北米の急性骨髄性白血病診断市場、製品タイプ別(機器および消耗品と付属品)、検査タイプ別(画像検査、血液検査、骨髄検査、バイオマーカー検査、免疫表現型検査、遺伝子検査、その他)、がんの種類別(骨髄芽球性(M0)、骨髄芽球性(M1)、骨髄芽球性(M2)、前骨髄球性(M3)、骨髄単球性(M4)、単球性(M5)、赤白血病(M6)、巨核球性(M7))、年齢グループ別(21歳未満、21~29歳、30~65歳、65歳以上)、性別別(男性と女性)、エンドユーザー別(病院、関連ラボ、独立診断ラボ、診断画像センター、がん研究機関、その他)、流通チャネル別(直接入札と小売販売)業界動向と2020年までの予測2030年。
北米の急性骨髄性白血病診断市場の分析と洞察
医療費の増加により市場の成長が促進され、その結果、研究開発の機会がより多く提供される可能性があります。これとは別に、がんの診断のための臨床研究および診断研究所の増加により、市場が拡大しています。
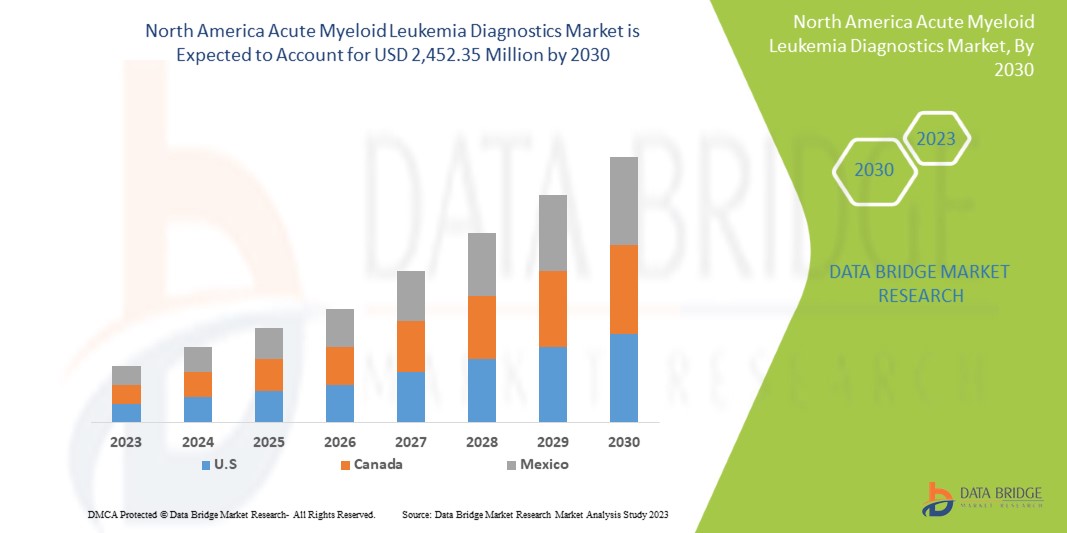
北米の急性骨髄性白血病診断市場は、2023年から2030年の予測期間に成長すると予想されています。データブリッジマーケットリサーチは、市場は2023年から2030年の予測期間に11.7%のCAGRで成長し、2030年までに24億5,235万米ドルに達すると分析しています。
|
レポートメトリック |
詳細 |
|
予測期間 |
2023年から2030年 |
|
基準年 |
2022 |
|
歴史的な年 |
2021 (2020~2016年にカスタマイズ可能) |
|
定量単位 |
収益(百万米ドル) |
|
対象セグメント |
製品タイプ(機器および消耗品と付属品)、検査タイプ(画像検査、血液検査、骨髄検査、バイオマーカー検査、免疫表現型検査、遺伝子検査、その他)、がんの種類(骨髄芽球性(M0)、骨髄芽球性(M1)、骨髄芽球性(M2)、前骨髄球性(M3)、骨髄単球性(M4)、単球性(M5)、赤白血病(M6)、巨核球性(M7))、年齢層(21歳未満、21~29歳、30~65歳、65歳以上)、性別(男性と女性)、エンドユーザー(病院、関連研究所、独立診断研究所、診断画像センター、がん研究機関、その他)、流通チャネル(直接入札と小売販売)別。 |
|
対象国 |
米国、カナダ、メキシコ。 |
|
対象となる市場プレーヤー |
Myriad Genetics, Inc.、Abbott、QIAGEN、Agilent Technologies, Inc.、Exact Sciences Corporation、Hologic Inc.、Illumina, Inc.、BD、Quest Diagnostics Incorporated、Bio-Rad Laboratories, Inc.、FONAR Corp.、Thermo Fisher Scientific Inc. など。 |
市場の定義
最も一般的な血液がんである急性骨髄性白血病 (AML) は、まれな白血病の 1 つでもあります。このがんは血液に侵入し、近くの臓器や身体系に広がります。専門家は、顕微鏡で細胞画像を調べ、注釈によってラベルを付けることで、がん細胞と非がん細胞を手動で診断する必要があります。
医療費の増加は、研究開発の機会の充実につながり、ビジネスの成長にもつながります。がんの事前検査のための臨床研究および診断研究所の増加により、市場が拡大しています。
がん患者数の増加と白血病に対する意識の高まりにより、市場の成長が加速しています。
北米の急性骨髄性白血病診断市場は、市場プレーヤーの増加と高度な画像機器および消耗品の入手可能性により、予測年度に成長しています。これに伴い、メーカーは市場に製品を投入するための研究開発活動に取り組んでいます。研究開発と事前テストの増加により、市場の成長がさらに促進されています。ただし、がん診断製品の承認と商品化および遅延診断に関する厳格な規制と基準は、市場の成長に対する課題となることが予想されます。
北米の急性骨髄性白血病診断市場の動向
このセクションでは、市場の推進要因、制約、機会、課題について理解します。これらについては、以下で詳しく説明します。
ドライバー
- 白血病がんの罹患率増加
白血病は、あらゆる年齢層で発症する可能性があります。白血病は、その兆候や症状が多岐にわたるにもかかわらず、非特異的であり、他のより広範な病状と関連している可能性があるため、診断が難しい場合があります。AML は、成人によく見られる 4 つの白血病の 1 つです。発症頻度はそれほど高くありません。女性よりも男性にわずかに多く見られますが、男女ともに AML を発症する平均生涯リスクは平均して約 0.5% です。
AML は、成人と小児で診断される白血病の中で 2 番目に多いタイプですが、ほとんどの症例は成人に発生します。どの年齢でも診断される可能性がありますが、45 歳未満で診断されることはまれです。診断される平均年齢は 68 歳です。母親の肥満率の上昇が、AML の有病率の上昇の一因である可能性があります。
さまざまなリスク要因により、AMLの発生率は上昇しており、重大な社会経済的問題となっています。これが市場成長の原動力となることが期待されています。
- 白血病診断における新たな技術的進歩
最も一般的な血液がんである AML は、まれな白血病の 1 つでもあります。このがんは血液に侵入し、近くの臓器や身体系に広がります。専門家は、顕微鏡で細胞画像を調べ、注釈でラベルを付けることで、がん細胞と非がん細胞を手動で診断する必要があります。ただし、この手動の顕微鏡検査は時間がかかり、誤った診断を下す可能性があります。
その後、コンピュータ化されたソフトウェアを使用することで、誤った薬を処方するリスクが軽減されました。白血病の壊滅的な影響を阻止するには、自動で信頼性の高い分類システムの作成が不可欠になりました。複数のセグメンテーション技術が、既存の白血病分類アルゴリズムの基礎を構成しました。
いくつかの新しい診断方法の開発により、多くの新しい高度な製品が発売され、市場の成長が加速します。したがって、市場で急性白血病癌の診断製品の需要が生まれることが期待されます。
拘束
- 白血病診断製品の承認と商品化に関する厳格な規制と基準
市場でのあらゆる製品の商品化に対する厳格な規制は、規制があり、規制手続きのための別の機関がある癌診断製品の製造業者にとって大きな課題となっていることが判明しています。
メーカーは、製品を市場に投入する前に、まず CE マークの承認を確認する必要があります。厳しい規制政策は、市場の成長と発展を妨げることが予想されます。
- 白血病の診断の遅れと予後不良
がんは世界における死亡原因の第 1 位です。しかし、早期に発見され、適切な治療を受ければ、一部のがんは治癒可能です。診断プロセスでは、がんの診断が遅れる場合があります。患者が潜在的ながんの兆候を見落としたり、反応しなかったりすると、診断が遅れます。診断が遅れる主な原因は、特にそのような症状が異常な場合、がんの早期兆候が一般に知られていないことです。
これらの要因により診断が遅れることが多く、予後不良につながるため、市場の成長が抑制されると予想されます。
機会/課題
- コスト、安全性、利便性の問題の増大
白血病は致命的な癌であり、白血病の診断プロセスにも安全性の問題があります。費用対効果が高くありません。治療に最も費用がかかる疾患の 1 つは癌です。癌患者は入院し、手術、放射線療法、全身療法などのさまざまな治療を受けます。癌患者の健康保険料は、以前よりも高くなっています。さらに、自己負担額、控除額、共同保険料も上昇しています。
今後、現在の白血病診断プロセスには安全性、コスト、利便性の面で問題があり、市場の成長に対する課題となることが予想されます。
最近の動向
- キヤノン株式会社は、2022年11月に半導体検出器モジュールの開発・製造に関する新技術創出で世界をリードするRedlen Technologies Inc.(以下、Redlen社)を買収しました。キヤノン株式会社のグループ会社であるキヤノンメディカルシステムズ株式会社(以下、キヤノンメディカル)は、Redlen社の先進技術を組み込んだ国産初の光子計数CT(PCCT)システムを開発しました。このシステムは、国立がん研究センター先端医療研究センターに設置され、現在、PCCTの臨床応用に向けた研究に使用されています。
- シスメックス株式会社 代表取締役会長 兼 CEO 家次 恒氏は、乳がん、大腸がん、胃がん、非小細胞肺がんのリンパ節転移検査用試薬として販売している遺伝子増幅試薬「リノアンプ CK19」について、増幅対象を子宮頸がん、子宮内膜がんに拡大し、日本での製造販売承認事項一部変更申請が2022年10月に承認されたことをお知らせします。
北米の急性骨髄性白血病診断市場の範囲
北米の急性骨髄性白血病診断市場は、製品タイプ、検査タイプ、がんの種類、年齢層、性別、エンドユーザー、流通チャネルに基づいて、7 つの主要なセグメントに分割されています。これらのセグメントの成長は、業界の主要な成長セグメントの分析に役立ち、ユーザーに貴重な市場の概要と市場洞察を提供して、コア市場アプリケーションを特定するための戦略的決定を下すのに役立ちます。
製品タイプ
- 楽器
- 消耗品とアクセサリー
製品タイプに基づいて、市場は機器と消耗品およびアクセサリに分類されます。
テストの種類
- 血液検査
- 画像検査
- 骨髄検査
- 遺伝子検査
- バイオマーカー検査
- 免疫表現型検査
- その他
検査の種類に基づいて、市場は血液検査、画像検査、骨髄検査、遺伝子検査、バイオマーカー検査、免疫表現型検査などに分類されます。
がんの種類
- 骨髄芽球性(M0)
- 骨髄芽球性(M1)
- 骨髄芽球性(M2)
- 前骨髄球性(M3)
- 骨髄単球性(M4)
- 単球性(M5)
- 赤白血病(M6)
- 巨核球(M7)
がんの種類に基づいて、市場は骨髄芽球性(M0)、骨髄芽球性(M1)、骨髄芽球性(M2)、前骨髄球性(M3)、骨髄単球性(M4)、単球性(M5)、赤白血病(M6)、および巨核球性(M7)に分類されます。
年齢層
- 21歳未満
- 21-29
- 30-65
- 65歳以上
年齢層に基づいて、市場は 21 歳未満、21 ~ 29 歳、30 ~ 65 歳、65 歳以上に分類されます。
性別
- 男
- 女性
性別に基づいて、市場は男性と女性に分割されます。
エンドユーザー
- 病院
- 関連ラボ
- 独立診断研究所
- 画像診断センター
- がん研究機関
- その他
エンドユーザーに基づいて、市場は病院、関連研究所、独立した診断研究所、診断画像センター、がん研究機関などに分類されます。
流通チャネル
- 直接入札
- 小売販売
流通チャネルに基づいて、市場は直接入札と小売販売に分類されます。
北米急性骨髄性白血病診断市場地域分析/洞察
北米の急性骨髄性白血病診断市場が分析され、製品タイプ、検査タイプ、がんの種類、年齢層、性別、エンドユーザー、流通チャネルに基づいて、国別に市場規模の洞察と傾向が提供されます。
この市場レポートで取り上げられている国は、上記のとおり米国、カナダ、メキシコです。
米国は、市場シェアと収益の面で北米の急性骨髄性白血病診断市場を支配しており、予測期間中もその優位性を維持し続けるでしょう。これは、この地域の診断における技術進歩の高まり、研究開発投資の増加、市場の成長を後押しする新製品の発売によるものです。
レポートの国別セクションでは、市場の現在および将来の傾向に影響を与える個別の市場影響要因と市場規制の変更も提供しています。新規および交換販売、国の人口統計、疾病疫学、輸出入関税などのデータ ポイントは、各国の市場シナリオを予測するために使用される主要な指標の一部です。さらに、国別データの予測分析を提供する際には、北米ブランドの存在と可用性、地元および国内ブランドとの競争により直面する課題、販売チャネルの影響が考慮されています。
競争環境と北米の急性骨髄性白血病診断市場シェア分析
北米の急性骨髄性白血病診断市場の競争状況は、競合他社ごとに詳細を提供します。含まれる詳細には、会社概要、会社の財務状況、収益、市場の可能性、研究開発への投資、新しい市場への取り組み、北米でのプレゼンス、生産拠点と施設、生産能力、会社の強みと弱み、製品の発売、製品の幅と広さ、アプリケーションの優位性などがあります。提供されている上記のデータ ポイントは、市場に関連する会社の焦点にのみ関連しています。
北米の急性骨髄性白血病診断市場で活動している主要企業としては、Myriad Genetics, Inc.、Abbott、QIAGEN、Agilent Technologies, Inc.、Exact Sciences Corporation、Hologic Inc.、Illumina, Inc.、BD、Quest Diagnostics Incorporated、Bio-Rad Laboratories, Inc.、FONAR Corp.、Thermo Fisher Scientific Inc. などがあります。
SKU-
世界初のマーケットインテリジェンスクラウドに関するレポートにオンラインでアクセスする
- インタラクティブなデータ分析ダッシュボード
- 成長の可能性が高い機会のための企業分析ダッシュボード
- カスタマイズとクエリのためのリサーチアナリストアクセス
- インタラクティブなダッシュボードによる競合分析
- 最新ニュース、更新情報、トレンド分析
- 包括的な競合追跡のためのベンチマーク分析のパワーを活用
目次
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 PRODUCT TYPE LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 MARKET END USER COVERAGE GRID
2.11 VENDOR SHARE ANALYSIS
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL ANALYSIS
4.2 PORTER'S 5 FORCES
5 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, REGULATIONS
5.1 REGULATORY SCENARIO IN THE U.S.
5.2 REGULATORY SCENARIO IN AUSTRALIA
5.3 REGULATORY SCENARIO IN JAPAN
5.4 REGULATORY SCENARIO IN CHINA
6 MARKET OVERVIEW
6.1 DRIVERS
6.1.1 GROWING PREVALENCE OF LEUKEMIA CANCER
6.1.2 NOVEL TECHNOLOGICAL ADVANCEMENTS IN LEUKEMIA DIAGNOSTICS
6.1.3 RISING PREFERENCE FOR PREVENTIVE HEALTH CHECK-UPS
6.1.4 INCREASE IN AWARENESS REGARDING LEUKEMIA CANCER
6.2 RESTRAINTS
6.2.1 STRICT REGULATIONS AND STANDARDS FOR THE APPROVAL AND COMMERCIALIZATION OF LEUKEMIA DIAGNOSTIC PRODUCTS
6.2.2 LATE DIAGNOSIS AND POOR PROGNOSIS OF LEUKEMIA
6.3 OPPORTUNITIES
6.3.1 INCREASE IN DIAGNOSTIC PRODUCTS FOR LEUKEMIA
6.3.2 RISE IN HEALTHCARE EXPENDITURE FOR CANCER DIAGNOSIS AND TREATMENT
6.3.3 GOVERNMENT INITIATIVES TOWARD CANCER DIAGNOSTICS
6.4 CHALLENGES
6.4.1 INCREASED COST, SAFETY, AND CONVENIENCE ISSUES
6.4.2 LACK OF SKILLED AND CERTIFIED PROFESSIONALS
7 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE
7.1 OVERVIEW
7.2 INSTRUMENTS
7.2.1 BIOPSY INSTRUMENTS
7.2.1.1 BONE MARROW BIOPSY
7.2.1.2 NEEDLE BIOPSY
7.2.1.3 SURGICAL BIOPSY
7.2.1.4 OTHERS
7.2.2 PATHOLOGY-BASED INSTRUMENTS
7.2.2.1 PCR INSTRUMENTS
7.2.2.2 SLIDE STAINING SYSTEMS
7.2.2.3 TISSUE PROCESSING SYSTEMS
7.2.2.4 CELL PROCESSORS
7.2.2.5 OTHER PATHOLOGY-BASED INSTRUMENTS
7.2.3 IMAGING INSTRUMENTS
7.2.3.1 ULTRASOUND SYSTEMS
7.2.3.2 CT SYSTEMS
7.2.3.3 MRI SYSTEMS
7.2.3.4 OTHERS
7.2.4 OTHERS
7.3 CONSUMABLES & ACCESSORIES
7.3.1 KITS
7.3.1.1 PCR KITS
7.3.1.2 DNA POLYMERASE KITS
7.3.1.3 NUCLEIC ACID ISOLATION KITS
7.3.1.4 OTHERS
7.3.2 REAGENTS
7.3.2.1 ASSAYS
7.3.2.2 BUFFERS
7.3.2.3 PRIMERS
7.3.2.4 OTHERS
7.3.3 PROBES
7.3.4 OTHER CONSUMABLES
8 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE
8.1 OVERVIEW
8.2 BLOOD TEST
8.2.1 COMPLETE BLOOD COUNT (CBC)
8.2.2 BLOOD CHEMISTRY TESTS
8.2.3 OTHERS
8.3 IMAGING TEST
8.3.1 COMPUTED TOMOGRAPHY (CT) SCAN
8.3.2 MRI
8.3.3 POSITRON EMISSION TOMOGRAPHY (PET) SCAN
8.3.4 OTHERS
8.4 BONE MARROW TESTS
8.4.1 BONE MARROW ASPIRATE
8.4.2 BONE MARROW BIOPSY
8.4.3 OTHERS
8.5 GENETIC TESTS
8.5.1 FLUORESCENCE IN SITU HYBRIDIZATION (FISH)
8.5.2 KARYOTYPING
8.5.3 OTHERS
8.6 BIOMARKER TEST
8.6.1 GENETIC ACUTE MYELOID LEUKEMIA (AML) BIOMARKER
8.6.2 EPIGENETIC ACUTE MYELOID LEUKEMIA (AML) BIOMARKER
8.6.3 PROTEOMIC ACUTE MYELOID LEUKEMIA (AML) BIOMARKER
8.7 IMMUNOPHENOTYPING
8.7.1 FLOW CYTOMETRY
8.7.2 IMMUNOHISTOCHEMISTRY
8.7.3 OTHERS
8.8 OTHERS
9 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY CANCER TYPE
9.1 OVERVIEW
9.2 MYELOBLASTIC (M0)
9.3 MYELOBLASTIC (M1)
9.4 MYELOBLASTIC (M2)
9.5 PROMYELOCYTIC (M3)
9.6 MYELOMONOCYTIC (M4)
9.7 MONOCYTIC (M5)
9.8 ERYTHROLEUKEMIA (M6)
9.9 MEGAKARYOCYTIC (M7)
10 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY AGE GROUP
10.1 OVERVIEW
10.2 65 AND ABOVE
10.3 30-65
10.4 BELOW 21
10.5 21-29
11 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY GENDER
11.1 OVERVIEW
11.2 MALE
11.3 FEMALE
12 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY END USER
12.1 OVERVIEW
12.2 HOSPITALS
12.3 ASSOCIATED LABS
12.4 INDEPENDENT DIAGNOSTIC LABORATORIES
12.5 DIAGNOSTIC IMAGING CENTERS
12.6 CANCER RESEARCH INSTITUTES
12.7 OTHERS
13 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL
13.1 OVERVIEW
13.2 DIRECT TENDER
13.3 RETAIL SALES
14 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION
14.1 NORTH AMERICA
14.1.1 U.S.
14.1.2 CANADA
14.1.3 MEXICO
15 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: COMPANY LANDSCAPE
15.1 COMPANY SHARE ANALYSIS: NORTH AMERICA
16 SWOT ANALYSIS
17 COMPANY PROFILE
17.1 CANON MEDICAL SYSTEMS CORPORATION.
17.1.1 COMPANY SNAPSHOT
17.1.2 REVENUE ANALYSIS
17.1.3 COMPANY SHARE ANALYSIS
17.1.4 PRODUCT PORTFOLIO
17.1.5 RECENT DEVELOPMENT
17.2 SYSMEX CORPORATION
17.2.1 COMPANY SNAPSHOT
17.2.2 REVENUE ANALYSIS
17.2.3 COMPANY SHARE ANALYSIS
17.2.4 PRODUCT PORTFOLIO
17.2.5 RECENT DEVELOPMENT
17.3 EPIGENOMICS AG.
17.3.1 COMPANY SNAPSHOT
17.3.2 REVENUE ANALYSIS
17.3.3 COMPANY SHARE ANALYSIS
17.3.4 PRODUCT PORTFOLIO
17.3.5 RECENT DEVELOPMENT
17.4 MYRIAD GENETICS, INC..
17.4.1 COMPANY SNAPSHOT
17.4.2 REVENUE ANALYSIS
17.4.3 COMPANY SHARE ANALYSIS
17.4.4 PRODUCT PORTFOLIO
17.4.5 RECENT DEVELOPMENT
17.5 F. HOFFMANN- LA ROCHE LTD
17.5.1 COMPANY SNAPSHOT
17.5.2 REVENUE ANALYSIS
17.5.3 COMPANY SHARE ANALYSIS
17.5.4 PRODUCT PORTFOLIO
17.5.5 RECENT DEVELOPMENT
17.6 ABBOTT
17.6.1 COMPANY SNAPSHOT
17.6.2 REVENUE ANALYSIS
17.6.3 PRODUCT PORTFOLIO
17.6.4 RECENT DEVELOPMENT
17.7 AGILENT TECHNOLOGIES, INC.
17.7.1 COMPANY SNAPSHOT
17.7.2 REVENUE ANALYSIS
17.7.3 PRODUCT PORTFOLIO
17.7.4 RECENT DEVELOPMENT
17.8 BD
17.8.1 COMPANY SNAPSHOT
17.8.2 REVENUE ANALYSIS
17.8.3 PRODUCT PORTFOLIO
17.8.4 RECENT DEVELOPMENT
17.9 BIOMERIEUX
17.9.1 COMPANY SNAPSHOT
17.9.2 PRODUCT PORTFOLIO
17.9.3 RECENT DEVELOPMENT
17.1 BIO-RAD LABORATORIES, INC.
17.10.1 COMPANY SNAPSHOT
17.10.2 REVENUE ANALYSIS
17.10.3 PRODUCT PORTFOLIO
17.10.4 RECENT DEVELOPMENT
17.11 DIASORIN S.P.A.
17.11.1 COMPANY SNAPSHOT
17.11.2 REVENUE ANALYSIS
17.11.3 PRODUCT PORTFOLIO
17.11.4 RECENT DEVELOPMENTS
17.12 EXACT SCIENCES CORPORATION
17.12.1 COMPANY SNAPSHOT
17.12.2 REVENUE ANALYSIS
17.12.3 PRODUCT PORTFOLIO
17.12.4 RECENT DEVELOPMENTS
17.13 FONAR CORP.
17.13.1 COMPANY SNAPSHOT
17.13.2 REVENUE ANALYSIS
17.13.3 PRODUCT PORTFOLIO
17.13.4 RECENT DEVELOPMENT
17.14 HOLOGIC INC.
17.14.1 COMPANY SNAPSHOT
17.14.2 REVENUE ANALYSIS
17.14.3 PRODUCT PORTFOLIO
17.14.4 RECENT DEVELOPMENT
17.15 ILLUMINA, INC.
17.15.1 COMPANY SNAPSHOT
17.15.2 REVENUE ANALYSIS
17.15.3 PRODUCT PORTFOLIO
17.15.4 RECENT DEVELOPMENT
17.16 KONINKLIJKE PHILIPS N.V.
17.16.1 COMPANY SNAPSHOT
17.16.2 REVENUE ANALYSIS
17.16.3 PRODUCT PORTFOLIO
17.16.4 RECENT DEVELOPMENT
17.17 MEDONICA CO. LTD
17.17.1 COMPANY SNAPSHOT
17.17.2 PRODUCT PORTFOLIO
17.17.3 RECENT DEVELOPMENT
17.18 MERCK KGAA
17.18.1 COMPANY SNAPSHOT
17.18.2 REVENUE ANALYSIS
17.18.3 PRODUCT PORTFOLIO
17.18.4 RECENT DEVELOPMENTS
17.19 MINFOUND MEDICAL SYSTEMS CO., LTD
17.19.1 COMPANY SNAPSHOT
17.19.2 PRODUCT PORTFOLIO
17.19.3 RECENT DEVELOPMENT
17.2 PLEXBIO
17.20.1 COMPANY SNAPSHOT
17.20.2 PRODUCT PORTFOLIO
17.20.3 RECENT DEVELOPMENTS
17.21 QIAGEN
17.21.1 COMPANY SNAPSHOT
17.21.2 REVENUE ANALYSIS
17.21.3 PRODUCT PORTFOLIO
17.21.4 RECENT DEVELOPMENTS
17.22 QUEST DIAGNOSTICS INCORPORATED
17.22.1 COMPANY SNAPSHOT
17.22.2 REVENUE ANALYSIS
17.22.3 PRODUCT PORTFOLIO
17.22.4 RECENT DEVELOPMENTS
17.23 SIEMENS HEALTHCARE GMBH
17.23.1 COMPANY SNAPSHOT
17.23.2 REVENUE ANALYSIS
17.23.3 PRODUCT PORTFOLIO
17.23.4 RECENT DEVELOPMENT
17.24 SONIC HEALTHCARE
17.24.1 COMPANY SNAPSHOT
17.24.2 PRODUCT PORTFOLIO
17.24.3 RECENT DEVELOPMENT
17.25 STERNMED GMBH
17.25.1 COMPANY SNAPSHOT
17.25.2 PRODUCT PORTFOLIO
17.25.3 RECENT DEVELOPMENTS
17.26 THERMO FISHER SCIENTIFIC INC.
17.26.1 COMPANY SNAPSHOT
17.26.2 REVENUE ANALYSIS
17.26.3 PRODUCT PORTFOLIO
17.26.4 RECENT DEVELOPMENT
17.27 TIME MEDICAL HOLDING
17.27.1 COMPANY SNAPSHOT
17.27.2 PRODUCT PORTFOLIO
17.27.3 RECENT DEVELOPMENT
18 QUESTIONNAIRE
19 RELATED REPORTS
表のリスト
TABLE 1 APPROVED DIAGNOSTICS OF LEUKEMIA
TABLE 2 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 3 NORTH AMERICA INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 4 NORTH AMERICA INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 5 NORTH AMERICA BIOPSY INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 6 NORTH AMERICA PATHOLOGY-BASED INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 7 NORTH AMERICA IMAGING INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 8 NORTH AMERICA CONSUMABLES & ACCESSORIES IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 9 NORTH AMERICA CONSUMABLES AND ACCESSORIES IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 10 NORTH AMERICA KITS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 11 NORTH AMERICA REAGENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 12 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 13 NORTH AMERICA BLOOD TEST IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 14 NORTH AMERICA BLOOD TEST IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 15 NORTH AMERICA IMAGING TEST IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 16 NORTH AMERICA IMAGING TEST IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 17 NORTH AMERICA BONE MARROW TESTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 18 NORTH AMERICA BONE MARROW TESTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 19 NORTH AMERICA GENETIC TESTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 20 NORTH AMERICA GENETIC TESTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 21 NORTH AMERICA BIOMARKER TEST IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 22 NORTH AMERICA BIOMARKER TEST IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 23 NORTH AMERICA IMMUNOPHENOTYPING IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 24 NORTH AMERICA IMMUNOPHENOTYPING IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 25 NORTH AMERICA OTHERS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 26 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 27 NORTH AMERICA MYELOBLASTIC (M0) IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 28 NORTH AMERICA MYELOBLASTIC (M1) IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 29 NORTH AMERICA MYELOBLASTIC (M2) IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 30 NORTH AMERICA PROMYELOCYTIC (M3) IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 31 NORTH AMERICA MYELOMONOCYTIC (M4) IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 32 NORTH AMERICA MONOCYTIC (M5) IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 33 NORTH AMERICA ERYTHROLEUKEMIA (M6) IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 34 NORTH AMERICA MEGAKARYOCYTIC (M7) ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 35 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY AGE GROUP, 2021-2030 (USD MILLION)
TABLE 36 NORTH AMERICA 65 AND ABOVE IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 37 NORTH AMERICA 30-65 IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 38 NORTH AMERICA BELOW 21 IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 39 NORTH AMERICA 21-29 IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 40 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY GENDER, 2021-2030 (USD MILLION)
TABLE 41 NORTH AMERICA MALE IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 42 NORTH AMERICA FEMALE IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 43 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 44 NORTH AMERICA HOSPITALS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 45 NORTH AMERICA ASSOCIATED LABS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 46 NORTH AMERICA INDEPENDENT DIAGNOSTIC LABORATORIES IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 47 NORTH AMERICA DIAGNOSTIC IMAGING CENTERS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 48 NORTH AMERICA CANCER RESEARCH INSTITUTES IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 49 NORTH AMERICA OTHERS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 50 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 51 NORTH AMERICA DIRECT TENDER IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 52 NORTH AMERICA RETAIL SALES IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 53 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY COUNTRY
TABLE 54 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 55 NORTH AMERICA INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 56 NORTH AMERICA PATHOLOGY-BASED INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 57 NORTH AMERICA IMAGING INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 58 NORTH AMERICA BIOPSY INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 59 NORTH AMERICA CONSUMABLES & ACCESSORIES IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 60 NORTH AMERICA KITS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 61 NORTH AMERICA REAGENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 62 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 63 NORTH AMERICA IMAGING TEST IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 64 NORTH AMERICA BONE MARROW TESTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 65 NORTH AMERICA BLOOD TEST IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 66 NORTH AMERICA BIOMARKER TEST IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 67 NORTH AMERICA IMMUNOPHENOTYPING IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 68 NORTH AMERICA GENETIC TESTING IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 69 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 70 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY AGE GROUP, 2021-2030 (USD MILLION)
TABLE 71 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY GENDER, 2021-2030 (USD MILLION)
TABLE 72 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 73 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 74 U.S. ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 75 U.S. INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 76 U.S. PATHOLOGY-BASED INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 77 U.S. IMAGING INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 78 U.S. BIOPSY INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 79 U.S. CONSUMABLES & ACCESSORIES IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 80 U.S. KITS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 81 U.S. REAGENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 82 U.S. ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 83 U.S. IMAGING TEST IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 84 U.S. BONE MARROW TESTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 85 U.S. BLOOD TEST IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 86 U.S. BIOMARKER TEST IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 87 U.S. IMMUNOPHENOTYPING IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 88 U.S. GENETIC TESTING IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 89 U.S. ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 90 U.S. ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY AGE GROUP, 2021-2030 (USD MILLION)
TABLE 91 U.S. ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY GENDER, 2021-2030 (USD MILLION)
TABLE 92 U.S. ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 93 U.S. ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 94 CANADA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 95 CANADA INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 96 CANADA PATHOLOGY-BASED INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 97 CANADA IMAGING INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 98 CANADA BIOPSY INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 99 CANADA CONSUMABLES & ACCESSORIES IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 100 CANADA KITS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 101 CANADA REAGENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 102 CANADA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 103 CANADA IMAGING TEST IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 104 CANADA BONE MARROW TESTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 105 CANADA BLOOD TEST IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 106 CANADA BIOMARKER TEST IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 107 CANADA IMMUNOPHENOTYPING IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 108 CANADA GENETIC TESTING IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 109 CANADA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 110 CANADA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY AGE GROUP, 2021-2030 (USD MILLION)
TABLE 111 CANADA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY GENDER, 2021-2030 (USD MILLION)
TABLE 112 CANADA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 113 CANADA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 114 MEXICO ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 115 MEXICO INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 116 MEXICO PATHOLOGY-BASED INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 117 MEXICO IMAGING INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 118 MEXICO BIOPSY INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 119 MEXICO CONSUMABLES & ACCESSORIES IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 120 MEXICO KITS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 121 MEXICO REAGENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 122 MEXICO ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 123 MEXICO IMAGING TEST IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 124 MEXICO BONE MARROW TESTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 125 MEXICO BLOOD TEST IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 126 MEXICO BIOMARKER TEST IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 127 MEXICO IMMUNOPHENOTYPING IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 128 MEXICO GENETIC TESTING IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 129 MEXICO ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 130 MEXICO ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY AGE GROUP, 2021-2030 (USD MILLION)
TABLE 131 MEXICO ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY GENDER, 2021-2030 (USD MILLION)
TABLE 132 MEXICO ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 133 MEXICO ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
図表一覧
FIGURE 1 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET : SEGMENTATION
FIGURE 2 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET : DATA TRIANGULATION
FIGURE 3 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET : DROC ANALYSIS
FIGURE 4 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET : NORTH AMERICA VS REGIONAL MARKET ANALYSIS
FIGURE 5 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET : COMPANY RESEARCH ANALYSIS
FIGURE 6 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET : INTERVIEW DEMOGRAPHICS
FIGURE 7 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET : DBMR MARKET POSITION GRID
FIGURE 8 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET : MARKET END USER COVERAGE GRID
FIGURE 9 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET : VENDOR SHARE ANALYSIS
FIGURE 10 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET : SEGMENTATION
FIGURE 11 THE GROWING PREVALENCE OF LEUKEMIA CANCER IS EXPECTED TO DRIVE THE NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET IN THE FORECAST PERIOD
FIGURE 12 THE INSTRUMENTS SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET IN 2023 & 2030
FIGURE 13 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF THE NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET
FIGURE 14 NUMBER OF PREVALENCE OF LEUKEMIA INCIDENCE WORLDWIDE (BOTH SEXES)
FIGURE 15 FIVE YEARS PREVALENCE LEUKEMIA INCIDENCE WORLDWIDE (BOTH SEXES)
FIGURE 16 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY PRODUCT TYPE, 2022
FIGURE 17 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY PRODUCT TYPE, 2023-2030 (USD MILLION)
FIGURE 18 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY PRODUCT TYPE, CAGR (2023-2030)
FIGURE 19 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY PRODUCT TYPE, LIFELINE CURVE
FIGURE 20 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY TEST TYPE, 2022
FIGURE 21 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY TEST TYPE, 2023-2030 (USD MILLION)
FIGURE 22 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY TEST TYPE, CAGR (2023-2030)
FIGURE 23 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY TEST TYPE, LIFELINE CURVE
FIGURE 24 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY CANCER TYPE, 2022
FIGURE 25 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY CANCER TYPE, 2023-2030 (USD MILLION)
FIGURE 26 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY CANCER TYPE, CAGR (2023-2030)
FIGURE 27 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY CANCER TYPE, LIFELINE CURVE
FIGURE 28 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY AGE GROUP, 2022
FIGURE 29 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY AGE GROUP, 2023-2030 (USD MILLION)
FIGURE 30 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY AGE GROUP, CAGR (2023-2030)
FIGURE 31 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY AGE GROUP, LIFELINE CURVE
FIGURE 32 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY GENDER, 2022
FIGURE 33 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY GENDER, 2023-2030 (USD MILLION)
FIGURE 34 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY GENDER, CAGR (2023-2030)
FIGURE 35 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY GENDER, LIFELINE CURVE
FIGURE 36 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY END USER, 2022
FIGURE 37 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY END USER, 2023-2030 (USD MILLION)
FIGURE 38 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY END USER, CAGR (2023-2030)
FIGURE 39 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY END USER, LIFELINE CURVE
FIGURE 40 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY DISTRIBUTION CHANNEL, 2022
FIGURE 41 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY DISTRIBUTION CHANNEL, 2023-2030 (USD MILLION)
FIGURE 42 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY DISTRIBUTION CHANNEL, CAGR (2023-2030)
FIGURE 43 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 44 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: SNAPSHOT (2022)
FIGURE 45 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY COUNTRY (2022)
FIGURE 46 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY COUNTRY (2023 & 2030)
FIGURE 47 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY COUNTRY (2022 & 2030)
FIGURE 48 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY PRODUCT TYPE (2023-2030)
FIGURE 49 NORTH AMERICA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: COMPANY SHARE 2022 (%)

調査方法
データ収集と基準年分析は、大規模なサンプル サイズのデータ収集モジュールを使用して行われます。この段階では、さまざまなソースと戦略を通じて市場情報または関連データを取得します。過去に取得したすべてのデータを事前に調査および計画することも含まれます。また、さまざまな情報ソース間で見られる情報の不一致の調査も含まれます。市場データは、市場統計モデルと一貫性モデルを使用して分析および推定されます。また、市場シェア分析と主要トレンド分析は、市場レポートの主要な成功要因です。詳細については、アナリストへの電話をリクエストするか、お問い合わせをドロップダウンしてください。
DBMR 調査チームが使用する主要な調査方法は、データ マイニング、データ変数が市場に与える影響の分析、および一次 (業界の専門家) 検証を含むデータ三角測量です。データ モデルには、ベンダー ポジショニング グリッド、市場タイムライン分析、市場概要とガイド、企業ポジショニング グリッド、特許分析、価格分析、企業市場シェア分析、測定基準、グローバルと地域、ベンダー シェア分析が含まれます。調査方法について詳しくは、お問い合わせフォームから当社の業界専門家にご相談ください。
カスタマイズ可能
Data Bridge Market Research は、高度な形成的調査のリーダーです。当社は、既存および新規のお客様に、お客様の目標に合致し、それに適したデータと分析を提供することに誇りを持っています。レポートは、対象ブランドの価格動向分析、追加国の市場理解 (国のリストをお問い合わせください)、臨床試験結果データ、文献レビュー、リファービッシュ市場および製品ベース分析を含めるようにカスタマイズできます。対象競合他社の市場分析は、技術ベースの分析から市場ポートフォリオ戦略まで分析できます。必要な競合他社のデータを、必要な形式とデータ スタイルでいくつでも追加できます。当社のアナリスト チームは、粗い生の Excel ファイル ピボット テーブル (ファクト ブック) でデータを提供したり、レポートで利用可能なデータ セットからプレゼンテーションを作成するお手伝いをしたりすることもできます。