTIONNAIRE
21 RELATED REPORTS
表のリスト
TABLE 1 ESTIMATED NEW CANCER CASES AND DEATHS
TABLE 2 APPROVED DIAGNOSTICS OF KIDNEY CANCER
TABLE 3 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 4 NORTH AMERICA IMAGING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 5 NORTH AMERICA IMAGING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 6 NORTH AMERICA BLOOD TEST IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 7 NORTH AMERICA BIOPSY IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 8 NORTH AMERICA BIOPSY IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 9 NORTH AMERICA BIOMARKER TEST IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 10 NORTH AMERICA BIOMARKER TEST IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 11 NORTH AMERICA GENETIC TEST IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 12 NORTH AMERICA OTHERS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 13 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 14 NORTH AMERICA STAGE I IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 15 NORTH AMERICA STAGE II IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 16 NORTH AMERICA STAGE III IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 17 NORTH AMERICA STAGE IV IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 18 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 19 NORTH AMERICA RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 20 NORTH AMERICA RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 21 NORTH AMERICA CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 22 NORTH AMERICA CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 23 NORTH AMERICA NON CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 24 NORTH AMERICA NON CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 25 NORTH AMERICA PAPILLARY RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 26 NORTH AMERICA CHROMOPHOBE RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 27 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 28 NORTH AMERICA INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 29 NORTH AMERICA INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 30 NORTH AMERICA INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 31 NORTH AMERICA INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 32 NORTH AMERICA PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 33 NORTH AMERICA PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 34 NORTH AMERICA PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 35 NORTH AMERICA PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 36 NORTH AMERICA KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 37 NORTH AMERICA KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 38 NORTH AMERICA KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 39 NORTH AMERICA KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 40 NORTH AMERICA OTHER CONSUMABLES IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 41 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 42 NORTH AMERICA FLUORESCENT IN SITU HYBRIDIZATION IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 43 NORTH AMERICA NEXT GENERATION SEQUENCING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 44 NORTH AMERICA FLUORIMMUNOASSAY IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 45 NORTH AMERICA COMPARATIVE GENOMIC HYBRIDIZATION IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 46 NORTH AMERICA IMMUNOHISTOCHEMICAL IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 47 NORTH AMERICA OTHERS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 48 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 49 NORTH AMERICA SCREENING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 50 NORTH AMERICA SCREENING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 51 NORTH AMERICA DIAGNOSTIC AND PREDICTIVE IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 52 NORTH AMERICA DIAGNOSTIC AND PREDICTIVE IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 53 NORTH AMERICA PROGNOSTIC IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 54 NORTH AMERICA PROGNOSTIC IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 55 NORTH AMERICA RESEARCH IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 56 NORTH AMERICA RESEARCH IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 57 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 58 NORTH AMERICA HOSPITALS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 59 NORTH AMERICA DIAGNOSTIC CENTERS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 60 NORTH AMERICA CANCER RESEARCH CENTERS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 61 NORTH AMERICA ACADEMIC INSTITUTES IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 62 NORTH AMERICA AMBULATORY SURGICAL CENTERS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 63 NORTH AMERICA OTHERS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 64 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 65 NORTH AMERICA DIRECT TENDER IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 66 NORTH AMERICA RETAIL SALES IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 67 NORTH AMERICA OTHERS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 68 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET, BY COUNTRY, 2021-2030 (USD MILLION)
TABLE 69 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 70 NORTH AMERICA IMAGING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 71 NORTH AMERICA BIOPSY IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 72 NORTH AMERICA BIOMARKER TEST IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 73 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 74 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 75 NORTH AMERICA RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 76 NORTH AMERICA CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 77 NORTH AMERICA NON CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 78 NORTH AMERICA PAPILLARY RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 79 NORTH AMERICA CHROMOPHOBE RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 80 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 81 NORTH AMERICA INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 82 NORTH AMERICA INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 83 NORTH AMERICA INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 84 NORTH AMERICA PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 85 NORTH AMERICA PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 86 NORTH AMERICA PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 87 NORTH AMERICA KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 88 NORTH AMERICA KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 89 NORTH AMERICA KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 90 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 91 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 92 NORTH AMERICA SCREENING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 93 NORTH AMERICA DIAGNOSTIC AND PREDICTIVE IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 94 NORTH AMERICA PROGNOSTIC IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 95 NORTH AMERICA RESEARCH IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 96 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 97 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 98 U.S. KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 99 U.S. IMAGING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 100 U.S. BIOPSY IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 101 U.S. BIOMARKER TEST IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 102 U.S. KIDNEY CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 103 U.S. KIDNEY CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 104 U.S. RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 105 U.S. CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 106 U.S. NON CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 107 U.S. PAPILLARY RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 108 U.S. CHROMOPHOBE RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 109 U.S. KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 110 U.S. INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 111 U.S. INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 112 U.S. INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 113 U.S. PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 114 U.S. PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 115 U.S. PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 116 U.S. KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 117 U.S. KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 118 U.S. KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 119 U.S. KIDNEY CANCER DIAGNOSTICS MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 120 U.S. KIDNEY CANCER DIAGNOSTICS MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 121 U.S. SCREENING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 122 U.S. DIAGNOSTIC AND PREDICTIVE IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 123 U.S. PROGNOSTIC IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 124 U.S. RESEARCH IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 125 U.S. KIDNEY CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 126 U.S. KIDNEY CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 127 CANADA KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 128 CANADA IMAGING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 129 CANADA BIOPSY IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 130 CANADA BIOMARKER TEST IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 131 CANADA KIDNEY CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 132 CANADA KIDNEY CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 133 CANADA RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 134 CANADA CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 135 CANADA NON CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 136 CANADA PAPILLARY RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 137 CANADA CHROMOPHOBE RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 138 CANADA KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 139 CANADA INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 140 CANADA INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 141 CANADA INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 142 CANADA PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 143 CANADA PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 144 CANADA PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 145 CANADA KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 146 CANADA KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 147 CANADA KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 148 CANADA KIDNEY CANCER DIAGNOSTICS MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 149 CANADA KIDNEY CANCER DIAGNOSTICS MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 150 CANADA SCREENING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 151 CANADA DIAGNOSTIC AND PREDICTIVE IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 152 CANADA PROGNOSTIC IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 153 CANADA RESEARCH IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 154 CANADA KIDNEY CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 155 CANADA KIDNEY CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 156 MEXICO KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 157 MEXICO IMAGING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 158 MEXICO BIOPSY IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 159 MEXICO BIOMARKER TEST IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 160 MEXICO KIDNEY CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 161 MEXICO KIDNEY CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 162 MEXICO RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 163 MEXICO CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 164 MEXICO NON CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 165 MEXICO PAPILLARY RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 166 MEXICO CHROMOPHOBE RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 167 MEXICO KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 168 MEXICO INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 169 MEXICO INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 170 MEXICO INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 171 MEXICO PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 172 MEXICO PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 173 MEXICO PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 174 MEXICO KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 175 MEXICO KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 176 MEXICO KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 177 MEXICO KIDNEY CANCER DIAGNOSTICS MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 178 MEXICO KIDNEY CANCER DIAGNOSTICS MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 179 MEXICO SCREENING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 180 MEXICO DIAGNOSTIC AND PREDICTIVE IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 181 MEXICO PROGNOSTIC IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 182 MEXICO RESEARCH IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 183 MEXICO KIDNEY CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 184 MEXICO KIDNEY CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
図表一覧
FIGURE 1 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET: SEGMENTATION
FIGURE 2 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET: DATA TRIANGULATION
FIGURE 3 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET: DROC ANALYSIS
FIGURE 4 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET: NORTH AMERICA VS REGIONAL MARKET ANALYSIS
FIGURE 5 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET: MARKET APPLICATION COVERAGE GRID
FIGURE 8 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET: DBMR MARKET POSITION GRID
FIGURE 9 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET: SEGMENTATION
FIGURE 11 GROWING AWARENESS OF KIDNEY CANCER AND INCREASING HEALTHCARE EXPENDITURE ARE EXPECTED TO DRIVE THE GROWTH OF THE NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET FROM 2023 TO 2030
FIGURE 12 THE IMAGING TEST SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET IN 2023 & 2030
FIGURE 13 DRIVERS, RESTRAINTS, OPPORTUNITIES AND CHALLENGES OF NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET
FIGURE 14 KIDNEY CANCER INCIDENCE, BOTH SEXES, BY REGION (2020)
FIGURE 15 KIDNEY CANCER MORTALITY, BOTH SEXES, BY REGION (2020)
FIGURE 16 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET : BY TEST TYPE, 2022
FIGURE 17 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET : BY TEST TYPE, 2023-2030 (USD MILLION)
FIGURE 18 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET : BY TEST TYPE, CAGR (2023-2030)
FIGURE 19 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET : BY TEST TYPE, LIFELINE CURVE
FIGURE 20 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET : BY CANCER STAGE , 2022
FIGURE 21 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET : BY CANCER STAGE, 2023-2030 (USD MILLION)
FIGURE 22 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET : BY CANCER STAGE, CAGR (2023-2030)
FIGURE 23 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET : BY CANCER STAGE, LIFELINE CURVE
FIGURE 24 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET : BY TUMOR TYPE, 2022
FIGURE 25 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET : BY TUMOR TYPE, 2023-2030 (USD MILLION)
FIGURE 26 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET : BY TUMOR TYPE, CAGR (2023-2030)
FIGURE 27 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET : BY TUMOR TYPE, LIFELINE CURVE
FIGURE 28 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET : BY PRODUCT, 2022
FIGURE 29 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET : BY PRODUCT, 2023-2030 (USD MILLION)
FIGURE 30 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET : BY PRODUCT, CAGR (2023-2030)
FIGURE 31 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET : BY PRODUCT, LIFELINE CURVE
FIGURE 32 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET : BY TECHNOLOGY, 2022
FIGURE 33 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET : BY TECHNOLOGY, 2023-2030 (USD MILLION)
FIGURE 34 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET : BY TECHNOLOGY, CAGR (2023-2030)
FIGURE 35 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET : BY TECHNOLOGY, LIFELINE CURVE
FIGURE 36 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET : BY APPLICATION, 2022
FIGURE 37 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET : BY APPLICATION, 2023-2030 (USD MILLION)
FIGURE 38 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET : BY APPLICATION, CAGR (2023-2030)
FIGURE 39 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET : BY APPLICATION, LIFELINE CURVE
FIGURE 40 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET : BY END USER, 2022
FIGURE 41 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET : BY END USER, 2023-2030 (USD MILLION)
FIGURE 42 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET : BY END USER, CAGR (2023-2030)
FIGURE 43 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET : BY END USER, LIFELINE CURVE
FIGURE 44 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET : BY DISTRIBUTION CHANNEL, 2022
FIGURE 45 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET : BY DISTRIBUTION CHANNEL, 2023-2030 (USD MILLION)
FIGURE 46 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET : BY DISTRIBUTION CHANNEL, CAGR (2023-2030)
FIGURE 47 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET : BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 48 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET: SNAPSHOT (2022)
FIGURE 49 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET: BY COUNTRY (2022)
FIGURE 50 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET: BY COUNTRY (2023 & 2030)
FIGURE 51 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET: BY COUNTRY (2022 & 2030)
FIGURE 52 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET: TEST TYPE (2023-2030)
FIGURE 53 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET: COMPANY SHARE 2022 (%)

調査方法
データ収集と基準年分析は、大規模なサンプル サイズのデータ収集モジュールを使用して行われます。この段階では、さまざまなソースと戦略を通じて市場情報または関連データを取得します。過去に取得したすべてのデータを事前に調査および計画することも含まれます。また、さまざまな情報ソース間で見られる情報の不一致の調査も含まれます。市場データは、市場統計モデルと一貫性モデルを使用して分析および推定されます。また、市場シェア分析と主要トレンド分析は、市場レポートの主要な成功要因です。詳細については、アナリストへの電話をリクエストするか、お問い合わせをドロップダウンしてください。
DBMR 調査チームが使用する主要な調査方法は、データ マイニング、データ変数が市場に与える影響の分析、および一次 (業界の専門家) 検証を含むデータ三角測量です。データ モデルには、ベンダー ポジショニング グリッド、市場タイムライン分析、市場概要とガイド、企業ポジショニング グリッド、特許分析、価格分析、企業市場シェア分析、測定基準、グローバルと地域、ベンダー シェア分析が含まれます。調査方法について詳しくは、お問い合わせフォームから当社の業界専門家にご相談ください。
カスタマイズ可能
Data Bridge Market Research は、高度な形成的調査のリーダーです。当社は、既存および新規のお客様に、お客様の目標に合致し、それに適したデータと分析を提供することに誇りを持っています。レポートは、対象ブランドの価格動向分析、追加国の市場理解 (国のリストをお問い合わせください)、臨床試験結果データ、文献レビュー、リファービッシュ市場および製品ベース分析を含めるようにカスタマイズできます。対象競合他社の市場分析は、技術ベースの分析から市場ポートフォリオ戦略まで分析できます。必要な競合他社のデータを、必要な形式とデータ スタイルでいくつでも追加できます。当社のアナリスト チームは、粗い生の Excel ファイル ピボット テーブル (ファクト ブック) でデータを提供したり、レポートで利用可能なデータ セットからプレゼンテーションを作成するお手伝いをしたりすることもできます。