北米B型肝炎感染症市場規模、シェア、トレンド分析レポート
Market Size in USD Billion
CAGR :
% 
 USD
2.46 Billion
USD
3.61 Billion
2024
2032
USD
2.46 Billion
USD
3.61 Billion
2024
2032
| 2025 –2032 | |
| USD 2.46 Billion | |
| USD 3.61 Billion | |
|
|
|
|
北米B型肝炎感染症市場のセグメンテーション、タイプ別(慢性および急性)、治療別(ワクチン、抗ウイルス薬、免疫調節 薬、手術) - 2032年までの業界動向と予測
北米のB型肝炎感染症市場規模
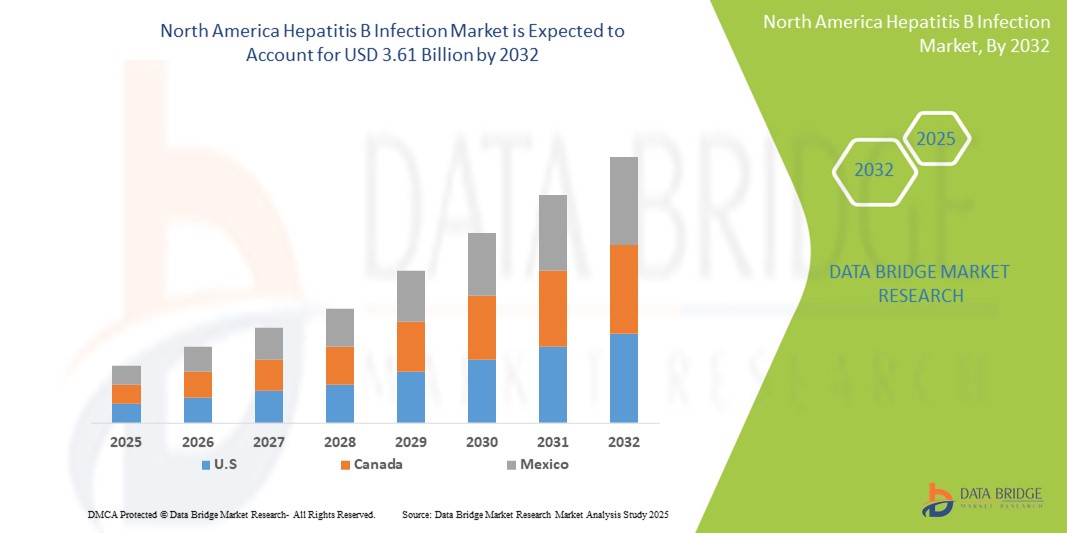
- 北米のB型肝炎感染市場規模は2024年に24億6000万米ドルと評価され、予測期間中に4.90%のCAGRで成長し、2032年には36億1000万米ドル に達すると予想されています 。
- 市場の成長は、B型肝炎に対する高度な診断技術と治療の革新の採用の増加と、欧州全域での電子医療システムのデジタル化と統合の増加によって主に推進されている。
- さらに、正確でアクセスしやすく、予防的なソリューションに対する消費者と公衆衛生の需要の高まりにより、B型肝炎管理プロトコルは医療政策の中心的な焦点として確立されつつあります。これらの要因が重なり、ワクチン接種、スクリーニング、抗ウイルス療法の導入が加速し、地域全体のB型肝炎感染症市場の成長を大幅に押し上げています。
北米B型肝炎感染症市場分析
- B型肝炎の治療と診断は、感染に関する意識の高まり、検査へのアクセス性の向上、抗ウイルス療法の進歩により、特に病院と外来の両方の環境で、北米の公衆衛生インフラのますます重要な要素となっています。
- 効果的なB型肝炎管理に対する需要の高まりは、主に予防接種の普及、HBV-HDVの重複感染スクリーニングの増加、そして特に高齢化社会と移民コミュニティにおける慢性肝疾患の負担の増加によって促進されています。
- 米国は、強力な公衆衛生政策、先進的な診断技術の早期導入、そして高いHBV検査率を特徴とする、2024年における北米のB型肝炎感染市場において、57.9%という最大の収益シェアを獲得し、市場を席巻しました。また、啓発活動、州レベルの撲滅計画、そして肝炎治療に対する保険適用拡大に後押しされ、特に高リスク層における治療の普及率も大幅に向上しました。
- メキシコは、都市化の進展、ワクチン接種率の向上、肝炎撲滅のための政府資金の拡大、都市部と農村部の両方での医療サービスへのアクセスの向上により、予測期間中に北米のB型肝炎感染市場の中で最も急速に成長する国になると予想されています。
- 慢性B型肝炎セグメントは、病気の持続性、長期の抗ウイルス療法の必要性、標的スクリーニングプログラムによる検出の改善、および肝炎検査のプライマリケアへの統合により、2024年に北米のB型肝炎感染市場で62.4%の市場シェアを占めました。
レポートの範囲と北米のB型肝炎感染症市場のセグメンテーション
|
属性 |
北米におけるB型肝炎感染症の主要市場分析 |
|
対象セグメント |
|
|
対象国 |
北米
|
|
主要な市場プレーヤー |
|
|
市場機会 |
|
|
付加価値データ情報セット |
データブリッジマーケットリサーチがまとめた市場レポートには、市場価値、成長率、セグメンテーション、地理的範囲、主要プレーヤーなどの市場シナリオに関する洞察に加えて、専門家による詳細な分析、価格設定分析、ブランドシェア分析、消費者調査、人口統計分析、サプライチェーン分析、バリューチェーン分析、原材料/消耗品の概要、ベンダー選択基準、PESTLE分析、ポーター分析、規制の枠組みも含まれています。 |
北米のB型肝炎感染症市場動向
「統合ケアと高度な治療アクセスによる利便性の向上」
- 北米のB型肝炎感染症市場において、重要かつ加速しているトレンドとして、多科医療モデルの統合と、集中型医療システムによる高度な治療へのアクセスの拡大が挙げられます。このトレンドは、一般開業医、肝臓専門医、公衆衛生機関間のシームレスなコミュニケーションを可能にすることで、患者の転帰と治療遵守率を大幅に向上させています。
- 例えば、西欧諸国のいくつかの国では、患者が単一の調整された枠組みの下で早期診断、抗ウイルス治療、定期的なフォローアップケアを受けられるよう、国家肝炎行動計画を実施しています。例えばドイツの統合ケアモデルは、診断から治療までの効率的な連携を可能にし、病気の進行率を低下させています。
- 集中化された患者登録、デジタル健康記録システム、合理化された紹介経路などの取り組みにより、タイムリーな介入とモニタリングが可能になり、B型肝炎感染管理が最適化されています。これらのシステムにより、医療従事者は肝機能、治療への反応、そしてD型肝炎などの合併感染をリアルタイムで追跡できます。
- 高度な診断と日常的なプライマリケアサービスの統合により、急性期および慢性期の症例の早期発見が容易になります。この集中的なアプローチと、より安価な抗ウイルス療法へのアクセスを組み合わせることで、個々の患者ケアとより広範な公衆衛生監視の両方が向上します。
- より合理化され、調整され、テクノロジーに支えられたB型肝炎ケアへのこの傾向は、各国の医療制度における期待を根本的に変革しつつあります。その結果、多くの欧州政府は、移民、静脈内薬物使用者、高齢者といった脆弱でリスクの高い集団を中心に、ウイルス性肝炎スクリーニングへのアクセスを拡大しています。
- 関係者が長期的な疾病管理と世界保健機関の2030年肝炎撲滅目標との整合性にますます重点を置くようになるにつれ、アクセスしやすく、効率的で統合的なB型肝炎治療モデルの需要は、公的および民間の医療部門の両方で急速に高まっています。
北米のB型肝炎感染症市場の動向
ドライバ
「疾病負担の増加と予防医療の導入によるニーズの高まり」
- ヨーロッパ全土におけるB型肝炎感染の増加と肝疾患に対する意識の高まりにより、早期診断、ワクチン接種、治療ソリューションの需要が大幅に高まっています。
- 例えば、2024年4月、グラクソ・スミスクライン(GSK)は、地域の医療システムとの戦略的提携を通じて、欧州におけるB型肝炎ワクチンの供給を拡大し、高リスク集団の予防接種率の向上を目指しました。主要市場プレーヤーによるこのような取り組みは、予測期間中の北米B型肝炎感染症市場の成長を促進すると予想されます。
- 公衆衛生当局と消費者が、肝硬変や肝臓がんなどの慢性B型肝炎に関連する長期合併症についてより深く認識するにつれて、ワクチン接種や早期スクリーニングなどの予防戦略の採用が増加し続けています。
- さらに、B型肝炎検査を日常の健康診断に統合し、ポイントオブケア診断技術の普及が進むことで、B型肝炎管理はヨーロッパ全体でよりアクセスしやすく、効率的になっています。
- 効果的なワクチン、経口抗ウイルス薬、そして高度な免疫調節薬の開発により、疾病管理の改善が可能になっています。政府の資金援助、保険償還政策、そしてWHO主導の肝炎撲滅目標も、公的医療現場と民間医療現場の両方で導入率を押し上げています。
抑制/挑戦
「治療へのアクセス性と先進治療の高額な費用に関する懸念」
- 医学の進歩にもかかわらず、北米の一部の地域では、高度な抗ウイルス療法や免疫調節剤へのアクセスが限られており、特に医療格差が残る北米東部と南部では依然として課題が残っている。
- 例えば、2024年初頭に発表された研究によると、一部のEU加盟国では依然としてB型肝炎ワクチンの不足や、調達と償還の問題による新しい治療法へのアクセスの制限に直面している。
- このギャップを埋めるには、EUレベルの資金援助、価格交渉、規制承認の合理化などを通じて、すべての欧州諸国でB型肝炎治療基準を調和させるための政策レベルの取り組みが必要である。
- さらに、第一選択の抗ウイルス薬はより手頃な価格になってきている一方で、有効性が向上した新世代の治療法はコストが高くなることが多く、無保険者や低所得者層への普及が制限される可能性がある。
- 特にパンデミック後のヨーロッパでは、国民の不信感やワクチンへの躊躇が、啓発キャンペーンや医療提供者の関与を通じて対処しなければならないもう一つの障壁となっている。
- 保険適用範囲の拡大、官民連携、地域医療インフラへの投資増加を通じてこれらの課題を克服することが、北米のB型肝炎感染症市場の長期的な成長を持続させる上で極めて重要となる。
北米のB型肝炎感染症市場の範囲
市場はタイプと処理に基づいて細分化されています。
• タイプ別
北米のB型肝炎感染市場は、種類別に慢性と急性に分類されます。慢性セグメントは、主に慢性HBV症例の有病率の高さと、抗ウイルス療法とモニタリングによる生涯にわたる疾患管理の必要性により、2024年には62.4%という最大の市場収益シェアを占めました。
急性セグメントは、早期スクリーニングの取り組みの強化、公衆衛生イニシアチブ、およびタイムリーな診断と治療につながる意識の高まりにより、2025年から2032年にかけて6.4%のCAGRで最も高い成長率を示すことが予想されています。
• 治療によって
北米のB型肝炎感染症市場は、治療に基づいて、ワクチン、抗ウイルス薬、免疫調節薬、手術に分類されます。ワクチン分野は、国家的なワクチン接種推進、出生時予防接種の増加、そして高リスク成人層における接種率の上昇に支えられ、2024年には41.2%と最大の収益シェアを獲得しました。
抗ウイルス薬セグメントは、慢性 HBV 患者プールの拡大、経口療法の進歩、有利な償還ポリシーにより、2025 年から 2032 年にかけて 7.1% という最も高い CAGR を達成すると予測されています。
北米B型肝炎感染症市場の地域分析
- 北米は、強力な公衆衛生インフラ、広範なワクチン接種プログラム、B型肝炎の感染と予防に関する意識の高まりにより、2024年に33.27%という最大の収益シェアでB型肝炎感染市場を支配しました。
- この地域は、最先端の診断技術、確立された予防接種プロトコル、そして政府主導の積極的な肝炎監視イニシアチブを特徴としています。
- この予防および治療戦略の強力な採用は、広範な医療アクセス、継続的な研究開発資金、早期発見と疾患管理への重点的な取り組みによってさらにサポートされており、北米が世界のB型肝炎感染市場への主要な貢献者としての役割を強固なものにしています。
米国B型肝炎感染症市場の洞察
米国のB型肝炎感染症市場は、先進的な医療インフラ、広範なHBVワクチン接種率、そしてスクリーニングおよび診断プロトコルの早期導入により、2024年には北米市場において最大の収益シェア(57.9%)を占めました。CDC推奨のユニバーサルスクリーニングガイドラインなどの政府の取り組みや、HBV-HDV重複感染検査への強力な支援が市場の成長を牽引しています。さらに、大規模な啓発キャンペーンと民間セクターによる抗ウイルス療法の革新も、安定した需要に貢献しています。
カナダのB型肝炎感染症市場に関する洞察
カナダのB型肝炎感染症市場は、2024年に北米市場収益の25.6%を占めました。カナダは、公的資金による医療制度、小児を対象としたユニバーサルワクチン接種プログラム、そして移民スクリーニングと母体検査の重視といった恩恵を受けています。政府と研究機関の継続的な協力により、特に医療サービスが行き届いていない地域や先住民コミュニティにおいて、慢性HBVの診断と治療へのアクセスが向上しています。
メキシコのB型肝炎感染症市場に関する洞察
メキシコのB型肝炎感染症市場は、2024年に地域市場シェアの16.5%を占め、予測期間中に6.9%という最も高いCAGRを達成すると予想されています。この成長は、感染症予防への政府投資の増加、乳幼児および青少年向けのワクチン接種プログラムの強化、そして地方における診断アクセスの拡大によって支えられています。官民パートナーシップと教育イニシアチブもまた、B型肝炎に関する意識向上と偏見の軽減に役立っており、早期発見と治療成果の向上に貢献しています。
北米のB型肝炎感染症市場シェア
北米の B 型肝炎感染症業界は、主に、次のような定評のある企業によって牽引されています。
- ギリアド・サイエンシズ(米国)
- GSK plc(英国)
- ダイナバックス・テクノロジーズ(米国)
- F. ホフマン・ラ・ロシュ社(スイス)
- ブリストル・マイヤーズ スクイブ社(米国)
- メルク社(米国)
- ノバルティスAG(スイス)
- アローヘッド・ファーマシューティカルズ社(米国)
- アルビュータス・バイオファーマ(カナダ)
- テバ・ファーマシューティカルズ社(イスラエル)
- ザイダス・ファーマシューティカルズ(インド)
- オーロビンドファーマ(インド)
- ルピン・ファーマシューティカルズ社(インド)
北米B型肝炎感染症市場の最新動向
- 2024年9月、ギリアド・サイエンシズとジェネシス・セラピューティクスは、ジェネシスのGEMS AIプラットフォームを活用した新規低分子治療薬の発見と開発に向けた戦略的提携を発表しました。ギリアドはこの提携により、製品の開発および商業化に関する独占的権利を取得しました。
- 2024年7月、ギリアド・サイエンシズ社は、ヒスパニック系/ラテン系の人々や併存疾患を有する高齢者を含む、多様なHIV感染者集団におけるビクタルビの長期的な有効性と安全性を示す研究データを発表しました。また、1日1回投与および週1回投与の治験薬レジメンについても強調されました。
- GSKは2024年2月、重症喘息治療薬として有望なモノクローナル抗体AIO-001を含むAiolos Bioの買収を完了しました。GSKは10億ドルの前払金と最大4億ドルのマイルストーンペイメントを支払い、呼吸器系生物製剤のポートフォリオを拡大しました。
SKU-
世界初のマーケットインテリジェンスクラウドに関するレポートにオンラインでアクセスする
- インタラクティブなデータ分析ダッシュボード
- 成長の可能性が高い機会のための企業分析ダッシュボード
- カスタマイズとクエリのためのリサーチアナリストアクセス
- インタラクティブなダッシュボードによる競合分析
- 最新ニュース、更新情報、トレンド分析
- 包括的な競合追跡のためのベンチマーク分析のパワーを活用
目次
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF NORTH AMERICA HEPATITIS B INFECTION MARKET
1.4 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 THERAPEUTICS LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 VENDOR SHARE ANALYSIS
2.11 SECONDARY SOURCES
2.12 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHT
4.1 PESTAL ANALYSIS
4.2 PORTER FIVE FORCES
5 NORTH AMERICA HEPATITIS B INFECTION MARKET: REGULATIONS
5.1 REGULATORY AUTHORITIES IN THE ASIA-PACIFIC REGION
5.2 NORTH AMERICA REGULATORY SCENARIO
5.3 EUROPE REGULATORY SCENARIO
5.4 MIDDLE EAST AND AFRICA REGULATORY SCENARIO
5.5 SOUTH AMERICA REGULATORY SCENARIO
6 PIPELINE ANALYSIS
7 EPIDEMILIOGY
8 MARKET OVERVIEW
8.1 DRIVERS
8.1.1 INCREASING PREVALENCE OF HEPATITIS B INFECTIONS
8.1.2 TECHNOLOGICAL ADVANCEMENTS IN DIAGNOSTICS
8.1.3 DEVELOPMENT OF COMBINATION THERAPIES FOR HEPATITIS B
8.1.4 STRATEGIC INITIATIVES BY COMPANIES FOR HEPATITIS B INFECTION
8.2 RESTRAINTS
8.2.1 SIDE EFFECTS AND DRUG RESISTANCE
8.2.2 INSUFFICIENT VACCINE COVERAGE FOR HEPATITIS B INFECTION
8.3 OPPORTUNITY
8.3.1 RISING NEW DRUG RELEASES AND INCREASING NEW DRUG PERMITS FOR HEPATITIS B
8.3.2 GOVERNMENT PROGRAMS TO RAISE AWARENESS OF HEPATITIS B INFECTION
8.3.3 ADVANCED RESEARCH AND DEVELOPMENT FOR CLINICAL TRIALS
8.4 CHALLENGES
8.4.1 THE COST OF HEPATITIS B TREATMENTS IS HIGH
8.4.2 STRINGENT REGULATORY POLICIES AND REGIONAL DISPARITIES IN TREATMENT ACCESS
9 NORTH AMERICA HEPATITIS B INFECTION MARKET, BY TYPE
9.1 OVERVIEW
9.2 CHRONIC
9.3 ACUTE
10 NORTH AMERICA HEPATITIS B INFECTION MARKET, BY TREATMENT
10.1 OVERVIEW
10.2 VACCINE
10.2.1 HOSPITAL PHARMACIES
10.2.2 DRUGS STORES AND RETAIL PHARMACIES
10.2.3 ONLINE PHARMACIES
10.3 ANTIVIRAL DRUGS
10.3.1 TENOFOVIR ALAFENAMIDE FUMARATE (TAF)
10.3.2 TENOFOVIR DISOPROXIL FUMARATE (TDF)
10.3.3 ENTECAVIR
10.3.4 OTHERS
10.4 IMMUNE MODULATOR DRUGS
10.4.1 PEGYLATED INTERFERON
10.4.2 INTERFERON ALPHA
10.5 SURGERY
11 NORTH AMERICA HEPATITIS B INFECTION MARKET, BY REGION
11.1 NORTH AMERICA
11.1.1 U.S
11.1.2 CANADA
11.1.3 MEXICO
12 NORTH AMERICA HEPATITIS B TREATMENT MARKET: COMPANY LANDSCAPE
12.1 COMPANY SHARE ANALYSIS: NORTH AMERICA
13 SWOT ANALYSIS
14 COMPANY PROFILE
14.1 GILEAD SCIENCES, INC.
14.1.1 COMPANY SNAPSHOT
14.1.2 REVENUE ANALYSIS
14.1.3 COMPANY SHARE ANALYSIS
14.1.4 PRODUCT PORTFOLIO
14.1.5 RECENT DEVELOPMENT
14.2 GLAXOSMITHKLINE PLC
14.2.1 COMPANY SNAPSHOT
14.2.2 REVENUE ANALYSIS
14.2.3 COMPANY SHARE ANALYSIS
14.2.4 PRODUCT PORTFOLIO
14.2.5 RECENT DEVELOPMENT
14.3 DYNAVAX TECHNOLOGIES CORPORATION
14.3.1 COMPANY SNAPSHOT
14.3.2 REVENUE ANALYSIS
14.3.3 COMPANY SHARE ANALYSIS
14.3.4 PRODUCT PORTFOLIO
14.3.5 RECENT DEVELOPMENTS
14.4 F. HOFFMAN-LA ROCHE LTD.
14.4.1 COMPANY SNAPSHOT
14.4.2 REVENUE ANALYSIS
14.4.3 COMPANY SHARE ANALYSIS
14.4.4 PRODUCT PORTFOLIO
14.4.5 RECENT DEVELOPMENTS
14.5 BRISTOL-MYERS SQUIBB COMPANY
14.5.1 COMPANY SNAPSHOT
14.5.2 REVENUE ANALYSIS
14.5.3 COMPANY SHARE ANALYSIS
14.5.4 PRODUCT PORTFOLIO
14.5.5 RECENT DEVELOPMENTS
14.6 ARROWHEAD PHARMACEUTICALS, INC.
14.6.1 COMPANY SNAPSHOT
14.6.2 REVENUE ANALYSIS
14.6.3 PRODUCT PORTFOLIO
14.6.4 RECENT DEVELOPMENTS
14.7 ARBUTUS BIOPHARMA
14.7.1 COMPANY SNAPSHOT
14.7.2 REVENUE ANALYSIS
14.7.3 PRODUCT PORTFOLIO
14.7.4 RECENT UPDATES
14.8 AUROBINDO PHARMA
14.8.1 COMPANY SNAPSHOT
14.8.2 REVENUE ANALYSIS
14.8.3 PRODUCT PORTFOLIO
14.8.4 RECENT UPDATES
14.9 LUPIN PHARMACEUTICALS, INC.
14.9.1 COMPANY SNAPSHOT
14.9.2 PRODUCT PORTFOLIO
14.9.3 RECENT UPDATES
14.1 MERCK & CO., INC.,
14.10.1 COMPANY SNAPSHOT
14.10.2 REVENUE ANALYSIS
14.10.3 PRODUCT PORTFOLIO
14.10.4 RECENT DEVELOPMENTS
14.11 NOVARTIS AG
14.11.1 COMPANY SNAPSHOT
14.11.2 REVENUE
14.11.3 PRODUCT PORTFOLIO
14.11.4 RECENT DEVELOPMENT
14.12 TEVA PHARMACEUTICAL INDUSTRIES
14.12.1 COMPANY SNAPSHOT
14.12.2 PRODUCT PORTFOLIO
14.12.3 RECENT DEVELOPMENTS
14.13 ZYDUS PHARMACEUTICALS, INC.
14.13.1 COMPANY SNAPSHOT
14.13.2 PRODUCT PORTFOLIO
14.13.3 REVENUE
14.13.4 RECENT DEVELOPMENT
15 QUESTIONNAIRE
16 RELATED REPORTS
表のリスト
TABLE 1 NORTH AMERICA CLINICAL TRIAL AND PIPELINE A-LYSIS AS PER THE COMPANY
TABLE 2 DISTRIBUTION OF PRODUCTS OR PROJECTS BY PHASE
TABLE 3 COUNTRY WISE EPIDEMIOLOGY FOR HEPATITIS B
TABLE 4 COST OF HEPATITIS B MEDICATIONS: BRAND VS. GENERIC PRICES
TABLE 5 NORTH AMERICA HEPATITIS B INFECTION MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 6 NORTH AMERICA CHRONIC IN HEPATITIS B INFECTION MARKET, BY REGION, 2022-2031 (USD MILLION)
TABLE 7 NORTH AMERICA ACUTE IN HEPATITIS B INFECTION MARKET, BY REGION, 2022-2031 (USD MILLION)
TABLE 8 NORTH AMERICA HEPATITIS B INFECTION MARKET, BY TREATMENT, 2022-2031 (USD MILLION)
TABLE 9 NORTH AMERICA VACCINE IN HEPATITIS B INFECTION MARKET, BY REGION, 2022-2031 (USD MILLION)
TABLE 10 NORTH AMERICA VACCINE, ANTIVIRAL DRUGS, IMMUNE MODULATOR DRUGS IN HEPATITIS B INFECTION MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD MILLION)
TABLE 11 NORTH AMERICA ANTIVIRAL DRUGS IN HEPATITIS B INFECTION MARKET, BY REGION, 2022-2031 (USD MILLION)
TABLE 12 NORTH AMERICA ANTIVIRAL DRUGS IN HEPATITIS B INFECTION MARKET, BY TREATMENT, 2022-2031 (USD MILLION)
TABLE 13 NORTH AMERICA IMMUNE MODULATOR DRUGS IN HEPATITIS B INFECTION MARKET, BY REGION, 2022-2031 (USD MILLION)
TABLE 14 NORTH AMERICA IMMUNE MODULATOR DRUGS IN HEPATITIS B INFECTION MARKET, BY TREATMENT, 2022-2031 (USD MILLION)
TABLE 15 NORTH AMERICA SURGERY IN HEPATITIS B INFECTION MARKET, BY REGION, 2022-2031 (USD MILLION)
TABLE 16 NORTH AMERICA HEPATITIS B INFECTION MARKET, BY COUNTRY, 2022-2031 (USD MILLION)
TABLE 17 NORTH AMERICA HEPATITIS B INFECTION MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 18 NORTH AMERICA HEPATITIS B INFECTION MARKET, BY TREATMENT, 2022-2031 (USD MILLION)
TABLE 19 NORTH AMERICA ANTIVIRAL DRUGS IN HEPATITIS B INFECTION MARKET, BY TREATMENT, 2022-2031 (USD MILLION)
TABLE 20 NORTH AMERICA IMMUNE MODULATOR DRUGS IN HEPATITIS B INFECTION MARKET, BY TREATMENT, 2022-2031 (USD MILLION)
TABLE 21 NORTH AMERICA VACCINE, ANTIVIRAL DRUGS, IMMUNE MODULATOR DRUGS IN HEPATITIS B INFECTION MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD MILLION)
TABLE 22 U.S. HEPATITIS B INFECTION MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 23 U.S. HEPATITIS B INFECTION MARKET, BY TREATMENT, 2022-2031 (USD MILLION)
TABLE 24 U.S. ANTIVIRAL DRUGS IN HEPATITIS B INFECTION MARKET, BY TREATMENT, 2022-2031 (USD MILLION)
TABLE 25 U.S. IMMUNE MODULATOR DRUGS IN HEPATITIS B INFECTION MARKET, BY TREATMENT, 2022-2031 (USD MILLION)
TABLE 26 U.S. VACCINE, ANTIVIRAL DRUGS, IMMUNE MODULATOR DRUGS IN HEPATITIS B INFECTION MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD MILLION)
TABLE 27 CANADA HEPATITIS B INFECTION MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 28 CANADA HEPATITIS B INFECTION MARKET, BY TREATMENT, 2022-2031 (USD MILLION)
TABLE 29 CANADA ANTIVIRAL DRUGS IN HEPATITIS B INFECTION MARKET, BY TREATMENT, 2022-2031 (USD MILLION)
TABLE 30 CANADA IMMUNE MODULATOR DRUGS IN HEPATITIS B INFECTION MARKET, BY TREATMENT, 2022-2031 (USD MILLION)
TABLE 31 CANADA VACCINE, ANTIVIRAL DRUGS, IMMUNE MODULATOR DRUGS IN HEPATITIS B INFECTION MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD MILLION)
TABLE 32 MEXICO HEPATITIS B INFECTION MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 33 MEXICO HEPATITIS B INFECTION MARKET, BY TREATMENT, 2022-2031 (USD MILLION)
TABLE 34 MEXICO ANTIVIRAL DRUGS IN HEPATITIS B INFECTION MARKET, BY TREATMENT, 2022-2031 (USD MILLION)
TABLE 35 MEXICO IMMUNE MODULATOR DRUGS IN HEPATITIS B INFECTION MARKET, BY TREATMENT, 2022-2031 (USD MILLION)
TABLE 36 MEXICO VACCINE, ANTIVIRAL DRUGS, IMMUNE MODULATOR DRUGS IN HEPATITIS B INFECTION MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD MILLION)
図表一覧
FIGURE 1 NORTH AMERICA HEPATITIS B INFECTION MARKET: SEGMENTATION
FIGURE 2 NORTH AMERICA HEPATITIS B INFECTION MARKET: DATA TRIANGULATION
FIGURE 3 NORTH AMERICA HEPATITIS B INFECTION MARKET: DROC ANALYSIS
FIGURE 4 NORTH AMERICA HEPATITIS B INFECTION MARKET: NORTH AMERICA VS REGIONAL MARKET ANALYSIS
FIGURE 5 NORTH AMERICA HEPATITIS B INFECTION MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 NORTH AMERICA HEPATITIS B INFECTION MARKET: MULTIVARIATE MODELLING
FIGURE 7 NORTH AMERICA HEPATITIS B INFECTION MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 8 NORTH AMERICA HEPATITIS B INFECTION MARKET: DBMR MARKET POSITION GRID
FIGURE 9 NORTH AMERICA HEPATITIS B INFECTION MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 NORTH AMERICA HEPATITIS B INFECTION MARKET: SEGMENTATION
FIGURE 11 TWO SEGMENTS COMPRISE THE NORTH AMERICA HEPATITIS B INFECTION MARKET, BY TYPE
FIGURE 12 EXECUTIVE SUMMARY
FIGURE 13 STRATEGIC DECISIONS
FIGURE 14 NORTH AMERICA HEPATITIS B INFECTION MARKET
FIGURE 15 CHRONIC SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE NORTH AMERICA HEPATITIS B INFECTION MARKET IN 2024 & 2031
FIGURE 16 DROC ANALYSIS
FIGURE 17 BURDEN OF HBV INFECTION IN THE GENERAL POPULATION BY WHO REGION, 2019
FIGURE 18 NORTH AMERICA HEPATITIS B INFECTION MARKET: BY TYPE, 2023
FIGURE 19 NORTH AMERICA HEPATITIS B INFECTION MARKET: BY TYPE, 2024-2031 (USD MILLION)
FIGURE 20 NORTH AMERICA HEPATITIS B INFECTION MARKET: BY TYPE, CAGR (2024-2031)
FIGURE 21 NORTH AMERICA HEPATITIS B INFECTION MARKET: BY TYPE, LIFELINE CURVE
FIGURE 22 NORTH AMERICA HEPATITIS B INFECTION MARKET: BY TREATMENT, 2023
FIGURE 23 NORTH AMERICA HEPATITIS B INFECTION MARKET: BY TREATMENT, 2024-2031 (USD MILLION)
FIGURE 24 NORTH AMERICA HEPATITIS B INFECTION MARKET: BY TREATMENT, CAGR (2024-2031)
FIGURE 25 NORTH AMERICA HEPATITIS B INFECTION MARKET BY TREATMENT, LIFELINE CURVE
FIGURE 26 NORTH AMERICA HEPATITIS B INFECTION MARKET, SNAPSHOT
FIGURE 27 NORTH AMERICA HEPATITIS B TREATMENT MARKET: COMPANY SHARE 2023 (%)

調査方法
データ収集と基準年分析は、大規模なサンプル サイズのデータ収集モジュールを使用して行われます。この段階では、さまざまなソースと戦略を通じて市場情報または関連データを取得します。過去に取得したすべてのデータを事前に調査および計画することも含まれます。また、さまざまな情報ソース間で見られる情報の不一致の調査も含まれます。市場データは、市場統計モデルと一貫性モデルを使用して分析および推定されます。また、市場シェア分析と主要トレンド分析は、市場レポートの主要な成功要因です。詳細については、アナリストへの電話をリクエストするか、お問い合わせをドロップダウンしてください。
DBMR 調査チームが使用する主要な調査方法は、データ マイニング、データ変数が市場に与える影響の分析、および一次 (業界の専門家) 検証を含むデータ三角測量です。データ モデルには、ベンダー ポジショニング グリッド、市場タイムライン分析、市場概要とガイド、企業ポジショニング グリッド、特許分析、価格分析、企業市場シェア分析、測定基準、グローバルと地域、ベンダー シェア分析が含まれます。調査方法について詳しくは、お問い合わせフォームから当社の業界専門家にご相談ください。
カスタマイズ可能
Data Bridge Market Research は、高度な形成的調査のリーダーです。当社は、既存および新規のお客様に、お客様の目標に合致し、それに適したデータと分析を提供することに誇りを持っています。レポートは、対象ブランドの価格動向分析、追加国の市場理解 (国のリストをお問い合わせください)、臨床試験結果データ、文献レビュー、リファービッシュ市場および製品ベース分析を含めるようにカスタマイズできます。対象競合他社の市場分析は、技術ベースの分析から市場ポートフォリオ戦略まで分析できます。必要な競合他社のデータを、必要な形式とデータ スタイルでいくつでも追加できます。当社のアナリスト チームは、粗い生の Excel ファイル ピボット テーブル (ファクト ブック) でデータを提供したり、レポートで利用可能なデータ セットからプレゼンテーションを作成するお手伝いをしたりすることもできます。