北米抗核抗体検査市場規模、シェア、トレンド分析レポート
Market Size in USD Billion
CAGR :
% 
 USD
1.63 Billion
USD
4.42 Billion
2025
2033
USD
1.63 Billion
USD
4.42 Billion
2025
2033
| 2026 –2033 | |
| USD 1.63 Billion | |
| USD 4.42 Billion | |
|
|
|
|
北米抗核抗体検査市場:抗体タイプ別(抽出核抗原(ENA)、抗DSDNAおよびヒストン、抗DFS70抗体、抗PM-SCL、抗セントロメア抗体、抗SP100、その他)、製品別(機器、消耗品、試薬、サービス)、技術別(ELISA、間接免疫蛍光法(IIF)、ブロッティング試験、抗原マイクロアレイ、ゲルベース技術、マルチプレックスアッセイ、フローサイトメトリー、受動血球凝集反応(PHA)、その他)、用途別(自己免疫疾患および感染症)、エンドユーザー別(病院、研究所、診断センター、研究機関、その他)、流通チャネル別(直接入札、小売販売、サードパーティ販売業者、その他) - 2033年までの業界動向と予測
北米の抗核抗体検査市場規模
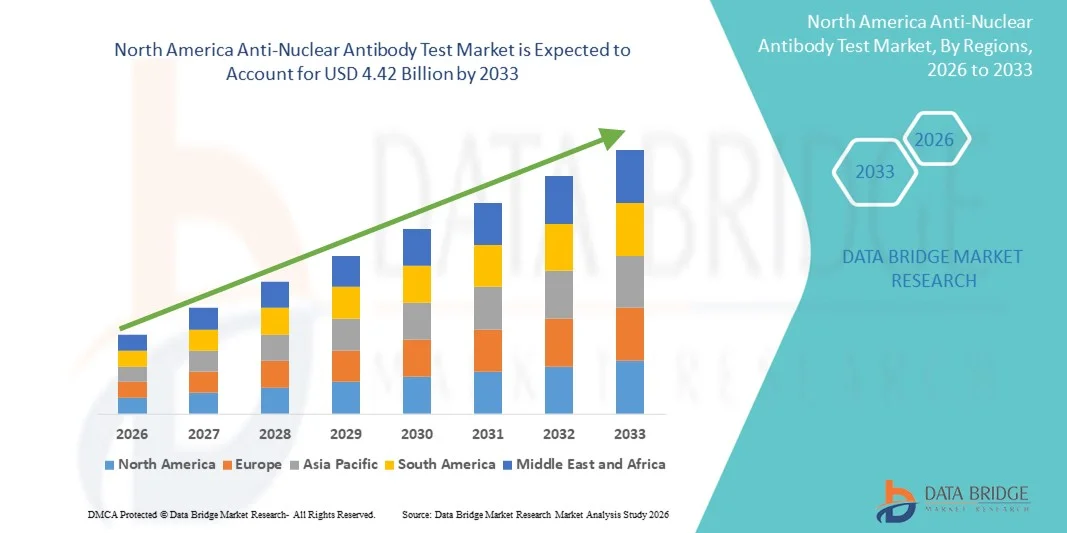
- 北米の抗核抗体検査市場規模は2025年に16億3000万米ドルと評価され、予測期間中に13.30%のCAGRで成長し、2033年までに44億2000万米ドル に達すると予想されています 。
- 市場の成長は、自己免疫疾患の罹患率の上昇、早期かつ正確な診断に対する意識の高まり、臨床検査技術の継続的な進歩によって、病院、診断研究所、専門クリニック全体で抗核抗体(ANA)検査の採用が拡大していることが主な要因です。
- さらに、ループス、関節リウマチ、強皮症などの自己免疫疾患に対する費用対効果が高く、信頼性が高く、迅速な診断ソリューションへの需要が高まっており、抗核抗体検査は日常診療および専門医療現場における重要な第一線スクリーニングツールとして確立されつつあります。これらの要因が相まって、抗核抗体検査ソリューションの普及が加速し、業界の成長を大きく後押ししています。
北米抗核抗体検査市場分析
- 自己免疫疾患に関連する自己抗体を検出するために使用される抗核抗体(ANA)検査は、全身性自己免疫疾患の早期発見とモニタリングにおける重要な役割のため、病院と専門研究所の両方で現代の診断においてますます不可欠なツールになりつつあります。
- ANA検査の需要の高まりは、主に全身性エリテマトーデス、関節リウマチ、強皮症などの疾患の世界的な罹患率の上昇と、医療従事者と患者の間で早期かつ正確な自己免疫疾患スクリーニングの重要性に対する意識の高まりによって推進されています。
- 米国は、高度な医療インフラ、高い診断検査率、強力な償還枠組み、大手診断企業の存在に支えられ、2025年には抗核抗体検査市場において34.6%という最大の収益シェアを獲得して市場を支配した。
- カナダは、医療アクセスの改善、診断ラボネットワークの拡大、自己免疫疾患の認識の高まり、医療インフラへの政府投資の増加により、予測期間中に抗核抗体検査市場で最も急速に成長する国となり、11.8%のCAGRを記録すると予想されています。
- 自己免疫疾患セグメントは、全身性エリテマトーデス、関節リウマチ、シェーグレン症候群、強皮症などの疾患の世界的な罹患率の増加により、2025年には約58.9%と最大の市場収益シェアを占めました。
レポートの範囲と抗核抗体検査市場のセグメンテーション
|
特性 |
抗核抗体検査の主要市場分析 |
|
対象セグメント |
|
|
対象国 |
北米
|
|
主要市場プレーヤー |
|
|
市場機会 |
|
|
付加価値データ情報セット |
Data Bridge Market Research がまとめた市場レポートには、市場価値、成長率、セグメンテーション、地理的範囲、主要プレーヤーなどの市場シナリオに関する洞察に加えて、専門家による詳細な分析、患者の疫学、パイプライン分析、価格分析、規制の枠組みも含まれています。 |
北米の抗核抗体検査市場動向
自己免疫疾患の早期発見と高度な診断導入の重要性の高まり
- 北米の抗核抗体検査市場において、全身性エリテマトーデス、関節リウマチ、強皮症といった自己免疫疾患および全身性炎症疾患の早期発見への関心が高まっていることは、重要な加速トレンドです。こうした臨床的関心の高まりにより、診断・研究環境における信頼性と高精度なANA検査の需要が大幅に高まっています。
- 例えば、北米ではますます多くの病院や診断検査室が、高度な免疫蛍光法や酵素結合免疫吸着法(ELISA)に基づくANA検査プラットフォームを導入し、検出感度の向上と診断時間の短縮を図っています。こうした広範な統合により、自己免疫疾患の日常的なスクリーニングと早期診断におけるANA検査の役割が強化されています。
- 検査自動化とハイスループット検査システムの導入により、検査室はより多くの検査量を管理しながらも、一貫した結果精度を維持することが可能になっています。一部の先進的なプラットフォームは、複数の自己抗体パターンを同時に検出できるようになり、根底にある自己免疫活動に関するより包括的な知見を提供し、より的を絞った臨床判断を支援しています。
- 臨床検査室、研究機関、製薬会社間の連携の拡大は、高度なANA検査キットの開発と導入をさらに促進しています。こうした連携を通じて、医療従事者は疾患モニタリングを強化し、治療反応をより効果的に分析し、自己免疫疾患における患者管理全体を改善することができます。
- より効率的、高感度、かつ信頼性の高い自己免疫診断ツールへの移行は、疾患の早期発見と長期的な疾患管理への期待を再構築しています。その結果、免疫診断を専門とする企業は、病院と臨床検査室の両方において、一貫性、再現性、そして臨床的に正確な結果を提供するように設計された、強化されたANA検査キットの開発をますます進めています。
- 医療システムが正確な診断、患者の転帰の改善、自己免疫疾患の効果的な長期管理を優先するにつれ、高度なANA検査ソリューションに対する需要は病院、診断センター、専門クリニック全体で増加し続けています。
北米の抗核抗体検査市場の動向
ドライバー
自己免疫疾患の有病率の増加と診断に対する認識の拡大
- 自己免疫疾患の罹患率の増加と、早期診断および疾患管理への意識の高まりは、抗核抗体検査市場の主要な牽引力となっています。リウマチ科の検査を受ける人が増えており、主要な診断ツールとしてのANA検査の需要が高まっています。
- 例えば近年、医療当局や医師会は自己免疫疾患の早期スクリーニングの重要性を強調し、疲労、関節痛、炎症などの原因不明の慢性症状を呈する患者に対し、プライマリケア医や専門医に対し、診断プロトコルにANA検査を含めるよう奨励しています。こうした取り組みは、予測期間中の市場の着実な成長を支えると期待されています。
- 患者が慢性疾患や長期的な健康状態への意識が高まるにつれ、根本的な免疫系の異常を特定できる包括的な血液検査への関心が高まっています。ANA検査は自己免疫活性を特定するための重要な第一歩であり、診断経路の不可欠な要素となっています。
- さらに、専門のリウマチ科クリニックや診断ラボの拡大により、ANA検査サービスへのアクセスが向上しています。医療インフラと検査室の能力への投資の増加により、都市部と準都市部の両方でこれらの検査がより広く利用可能になっています。
- 臨床研究、医薬品開発、自己免疫疾患モニタリングにおけるANA検査の利用増加も、市場の持続的な成長に貢献しています。製薬会社は、免疫関連試験における患者の適格性と治療効果を評価するために、臨床試験においてこれらの検査をますます活用しています。
拘束/挑戦
検査の解釈と高額な診断費用に関する懸念
- ANA検査結果の解釈に関する懸念は、市場への普及拡大にとって大きな課題となっています。ANA検査結果は健康な人でも陽性となる場合があり、誤った解釈は不必要な不安、誤診、追加検査につながる可能性があり、臨床医と患者の間で、この検査のみに過度に依存することへの躊躇が生じています。
- 例えば、免疫蛍光検査においては、検査技術、試薬の品質、そして主観的な解釈に基づく検査結果のばらつきが、場合によっては結果の一貫性を欠く原因となっています。このばらつきは臨床的意思決定を複雑にし、診断を裏付ける証拠がないままANA検査のみを使用することの信頼性を低下させる可能性があります。
- 標準化された検査プロトコル、検査専門家への研修の改善、そして検査精度の向上を通じてこれらの課題に対処することは、医療提供者間の信頼を強化するために不可欠です。さらに、高度なANA検査方法、特にマルチプレックスシステムや自動化システムは比較的高額であるため、特に発展途上地域においては、小規模な診療所やコストに敏感な医療施設にとって、アクセスが制限される可能性があります。基本的な検査オプションは利用可能ですが、より包括的で高感度なプラットフォームは、多くの機関にとって依然として経済的に手の届かないものとなっています。
- 技術の進歩とメーカー間の競争によりコストは徐々に低下していますが、公的医療制度の予算制約や償還の問題により、依然として普及が制限される可能性があります。多くの医療提供者は、日常的に使用するANA検査ソリューションを選択する際に、費用対効果と診断精度のバランスを慎重に取る必要があります。
- より良い標準化、費用対効果の高い製品開発、臨床医のトレーニングの強化、医療資金の改善を通じてこれらの課題を克服することは、抗核抗体検査市場の世界的な持続的な拡大に不可欠です。
北米の抗核抗体検査市場の展望
市場は、抗体の種類、製品、技術、用途、エンドユーザー、流通チャネルに基づいてセグメント化されています。
- 抗体の種類別
抗体の種類に基づいて、抗核抗体検査市場は、抽出核抗原(ENA)、抗dsDNAおよびヒストン、抗DFS70抗体、抗PM-SCL抗体、抗セントロメア抗体、抗SP100抗体、その他に分類されます。抗dsDNAおよびヒストン抗体セグメントは、全身性エリテマトーデス(SLE)などの全身性自己免疫疾患の診断における重要な役割に牽引され、2025年には約36.8%という最大の市場収益シェアを占めました。これらの抗体は、高い特異性と臨床的意義から、病院や診断検査室で広く利用されています。医師の認知度の高まり、高い検査精度、そして自己免疫パネルへの日常的な組み込みが、このセグメントの優位性をさらに支えています。狼瘡と関節リウマチの世界的な有病率の増加も、採用率の上昇に貢献していますさらに、アッセイの感度と標準化の向上により、結果に対する信頼性が高まり、より広範な臨床使用が促進されます。
抗ENA分野は、シェーグレン症候群、強皮症、多発性筋炎といった複数の結合組織疾患の検出における用途拡大により、2026年から2033年にかけて約10.7%という最も高いCAGR(年平均成長率)を達成すると予想されています。ENAパネルは、複雑な自己免疫疾患の早期発見と鑑別を可能にするため、高度な診断において非常に有用です。マルチプレックスプラットフォームの導入拡大と包括的なENA検査パネルの利用可能性が成長を牽引しています。さらに、医療投資の増加、診断インフラの拡大、そして発展途上国におけるスクリーニングプログラムの増加も需要を加速させています。ENA検査と自動化プラットフォームの統合も効率性の向上に寄与しており、急速な成長軌道にさらに貢献しています。
- 製品別
製品に基づいて、市場は機器、消耗品・試薬、およびサービスに分類されます。消耗品・試薬セグメントは、実施されるすべての検査に必要となるため、2025年には約47.3%と最大の収益シェアを占めました。試薬、緩衝液、抗体、およびアッセイキットの継続的な要件により、このセグメントはメーカーにとって安定した収益源となっています。検査数の増加、自己免疫疾患の有病率の上昇、および検査室ネットワークの拡大により、需要が大幅に増加しました。さらに、試薬の処方とキットの信頼性の継続的な進歩により、日常的な診断における試薬の使用が強化されています。病院や検査室による院内検査への移行は、このセグメントの長期的な成長をさらに支えています
サービス分野は、専門検査機関への診断検査のアウトソーシングの増加傾向に牽引され、2026年から2033年にかけて約11.2%という最も高いCAGRを記録すると予測されています。小規模な病院や診療所では、院内インフラが限られているため、サードパーティのサービスプロバイダーへの依存が高まっています。診断サービスチェーンの拡大とハイスループット検査機関への投資が急速な成長を牽引しています。さらに、個別化医療の台頭と高度な検査解釈サービスへの需要の高まりも重要な役割を果たしています。デジタル統合と遠隔検査モデルも、この分野のアクセス性と拡大を促進しています。
- テクニック別
技術に基づいて、市場はELISA、間接免疫蛍光法(IIF)、ブロッティング試験、抗原マイクロアレイ、ゲルベース技術、マルチプレックスアッセイ、フローサイトメトリー、受動血球凝集反応(PHA)、その他に分類されます。間接免疫蛍光法(IIF)セグメントは、2025年には約41.5%の収益シェアで市場を席巻し、ANA検査における世界的なゴールドスタンダードであり続けています。IIFは、幅広い自己抗体を高い感度で検出し、診断解釈に不可欠な明確なパターン可視化を提供します。ほとんどの臨床ガイドラインは、引き続きIIFを主要なスクリーニング方法として推奨しています。病院の検査室での広範な採用と強力な保険償還サポートは、その優位性をさらに強化しています。
マルチプレックスアッセイ分野は、複数の抗体を同時に検出できるため、2026年から2033年にかけて約12.4%という最も高いCAGR(年平均成長率)を達成すると予想されています。この技術は、ターンアラウンドタイムを大幅に短縮し、ワークフローの効率を向上させ、全体的な検査コストを削減します。自動化プラットフォームの導入拡大と、大規模検査室におけるハイスループット検査の需要の高まりが、この分野の成長を加速させています。さらに、技術の進歩とAIベースの分析ツールとの統合により、精度が向上し、臨床医の間での普及が進んでいます。
- 用途別
用途別に見ると、抗核抗体検査市場は自己免疫疾患と感染症に分類されます。自己免疫疾患セグメントは、全身性エリテマトーデス、関節リウマチ、シェーグレン症候群、強皮症などの疾患の世界的な有病率の増加に牽引され、2025年には約58.9%と最大の市場収益シェアを占めました。抗核抗体検査はこれらの疾患の診断と臨床モニタリングにおいて重要な役割を果たしており、病院や専門クリニックでは日常的に処方されています。患者と医師の意識の高まり、早期スクリーニングプログラムへのアクセスの改善、迅速な疾患特定への重点化が、需要を大幅に押し上げています。さらに、バイオマーカーの発見と自己免疫病態に関する継続的な研究の継続的な進歩により、疾患進行の追跡、治療反応評価、長期的な患者管理における抗核抗体検査の臨床的価値が拡大しています
感染症分野は、感染症と免疫系の調節異常の複雑な関係性を明らかにする新たな研究が相次いでいることから、2026年から2033年にかけて約9.6%という最も高いCAGRで成長すると予測されています。特にウイルスの蔓延や感染後合併症における免疫反応の理解への関心が高まり、ANA検査の臨床的意義が高まっています。さらに、ANA検査が包括的な免疫学的・診断パネルに統合されていること、そして感染症研究・監視プログラムへの世界的な投資増加も、この分野の急速な拡大に貢献しています。
- エンドユーザー別
エンドユーザーに基づいて、市場は病院、研究所、診断センター、研究機関、その他に分類されます。病院セグメントは、患者数の増加と病院内での統合診断システムの利用可能性に支えられ、2025年には約39.4%のシェアで市場を支配しました。ANA検査は、自己免疫疾患や慢性炎症疾患の日常的な臨床評価の一環として一般的に実施されています。熟練した医療専門家の存在、高度な検査能力、そして学際的なケア環境は、正確な検査と結果の解釈を保証します。さらに、医療インフラ、病院の近代化、専門部門の拡大への継続的な政府投資は、このセグメントのリーダーシップをさらに強化しています
診断センターセグメントは、専門性、信頼性、費用対効果の高い診断サービスに対する需要の拡大を背景に、2026年から2033年にかけて約10.9%という最も高いCAGRを記録すると予想されています。これらのセンターは、自動化技術と高度な検査プラットフォームの活用により、迅速なターンアラウンドタイムの提供に重点を置いています。都市部、準都市部、さらには医療サービスが行き届いていない地域における存在感の高まりは、ANA検査へのアクセスを大幅に向上させています。さらに、病院、診療所、研究機関との戦略的提携により、市場における地位が強化され、このセグメント全体の成長が加速しています。
- 流通チャネル別
流通チャネルに基づいて、市場は直接入札、小売販売、第三者販売業者、その他に分類されます。直接入札セグメントは、政府系病院、公衆衛生機関、大規模医療グループによる大量調達に牽引され、2025年には約44.6%という最大の市場シェアを占めました。このチャネルは、コスト効率、長期供給契約、そして一貫した製品供給を保証します。メーカーは安定した需要の恩恵を受け、医療機関はより良い価格設定と品質保証を得ることができます。さらに、直接入札は仲介マージンを削減し、医療機関がより効果的に資源を配分するのに役立ちます。政府が資金提供する診断プログラムや国家疾病スクリーニングイニシアチブの増加は、このセグメントの優位性をさらに強化しています
サードパーティディストリビューターセグメントは、ディストリビューターが地域的リーチを拡大し、サプライチェーン全体の効率性を向上させ続けることから、2026年から2033年にかけて約11.5%という最も高いCAGRで成長すると予想されています。確立されたローカルネットワークにより、これまで直接メーカーのサービスが行き届いていなかった農村部や未開発地域において、製品の入手性向上とサービス向上が可能になります。在庫管理、コールドチェーンの維持、ラストマイル配送におけるディストリビューターへの依存度の高まりは、ANA検査市場におけるディストリビューターの役割を大幅に強化しています。さらに、サードパーティディストリビューターは、技術サポート、トレーニング、アフターサービスを含むバンドルソリューションを提供することが多く、顧客満足度と顧客維持率を向上させています。直接調達能力に欠ける中小規模の診断ラボの増加も、これらのディストリビューターへの依存度を高めています。
北米抗核抗体検査市場地域分析
- 北米は、高度な医療インフラ、大量の診断検査、強力な償還枠組み、大手診断企業の存在に支えられ、2025年には抗核抗体検査市場において41.8%という最大の収益シェアを獲得し、市場を支配した。
- この地域では、間接蛍光抗体法(IIF)やELISA法といった高度なANA検査法が広く普及しており、その恩恵を受けています。ループス、関節リウマチ、シェーグレン症候群といった自己免疫疾患の有病率の上昇により、ANAの定期的かつ早期段階のスクリーニングに対する需要がさらに高まっています。
- この地域のリーダーシップは、医師と患者双方の高い意識、確立された検査ネットワーク、そして免疫検査における継続的な技術進歩によっても強化されています。北米の統合された病院と診断検査システムは、迅速な検査へのアクセスと正確な疾患モニタリングを確保しており、ANA検査は自己免疫疾患管理における日常的な要素となっています。
米国抗核抗体検査市場の洞察
米国の抗核抗体検査市場は、自己免疫疾患の負担増加と高度な診断施設の充実を背景に、2025年には最大の収益シェアを獲得しました。免疫蛍光法およびELISA技術の広範な利用、高い医療費、そして有利な償還政策が相まって、抗核抗体検査の普及を支えています。さらに、自己免疫バイオマーカーの継続的な研究、早期診断への関心の高まり、そして個別化医療へのアプローチの拡大は、米国における持続的な市場成長に大きく貢献しています。
カナダの抗核抗体検査市場の洞察
カナダの抗核抗体検査市場は、予測期間中に抗核抗体検査市場の中で最も急速に成長する国となり、年平均成長率(CAGR)11.8%を記録すると予想されています。この成長は、医療アクセスの向上、診断ラボのインフラ整備、自己免疫疾患への意識の高まり、そして医療開発への政府投資の増加に起因しています。カナダでは、疾患の早期発見への重点が高まっており、研究活動の拡大と高度な免疫診断技術の利用可能性の向上により、病院、診断センター、研究機関におけるANA検査の導入が加速しています。
北米の抗核抗体検査市場シェア
抗核抗体検査業界は、主に、以下を含む定評のある企業によって牽引されています。
• F. Hoffmann-La Roche Ltd.(スイス)
• Abbott(米国)
• Siemens Healthineers(ドイツ)
• Danaher Corporation(米国)
• bioMérieux SA(フランス)
• Thermo Fisher Scientific Inc.(米国)
• Becton, Dickinson and Company(米国)
• QuidelOrtho Corporation(米国)
• Werfen(スペイン)
• EUROIMMUN AG(ドイツ)
• Bio-Rad Laboratories, Inc.(米国)
• Inova Diagnostics(米国)
• Trinity Biotech(アイルランド)
• Genway Biotech, Inc.(米国)
• Arlington Scientific, Inc.(米国)
• Erba Diagnostics(ドイツ)
• Hycor Biomedical LLC(米国)
• Diagnostic Automation, Inc.(米国)
• Creative Diagnostics(米国)
• Snibe Diagnostic(中国)
北米抗核抗体検査市場の最新動向
- 2021年3月、世界のANA検査市場では、病院や診断研究室における自動化されたELISAおよび間接免疫蛍光(IIF)プラットフォームの採用が増加し、自己免疫疾患スクリーニングにおける精度が向上し、人的ミスが減少しました。
- 2022年7月、特に北米とヨーロッパで全身性エリテマトーデス、関節リウマチ、強皮症などの自己免疫疾患に対する意識が高まっていることを受けて、複数の大手診断会社がANA検査キットの生産能力を拡大しました。
- 2023年4月、EUROIMMUNは強化されたANA検査プロファイルを導入し、検査室がより広範囲の自己抗体をより正確に検出できるようにすることで、自己免疫疾患の早期診断と患者管理の改善を支援します。
- 2024年8月、サーモフィッシャーサイエンティフィックは、感度の向上と合理化されたラボワークフローを特徴とする、アップグレードされた自動化されたANA検査プラットフォームを発売し、大量検査センターのターンアラウンドタイムの短縮を可能にしました。
- 2025年1月、業界アナリストは、世界的な自己免疫疾患の蔓延と早期診断および疾患モニタリングの重要性の高まりにより、ANA検査の採用が継続的に増加していると報告した。
SKU-
世界初のマーケットインテリジェンスクラウドに関するレポートにオンラインでアクセスする
- インタラクティブなデータ分析ダッシュボード
- 成長の可能性が高い機会のための企業分析ダッシュボード
- カスタマイズとクエリのためのリサーチアナリストアクセス
- インタラクティブなダッシュボードによる競合分析
- 最新ニュース、更新情報、トレンド分析
- 包括的な競合追跡のためのベンチマーク分析のパワーを活用
調査方法
データ収集と基準年分析は、大規模なサンプル サイズのデータ収集モジュールを使用して行われます。この段階では、さまざまなソースと戦略を通じて市場情報または関連データを取得します。過去に取得したすべてのデータを事前に調査および計画することも含まれます。また、さまざまな情報ソース間で見られる情報の不一致の調査も含まれます。市場データは、市場統計モデルと一貫性モデルを使用して分析および推定されます。また、市場シェア分析と主要トレンド分析は、市場レポートの主要な成功要因です。詳細については、アナリストへの電話をリクエストするか、お問い合わせをドロップダウンしてください。
DBMR 調査チームが使用する主要な調査方法は、データ マイニング、データ変数が市場に与える影響の分析、および一次 (業界の専門家) 検証を含むデータ三角測量です。データ モデルには、ベンダー ポジショニング グリッド、市場タイムライン分析、市場概要とガイド、企業ポジショニング グリッド、特許分析、価格分析、企業市場シェア分析、測定基準、グローバルと地域、ベンダー シェア分析が含まれます。調査方法について詳しくは、お問い合わせフォームから当社の業界専門家にご相談ください。
カスタマイズ可能
Data Bridge Market Research は、高度な形成的調査のリーダーです。当社は、既存および新規のお客様に、お客様の目標に合致し、それに適したデータと分析を提供することに誇りを持っています。レポートは、対象ブランドの価格動向分析、追加国の市場理解 (国のリストをお問い合わせください)、臨床試験結果データ、文献レビュー、リファービッシュ市場および製品ベース分析を含めるようにカスタマイズできます。対象競合他社の市場分析は、技術ベースの分析から市場ポートフォリオ戦略まで分析できます。必要な競合他社のデータを、必要な形式とデータ スタイルでいくつでも追加できます。当社のアナリスト チームは、粗い生の Excel ファイル ピボット テーブル (ファクト ブック) でデータを提供したり、レポートで利用可能なデータ セットからプレゼンテーションを作成するお手伝いをしたりすることもできます。