中東およびアフリカの精神科/うつ病における薬理遺伝学検査市場、タイプ別(不安、気分障害、うつ病、双極性障害、精神病性障害、摂食障害)、検査タイプ別(全ゲノム配列解析、染色体アレイベースの検査)、患者タイプ別(小児、成人、高齢者)、遺伝子タイプ別(CYP2C19、CYP2C9、VKORC1、CYP2D6、HLA-B、HTR2A/C、HLA-A、CYP3A4、SLC6A4、MTHFR、COMT、その他)、製品別(機器、消耗品、ソフトウェアおよびサービス)、エンドユーザー別(病院および診療所、診断研究所、学術研究機関、その他)、流通チャネル別(直接入札、サードパーティ流通病院薬局、その他)– 業界動向および2025年までの予測2029
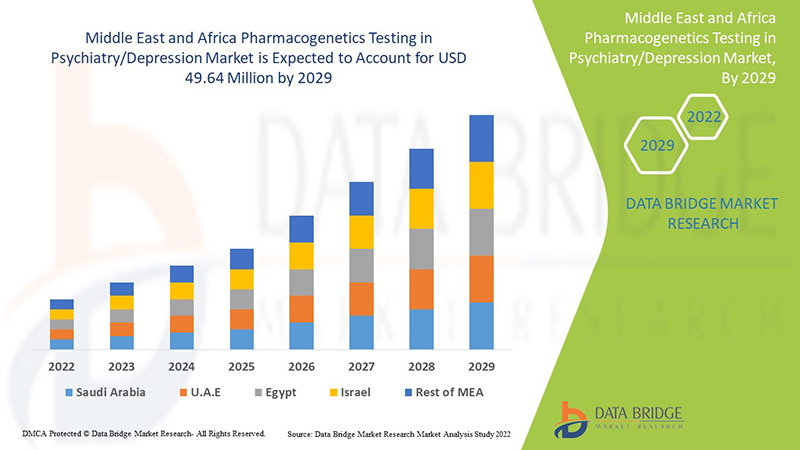
中東およびアフリカの精神医学/うつ病における薬理遺伝学検査の市場分析と洞察
薬理遺伝子検査は、人が薬剤をどのように代謝するかに関する情報を提供することで、医療専門家に役立ちます。この情報は、医師やその他の人々が 望ましくない結果をもたらす可能性のある抗うつ薬の処方を避けるのに役立ちます。薬理遺伝子学は、大うつ病性障害 (MDD) の治療における抗うつ薬の反応と忍容性を予測する上で有望であることが示されています。薬理遺伝子学は、抗うつ薬の選択と投与量を導くことで臨床結果を改善できます。バイオテクノロジー分野の成長と医療費の増加により、精神医学/うつ病における薬理遺伝子検査の需要が加速しています。
癌の罹患率の増加、うつ病やその他の精神疾患の治療における新技術により、精神科/うつ病の機器や処置における薬理遺伝学検査の採用が増加しており、非外科的処置に対する好みが高まっていることが、予測期間中の市場の需要を押し上げる主な要因となっています。ただし、検査に関連する高コスト、厳格な規制、認識不足により、予測期間中の精神科/うつ病市場における薬理遺伝学検査の成長が妨げられる可能性があります。
Data Bridge Market Research の分析によると、中東およびアフリカの精神医学/うつ病市場における薬物遺伝学検査は、予測期間中に 7.1% の CAGR で成長し、2029 年までに 4,964 万米ドルに達すると予想されています。中東およびアフリカの人口におけるうつ病率の上昇により、不安障害が市場で最大のセグメントを占めています。この市場レポートでは、価格分析、特許分析、技術進歩についても詳細に取り上げています。
|
レポートメトリック |
詳細 |
|
予測期間 |
2022年から2029年 |
|
基準年 |
2021 |
|
歴史的な年 |
2020 |
|
定量単位 |
売上高(百万米ドル)、販売数量(個数)、価格(米ドル) |
|
対象セグメント |
中東およびアフリカの精神医学/うつ病における薬理遺伝学検査市場、用途別(新薬候補、薬物の最適化と転用、前臨床試験および承認、薬物モニタリング、新しい疾患関連ターゲットと経路の発見、疾患メカニズムの理解、情報の集約と統合、仮説の形成と適格性、新規薬物設計、旧薬の薬物ターゲットの発見など)、技術別(機械学習、ディープラーニング、自然言語処理など)、薬物タイプ別(小分子、大分子)、提供内容別(ソフトウェアおよびサービス)、適応症別(免疫腫瘍学、神経変性疾患、心血管疾患、代謝性疾患など)、最終用途別(開発業務受託機関(CRO)、製薬およびバイオテクノロジー企業、研究センターおよび学術機関など) |
|
対象国 |
UAE、イスラエル、南アフリカ、エジプト、クウェート、その他の中東およびアフリカ |
|
対象となる市場プレーヤー |
Genelex(Invitae corporationの一部)、Genewiz(Azenta Life Sciencesの一部)、MD Labs、BiogeneiQ, Inc.、ONEOME, LLC、Myriad Genetics, Inc.、GenXys、Castle Biosciences, Inc.、PacBio、QIAGEN、Thermo Fisher Scientific Inc.、AB-Biotics, SA、Coriell Life Sciences、Eurofins Scientific、Illumina, Inc.、Dynamic DNA Laboratories、STADAPHARM GmbH、Color、cnsdose、Genomind, Inc.、Healthspek、myDNA Life Australia Pty Ltd.、HudsonAlpha、Sonic Healthcare Limitedなど。 |
中東およびアフリカの精神医学/うつ病における薬物遺伝学検査市場の定義
薬理ゲノム検査は最近、拡張可能になり、大うつ病性障害 (MDD) の診断に利用できるようになりました。臨床医は、薬理ゲノム (PGx) 検査を精神疾患の投薬決定を導くための必須ツールとしてますます認識しています。予測期間中、PGx 検査の広範な導入が市場を牽引します。
個別化医療、階層化医療、精密医療という用語は薬理遺伝学と近い関係にありますが、これらはより広い用語であり、追加の非遺伝的要因もカバーしています。それでも、薬理遺伝学はこれらの分野の重要な要素です。薬理遺伝学は主にヒトの生殖細胞系列 DNA の変異に関係していますが、気分障害や精神疾患の理解においても最近重要な進歩がありました。
薬理遺伝学検査は、薬物と人の遺伝子反応の相互作用を研究し、薬物の効果に影響を与える遺伝子変異を探します。多くの研究者や科学者が薬物と個々の遺伝子の独特な相互作用を特定し、カスタマイズされた医薬品や個人向け医薬品の開発に使用できる貴重な洞察を提供しているため、この検査の需要が高まっています。
中東およびアフリカの精神医学/うつ病における薬理遺伝学検査市場の動向
This section deals with understanding the market drivers, advantages, opportunities, restraints, and challenges. All of this is discussed in detail below:
Drivers
- INCREASE IN THE NUMBER OF PATIENTS SUFFERING FROM PSYCHIATRIC AND DEPRESSION DISORDER
Depression is a common illness worldwide, with an estimated 3.8% of the population affected, including 5.0% among adults and 5.7% among adults older than 60 years. Depression can become a serious health condition of mild to extreme severity, affecting the person to suffer greatly and can lead to suicide in the worst cases. Although over 45 antidepressants are available, suboptimal response poses a challenge and is considered a result of genetic variation, psychiatry/depression. Depending on the severity and pattern of depressive episodes over time, healthcare providers may offer psychological diagnosis such as behavioral activation, cognitive behavioral therapy, interpersonal psychotherapy, and/or antidepressant medication such as selective serotonin reuptake inhibitors (SSRIs) and tricyclic antidepressants (TCAs). Different drugs are used for this kind of mental disorder.
With the growth in the prevalence of depression, the demand for pharmacogenetics testing is also increasing as it studies the effect of genetic variants intending to furnish tailored diagnosis. The market is expected to grow in foresting period.
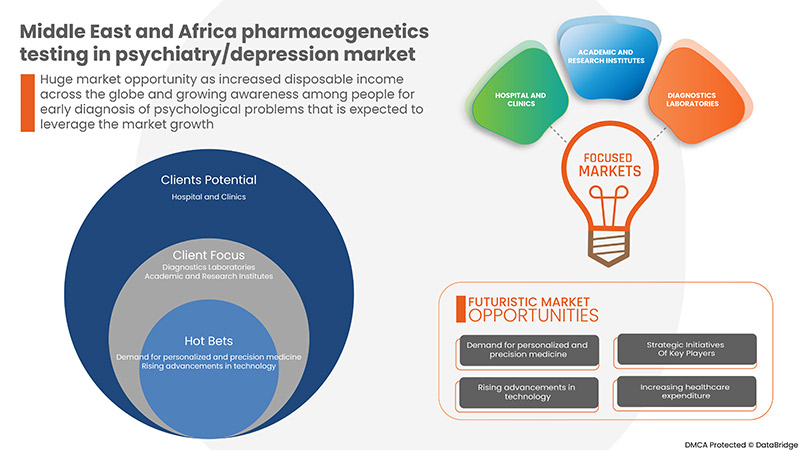
- RISE IN DEMAND FOR PERSONALIZED AND PRECISION MEDICINE
Pharmacogenetics test aids the medical professional in choosing the best medicine for the person because the test searches for the gene variant that may be responsible for influencing the effect of the drug.
Medicine is beginning to get personal, and patients are gradually expressing interest in improved outcomes and less adverse effects with personalized medications. Personalized medicine has the potential to tailor the therapy with a high safety margin and the best response. This trend is largely driven by genome sequencing improvements.
The move toward personal healthcare means changes in the manufacturing of medicines. Manufacturers are moving from creating small molecules to the combination of small molecule and gene therapies. Sponsors focus on replacing inefficient large-scale batch production with investment in new technology and producing personalized drugs.
Restraint
- Lack of skilled medical and genomic expert
Most clinicians still lack confidence in pharmacogenetic (PGx) testing and subsequent data interpretation, indicating insufficient knowledge in this field. It emphasizes the need to improve literacy among healthcare professionals regarding expertise in and understanding of pharmacogenetic (PGx) testing.
Lack of practitioners awareness about the possibilities of pharmacogenetics and poor or insufficient explanation of the test results also reduce personalization technologies for patients. In addition to developing thematic training courses at medical universities, including the educational cycles in continuing professional education systems and free placement of information for practicing doctors are required: academic internet portals, webinars, etc. A clinical pharmacologist plays a crucial role in the implementation of pharmacogenetic testing.
The competence of a clinical pharmacologist in the field of pharmacogenetics is critical: he or she is the one who organizes the application of genotyping in clinical practice, interprets tests, and informs doctors about the possibilities of pharmacogenetics for patients with specific nosologies, that is, acts as the main link between the scientific world, the healthcare system, and practicing physicians in the process of introducing pharmacogenetics.
Opportunity
-
Rising advancements in technology
Advances in pharmacogenomics have introduced an increasing number of opportunities to bring personalized medicine into clinical practice for psychiatric disorders. Personalized medicine may be defined as a comprehensive, prospective approach to preventing, diagnosing, treating, and monitoring disease in ways that achieve optimal individual health care decisions. Over 100 medications now contain United States Food and Drug Administration (FDA) labelling related to potentially applicable pharmacogenomic biomarkers with technological advancements in healthcare. Also, new and advanced methods are being developed to promote pharmacogenetics testing in depression-like disorders. These tests use advanced genetic testing methods to give precise results to form a treatment regimen. The improvements in technology supporting tests improved accessibility of testing options, and the growing number of resources that help clinicians understand how to use this information when it is available are making this aspect of personalized or precision medicine a reality. Thus, providers need to become more aware of the scientific and clinical relevance of pharmacogenomic tests.
The tests also help to establish a meaningful relationship between a drug and the individual genetic makeup. This helps in deciding the drugs to be administered to the patient to treat major depressive disorders and other psychiatric conditions.
Challenge
- Strict government regulation on new products and instruments approval
The concerns regarding the efficacy and safety of products have caused most governments to develop regulatory agencies and policies to look after the development of new medical products or tests. The use of these medical products can be done after passing stringent regulatory standards, which ensure the product is safe, well studied, and has no adverse reactions.
The recent guidelines and the amendment have adequate guidance for manufacturers. International regulations such as food, drug, and administration play a major role in the new launch of the medical product or test into the market. Thus, it can be a major restraint for the market. Therefore, strict government regulation on new products and instrument approval will likely impact the market.
Post-COVID-19 Impact on the Middle East & Africa Pharmacogenetics Testing in Psychiatry/Depression Market
The COVID-19 outbreak had a beneficial impact on the expansion of the pharmacogenetics testing industry. The pandemic has had a negative impact on the pharmacogenomics market growth on account of the temporary halt in research activities in this field, coupled with the low influx of patients in hospitals and diagnostic centers. Since the second half of 2020, with the rising demand for research on certain drugs and testing kits for COVID-19, pharmacogenomic practices have been in vogue.
Manufacturers are making various strategic decisions to bounce back post-COVID-19. The players are conducting multiple R&D activities, product launches, and strategic partnerships to improve the technology and test results involved in the pharmacogenetics testing market.
Recent Developments
- In April 2022, Blue Care Network (BCN) launched a precision medicine program, Blue Cross Personalized Medicine, which leverages pharmacogenomics, or genetic testing, to personalize and tailor medication treatments more effectively for select members based on a review of their prescribed medications for various diagnosis including behavioral health, cardiology, cardiovascular, and oncology. OneOme LLC has helped BCN achieve its precision medicine program goals, reduce the total cost of care, and improve patient health outcomes by reducing adverse drug reactions. This has helped the company to enhance its product portfolio.
- In February 2022, PacBio, a leading provider of high-quality, highly accurate sequencing platforms, announced that it is supporting The Hospital for Sick Children (SickKids) in Toronto, Canada, in using HiFi whole genome sequencing (HiFi WGS) to potentially identify genetic variants that may be associated with medical and developmental conditions. Samples that are examined using HiFi WGS were previously sequenced using short-read DNA sequencing technology but still lack the identification of a disease-causing variant. This has helped the company to enhance the use of its products.
- In July 2022, according to a new nationwide study of nearly 2,000 veterans conducted by the U.S. Department of Veterans Affairs (VA), and Major Depressive Disorder (MDD) remission rates were significantly improved when clinicians had access to GeneSight Psychotropic test results from Myriad Genetics, Inc. in largest ever mental health PGx randomized controlled trial. This has helped the company to show its progress in pharmacogenetic testing.
- In January 2021, myDNA Life Australia Pty Ltd, announced a merger with the U.S., Houston-based consumer DNA test company, FamilyTreeDNA, and its parent company. Gene by Gene for revolutionizing the field of pharmacogenomics, making truly personalized medicine a reality, before expanding into nutrigenomics to deliver actionable, personalized nutrition, fitness, and skincare recommendations. This has helped the company to expand its business.
Middle East & Africa Pharmacogenetics Testing in Psychiatry/Depression Market Scope
Middle East & Africa pharmacogenetics testing in psychiatry/depression market is segmented into type, test type, gene type, patient type, product, end user, and distribution channel. The growth among segments helps you analyze niche pockets of growth and strategies to approach the market and determine your core application areas and the difference in your target markets.
- Middle East & Africa Pharmacogenetics Testing In Psychiatry/Depression Market, By Type
- Anxiety
- Mood Disorders
- Depression
- Bipolar Disorders
- Psychotic Disorders
- Eating Disorders
On the basis of type, Middle East & Africa pharmacogenetics testing in psychiatry/depression market is segmented into anxiety, mood disorders, depression, bipolar disorders, psychotic disorders, and eating disorders.
- Middle East & Africa Pharmacogenetics Testing In Psychiatry/Depression Market, By Test Type
- Whole Genome Sequencing
- Chromosomal Array-Based Tests
On the basis of test type, Middle East & Africa pharmacogenetics testing in psychiatry/depression market is segmented into whole genome sequencing, and chromosomal array-based tests.
- Middle East & Africa Pharmacogenetics Testing In Psychiatry/Depression Market, By Gene Type
- CYP2C19
- CYP2C9 AND VKORC1
- CYP2D6
- HLA-B
- HTR2A/C
- HLA-A
- CYP3A4
- SLC6A4
- MTHFR
- COMT
- OTHERS
On the basis of gene type, Middle East & Africa pharmacogenetics testing in psychiatry/depression market is segmented into CYP2C19, CYP2C9, VKORC1, CYP2D6, HLA-B, HTR2A/C, HLA-A, CYP3A4, SLC6A4, MTHFR, COMT, and others.
- Middle East & Africa Pharmacogenetics Testing In Psychiatry/Depression Market, By Patient Type
- Child
- Adult
- Geriatric
On the basis of patient type, the Middle East & Africa pharmacogenetics testing in psychiatry/depression market is segmented into child, adult, and geriatric.
- Middle East & Africa Pharmacogenetics Testing In Psychiatry/Depression Market, By Product
- Instruments
- Consumables
- Software & Services
On the basis of product type, Middle East & Africa pharmacogenetics testing in psychiatry/depression market is segmented into instruments, consumables, and software & services.
- Middle East & Africa Pharmacogenetics Testing In Psychiatry/Depression Market, By End User
- Hospitals & Clinics
- Dignostics Laboratories
- Academic And Research Institutes
- Others
エンドユーザーに基づいて、中東およびアフリカの精神医学/うつ病市場における薬物遺伝学検査は、病院と診療所、診断研究所、学術研究機関、その他に分類されます。
- 中東およびアフリカの精神科/うつ病市場における薬物遺伝学検査、流通チャネル別
- 直接入札
- サードパーティによる配布
- 病院薬局
- その他

流通チャネルに基づいて、中東およびアフリカの精神医学/うつ病における薬物遺伝学検査市場は、直接入札、サードパーティ流通病院薬局、およびその他のカテゴリに分類されます。
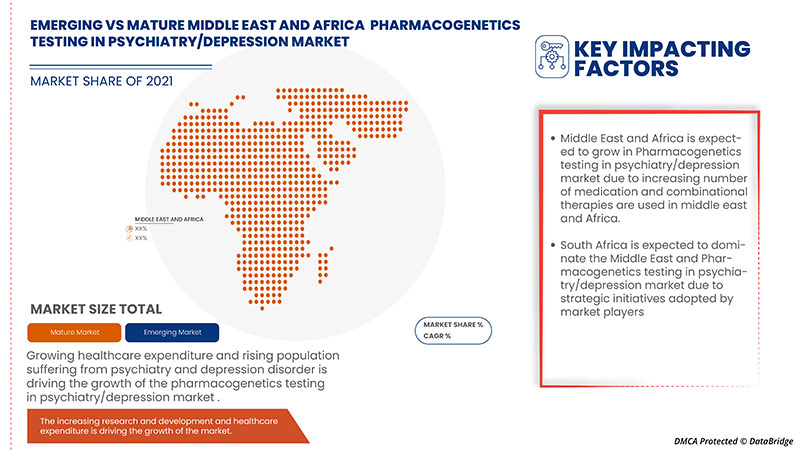
中東およびアフリカの精神医学/うつ病市場における薬物遺伝学検査の地域分析/洞察
中東およびアフリカの精神医学/うつ病市場における薬物遺伝学検査が分析され、市場規模の情報がタイプ、検査タイプ、遺伝子タイプ、患者タイプ、製品、エンドユーザー、流通チャネル別に提供されています。この市場レポートでカバーされている国は、UAE、イスラエル、南アフリカ、エジプト、クウェート、および中東およびアフリカのその他の国です。
2022年には、GDPの高い最大の消費者市場に主要な市場プレーヤーが存在するため、中東およびアフリカが優勢になります。南アフリカは、薬物遺伝子検査の技術進歩の高まりにより成長すると予想されています。
レポートの国別セクションでは、市場の現在および将来の動向に影響を与える国内市場における個別の市場影響要因と規制の変更も提供しています。新規販売、交換販売、国の人口統計、規制行為、輸出入関税などのデータ ポイントは、各国の市場シナリオを予測するために使用される主要な指標の一部です。また、中東およびアフリカのブランドの存在と入手可能性、地元および国内ブランドとの競争が激しいか少ないために直面する課題、販売チャネルの影響を考慮しながら、国別データの予測分析を提供します。
競争環境と中東およびアフリカの精神医学/うつ病における薬理遺伝学検査の市場シェア分析
中東およびアフリカの精神医学/うつ病における薬物遺伝学検査市場の競争環境は、競合他社の詳細を提供します。含まれる詳細には、会社概要、会社の財務、収益、市場の可能性、研究開発への投資、新しい市場への取り組み、生産拠点と施設、会社の強みと弱み、製品の発売、製品試験パイプライン、製品の承認、特許、製品の幅と幅、アプリケーションの優位性、技術ライフライン曲線などがあります。提供された上記のデータ ポイントは、中東およびアフリカの精神医学/うつ病における薬物遺伝学検査市場への会社の重点にのみ関連しています。
中東およびアフリカの精神医学/うつ病の薬物遺伝学検査市場で活動している主要企業には、
- Genelex (Invitae コーポレーションの一部)
- Genewiz(Azenta Life Sciencesの一部)
- MDラボ
- バイオジェネイQ株式会社
- オネオムLLC
- ミリアドジェネティクス株式会社
- ジェンクシス
- キャッスルバイオサイエンス株式会社
- パックバイオ
- キアゲン
- サーモフィッシャーサイエンティフィック株式会社
- AB-バイオティクスSA
- コリエルライフサイエンス
- ユーロフィンサイエンティフィック
- イルミナ株式会社
- ダイナミックDNAラボラトリーズ
- スタダファーム株式会社
- 色
- CNSDose の
- ジェノマインド株式会社
- ヘルススペック
- myDNA Life オーストラリア Pty Ltd.
- ハドソンアルファ
- ソニックヘルスケア株式会社
調査方法: 中東およびアフリカの精神医学/うつ病市場における薬物遺伝学検査
データ収集と基準年分析は、大規模なサンプル サイズのデータ収集モジュールを使用して行われます。市場データは、市場統計モデルとコヒーレント モデルを使用して分析および推定されます。さらに、市場シェア分析と主要トレンド分析は、市場レポートの主要な成功要因です。DBMR 調査チームが使用する主要な調査方法は、データ マイニング、データ変数の市場への影響の分析、および一次 (業界の専門家) 検証を含むデータ三角測量です。これとは別に、データ モデルには、ベンダー ポジショニング グリッド、市場タイムライン分析、市場概要とガイド、企業ポジショニング グリッド、企業市場シェア分析、測定基準、中東およびアフリカと地域、ベンダー シェア分析が含まれます。さらに問い合わせる場合は、アナリストへの電話をリクエストしてください。
SKU-
世界初のマーケットインテリジェンスクラウドに関するレポートにオンラインでアクセスする
- インタラクティブなデータ分析ダッシュボード
- 成長の可能性が高い機会のための企業分析ダッシュボード
- カスタマイズとクエリのためのリサーチアナリストアクセス
- インタラクティブなダッシュボードによる競合分析
- 最新ニュース、更新情報、トレンド分析
- 包括的な競合追跡のためのベンチマーク分析のパワーを活用
目次
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATIONS
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 DBMR TRIPOD DATA VALIDATION MODEL
2.5 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.6 MULTIVARIATE MODELLING
2.7 MARKET APPLICATION COVERAGE GRID
2.8 SOURCE LIFELINE CURVE
2.9 DBMR MARKET POSITION GRID
2.1 VENDOR SHARE ANALYSIS
2.11 SECONDARY SOURCES
2.12 ASSUMPTIONS
3 EXECUTIVE SUMMARY
3.1 PESTEL ANALYSIS
3.2 PORTER'S FIVE FORCES MODEL
3.3 INDUSTRIAL INSIGHTS:
3.4 PIPELINE ANALYSIS FOR MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET
3.5 EPIDEMIOLOGY
4 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, REGULATIONS
4.1 UNITED STATES (U.S.)
4.1.1 ROLE OF FDA
4.1.2 ROLE OF CDC AND HCFA
4.2 EUROPEAN UNION (EU)
4.3 FRANCE
4.4 AUSTRALIA
4.5 SOUTH KOREA
5 MARKET OVERVIEW
5.1 DRIVERS
5.1.1 INCREASE IN THE NUMBER OF PATIENTS SUFFERING FROM PSYCHIATRIC AND DEPRESSION DISORDER
5.1.2 RISE IN HEALTHCARE EXPENDITURE
5.1.3 RISE IN DEMAND FOR PERSONALIZED AND PRECISION MEDICINE
5.2 RESTRAINTS
5.2.1 LACK OF SKILLED MEDICAL AND GENOMIC EXPERT
5.2.2 LACK OF STRONG CLINICAL EVIDENCE
5.2.3 HIGH COST ASSOCIATED WITH THE DIAGNOSIS
5.3 OPPORTUNITIES
5.3.1 RISING ADVANCEMENTS IN TECHNOLOGY
5.3.2 INCREASING NUMBER OF KEY PLAYERS IN MARKET
5.4 CHALLENGES
5.4.1 STRICT GOVERNMENT REGULATION ON NEW PRODUCTS AND INSTRUMENTS APPROVAL
5.4.2 DEARTH OF SKILLED PERSONNEL
6 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TYPE
6.1 OVERVIEW
6.2 ANXIETY
6.3 DEPRESSION
6.4 MOOD DISORDERS
6.5 BIPOLAR DISORDERS
6.6 EATING DISORDERS
6.7 PSYCHOTIC DISORDERS
7 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT
7.1 OVERVIEW
7.2 CONSUMABLES
7.2.1 WHOLE GENOME SEQUENCING KITS
7.2.2 CHROMOSOMAL ARRAY BASED KITS
7.3 INSTRUMENTS
7.4 SOFTWARE AND SERVICES
8 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE
8.1 OVERVIEW
8.2 WHOLE GENOME SEQUENCING
8.2.1 EXOME SEQUENCING
8.2.1.1 SNP
8.2.1.2 CNV
8.2.1.3 RARE PTV MUTATION
8.2.1.4 ULTRA PTV MUTATION
8.2.1.5 OTHERS
8.2.2 KARYOTYPE
8.2.2.1 SNP
8.2.2.2 CNV
8.2.2.3 RARE PTV MUTATION
8.2.2.4 ULTRA PTV MUTATION
8.2.2.5 OTHERS
8.2.3 LOW COVERAGE WGS
8.2.3.1 SNP
8.2.3.2 CNV
8.2.3.3 RARE PTV MUTATION
8.2.3.4 ULTRA PTV MUTATION
8.2.3.5 OTHERS
8.2.4 DEEP COVERAGE WGS
8.2.4.1 SNP
8.2.4.2 CNV
8.2.4.3 RARE PTV MUTATION
8.2.4.4 ULTRA PTV MUTATION
8.2.4.5 OTHERS
8.2.5 MICROARRAY
8.2.5.1 SNP
8.2.5.2 CNV
8.2.5.3 RARE PTV MUTATION
8.2.5.4 ULTRA PTV MUTATION
8.2.5.5 OTHERS
8.2.6 OTHERS
8.3 CHROMOSOMAL ARRAY BASED TESTS
8.3.1 MICRODELETIONS
8.3.2 MICRO DUPLICATIONS
9 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY GENE TYPE
9.1 OVERVIEW
9.2 CYP2C19
9.3 CYP2C9 AND VKORC1
9.4 CYP2D6
9.5 HLA-B
9.6 HTR2A/C
9.7 HLA-A
9.8 CYP3A4
9.9 SLC6A4
9.1 MTHFR
9.11 COMT
9.12 OTHERS
10 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PATIENT TYPE
10.1 OVERVIEW
10.2 ADULT
10.3 GERIATRIC
10.4 CHILD
11 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET , BY END USER
11.1 OVERVIEW
11.2 HOSPITAL AND CLINICS
11.2.1 DRUG EFFECTIVENESS
11.2.2 SIDE EFFECTS
11.2.3 DOSAGE
11.3 DIAGNOSTICS LABORATORIES
11.3.1 DRUG EFFECTIVENESS
11.3.2 SIDE EFFECTS
11.3.3 DOSAGE
11.4 ACADEMIC AND RESEARCH INSTITUTES
11.4.1 DRUG EFFECTIVENESS
11.4.2 SIDE EFFECTS
11.4.3 DOSAGE
11.5 OTHERS
12 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY DISTRIBUTION CHANNEL
12.1 OVERVIEW
12.2 DIRECT TENDER
12.3 THIRD PARTY DISTRIBUTION
12.4 HOSPITAL PHARMACY
12.5 OTHERS
13 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION
13.1 MIDDLE EAST AND AFRICA
13.1.1 SOUTH AFRICA
13.1.2 SAUDI ARABIA
13.1.3 U.A.E
13.1.4 ISRAEL
13.1.5 EGYPT
13.1.6 REST OF MIDDLE EAST AND AFRICA
14 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: COMPANY LANDSCAPE
14.1 COMPANY SHARE ANALYSIS: MIDDLE EAST & AFRICA
15 SWOT ANALYSIS
16 COMPANY PROFILE
16.1 THERMO FISHER SCIENTIFIC INC.
16.1.1 COMPANY SNAPSHOT
16.1.2 RECENT FINANCIALS
16.1.3 COMPANY SHARE ANALYSIS
16.1.4 PRODUCT PORTFOLIO
16.1.5 RECENT DEVELOPMENT
16.2 ILLUMINA, INC.
16.2.1 COMPANY SNAPSHOT
16.2.2 REVENUE ANALYSIS
16.2.3 COMPANY SHARE ANALYSIS
16.2.4 PRODUCT PORTFOLIO
16.2.5 RECENT DEVELOPMENTS
16.3 MYRIAD GENETICS, INC.
16.3.1 COMPANY SNAPSHOT
16.3.2 COMPANY SHARE ANALYSIS
16.3.3 PRODUCT PORTFOLIO
16.3.4 RECENT DEVELOPMENT
16.4 SONIC HEALTHCARE LIMITED
16.4.1 COMPANY SNAPSHOT
16.4.2 REVENUE ANALYSIS
16.4.3 COMPANY SHARE ANALYSIS
16.4.4 PRODUCT PORTFOLIO
16.4.5 RECENT DEVELOPMENTS
16.5 QIAGEN
16.5.1 COMPANY SNAPSHOT
16.5.2 RECENT FINANCIALS
16.5.3 COMPANY SHARE ANALYSIS
16.5.4 PRODUCT PORTFOLIO
16.5.5 RECENT DEVELOPMENTS
16.6 EUROFINS SCIENTIFIC
16.6.1 COMPANY SNAPSHOT
16.6.2 REVENUE ANALYSIS
16.6.3 PRODUCT PORTFOLIO
16.6.4 RECENT DEVELOPMENT
16.7 AB-BIOTICS, S.A.
16.7.1 COMPANY SNAPSHOT
16.7.2 PRODUCT PORTFOLIO
16.7.3 RECENT DEVELOPMENTS
16.8 BIOGENIQ INC.
16.8.1 COMPANY SNAPSHOT
16.8.2 PRODUCT PORTFOLIO
16.8.3 RECENT DEVELOPMENT
16.9 CASTLE BIOSCIENCE, INC.
16.9.1 COMPANY SNAPSHOT
16.9.2 REVENUE ANALYSIS
16.9.3 PRODUCT PORTFOLIO
16.9.4 RECENT DEVELOPMENT
16.1 CNSDOSE
16.10.1 COMPANY SNAPSHOT
16.10.2 PRODUCT PORTFOLIO
16.10.3 RECENT DEVELOPMENT
16.11 COLOR HEALTH, INC.
16.11.1 COMPANY SNAPSHOT
16.11.2 PRODUCT PORTFOLIO
16.11.3 RECENT DEVELOPMENTS
16.12 CORIELL LIFE SCIENCES
16.12.1 COMPANY SNAPSHOT
16.12.2 PRODUCT PORTFOLIO
16.12.3 RECENT DEVELOPMENTS
16.13 DYNAMIC DNA LABORATORIES
16.13.1 COMPANY SNAPSHOT
16.13.2 PRODUCT PORTFOLIO
16.13.3 RECENT DEVELOPMENTS
16.14 GENELEX (SUBSIADIARY OF INVITAE CORPORATION.)
16.14.1 COMPANY SNAPSHOT
16.14.2 REVENUE ANALYSIS
16.14.3 PRODUCT PORTFOLIO
16.14.4 RECENT DEVELOPMENT
16.15 GENEWIZ (PART OF AZENTA LIFE SCIENCES)
16.15.1 COMPANY SNAPSHOT
16.15.2 REVENUE ANALYSIS
16.15.3 PRODUCT PORTFOLIO
16.15.4 RECENT DEVELOPMENTS
16.16 GENOMIND, INC.
16.16.1 COMPANY SNAPSHOT
16.16.2 PRODUCT PORTFOLIO
16.16.3 RECENT DEVELOPMENTS
16.17 GENXYS
16.17.1 COMPANY SNAPSHOT
16.17.2 PRODUCT PORTFOLIO
16.17.3 RECENT DEVELOPMENTS
16.18 HEALTHSPEK
16.18.1 COMPANY SNAPSHOT
16.18.2 PRODUCT PORTFOLIO
16.18.3 RECENT DEVELOPMENTS
16.19 HUDSONALPHA
16.19.1 COMPANY SNAPSHOT
16.19.2 PRODUCT PORTFOLIO
16.19.3 RECENT DEVELOPMENT
16.2 MD LABS
16.20.1 COMPANY SNAPSHOT
16.20.2 PRODUCT PORTFOLIO
16.20.3 RECENT DEVELOPMENTS
16.21 MYDNA LIFE AUSTRALIA PTY LTD.
16.21.1 COMPANY SNAPSHOT
16.21.2 PRODUCT PORTFOLIO
16.21.3 RECENT DEVELOPMENT
16.22 ONEOME, LLC
16.22.1 COMPANY SNAPSHOT
16.22.2 PRODUCT PORTFOLIO
16.22.3 RECENT DEVELOPMENTS
16.23 PACBIO
16.23.1 COMPANY SNAPSHOT
16.23.2 REVENUE ANALYSIS
16.23.3 PRODUCT PORTFOLIO
16.23.4 RECENT DEVELOPMENTS
16.24 STADAPHARM GMBH
16.24.1 COMPANY SNAPSHOT
16.24.2 PRODUCT PORTFOLIO
16.24.3 RECENT DEVELOPMENT
17 QUESTIONNAIRE
18 RELATED REPORTS
表のリスト
TABLE 1 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 2 MIDDLE EAST & AFRICA ANXIETY IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 3 MIDDLE EAST & AFRICA DEPRESSION IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 4 MIDDLE EAST & AFRICA MOOD DISORDERS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 5 MIDDLE EAST & AFRICA BIPOLAR DISORDERS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 6 MIDDLE EAST & AFRICA EATING DISORDERS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 7 MIDDLE EAST & AFRICA PSYCHOTIC DISORDERS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 8 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 9 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 VOLUME (UNITS)
TABLE 10 MIDDLE EAST & AFRICA CONSUMABLES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 11 MIDDLE EAST & AFRICA CONSUMABLES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 12 MIDDLE EAST & AFRICA CONSUMABLES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 VOLUME (UNITS)
TABLE 13 MIDDLE EAST & AFRICA INSTRUMENTS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 14 MIDDLE EAST & AFRICA SOFTWARE AND SERVICES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 15 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 16 MIDDLE EAST & AFRICA WHOLE GENOME SEQUENCING IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 17 MIDDLE EAST & AFRICA WHOLE GENOME SEQUENCING IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 18 MIDDLE EAST & AFRICA EXOME SEQUENCING IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 19 MIDDLE EAST & AFRICA KARYOTYPE IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 20 MIDDLE EAST & AFRICA LOW COVERAGE WGS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 21 MIDDLE EAST & AFRICA DEEP COVERAGE WGS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 22 MIDDLE EAST & AFRICA MICROARRAY IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 23 MIDDLE EAST & AFRICA CHROMOSOMAL ARRAY BASED TESTS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 24 MIDDLE EAST & AFRICA CHROMOSOMAL ARRAY BASED TESTS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 25 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY GENE TYPE, 2020-2029 (USD MILLION)
TABLE 26 MIDDLE EAST & AFRICA CYP2C19 IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 27 MIDDLE EAST & AFRICA CYP2C9 AND VKORC1 IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 28 MIDDLE EAST & AFRICA CYP2D6 IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 29 MIDDLE EAST & AFRICA HLA-B IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 30 MIDDLE EAST & AFRICA HTR2A/C IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 31 MIDDLE EAST & AFRICA HLA-A IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 32 MIDDLE EAST & AFRICA CYP3A4 IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 33 MIDDLE EAST & AFRICA SLC6A4 IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 34 MIDDLE EAST & AFRICA MTHFR IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 35 MIDDLE EAST & AFRICA COMT IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 36 MIDDLE EAST & AFRICA OTHERS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 37 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 38 MIDDLE EAST & AFRICA ADULT IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 39 MIDDLE EAST & AFRICA GERIATRIC IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 40 MIDDLE EAST & AFRICA CHILD IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 41 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 42 MIDDLE EAST & AFRICA HOSPITAL AND CLINICS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 43 MIDDLE EAST & AFRICA HOSPITAL AND CLINICS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 44 MIDDLE EAST & AFRICA DIAGNOSTICS LABORATORIES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 45 MIDDLE EAST & AFRICA DIAGNOSTICS LABORATORIES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 46 MIDDLE EAST & AFRICA ACADEMIC AND RESEARCH INSTITUTES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 47 MIDDLE EAST & AFRICA ACADEMIC AND RESEARCH INSTITUTES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 48 MIDDLE EAST & AFRICA OTHERS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 49 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 50 MIDDLE EAST & AFRICA DIRECT TENDER IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 51 MIDDLE EAST & AFRICA THIRD PARTY DISTRIBUTION IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 52 MIDDLE EAST & AFRICA HOSPITAL PHARMACY IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 53 MIDDLE EAST & AFRICA OTHERS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 54 MIDDLE EAST AND AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY COUNTRY, 2020-2029 (USD MILLION)
TABLE 55 MIDDLE EAST AND AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 56 MIDDLE EAST AND AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 57 MIDDLE EAST AND AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 VOLUME (UNITS)
TABLE 58 MIDDLE EAST AND AFRICA CONSUMABLES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 59 MIDDLE EAST AND AFRICA CONSUMABLES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 VOLUME (UNITS)
TABLE 60 MIDDLE EAST AND AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 61 MIDDLE EAST AND AFRICA WHOLE GENOME SEQUENCING IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 62 MIDDLE EAST AND AFRICA EXOME SEQUENCING IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 63 MIDDLE EAST AND AFRICA KARYOTYPE IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 64 MIDDLE EAST AND AFRICA LOW COVERAGE WGS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 65 MIDDLE EAST AND AFRICA DEEP COVERAGE WGS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 66 MIDDLE EAST AND AFRICA MICROARRAY IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 67 MIDDLE EAST AND AFRICA CHROMOSOMAL ARRAY BASED TESTS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 68 MIDDLE EAST AND AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY GENE TYPE, 2020-2029 (USD MILLION)
TABLE 69 MIDDLE EAST AND AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 70 MIDDLE EAST AND AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 71 MIDDLE EAST AND AFRICA HOSPITAL AND CLINICS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 72 MIDDLE EAST AND AFRICA DIAGNOSTIC LABORATORIES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 73 MIDDLE EAST AND AFRICA ACADEMIC AND RESEARCH INSTITUTES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 74 MIDDLE EAST AND AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 75 SOUTH AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 76 SOUTH AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 77 SOUTH AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 VOLUME (UNITS)
TABLE 78 SOUTH AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 ASP (USD)
TABLE 79 SOUTH AFRICA CONSUMABLES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 80 SOUTH AFRICA CONSUMABLES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 VOLUME (UNITS)
TABLE 81 SOUTH AFRICA CONSUMABLES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 ASP (USD)
TABLE 82 SOUTH AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 83 SOUTH AFRICA WHOLE GENOME SEQUENCING IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 84 SOUTH AFRICA EXOME SEQUENCING IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 85 SOUTH AFRICA KARYOTYPE IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 86 SOUTH AFRICA LOW COVERAGE WGS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 87 SOUTH AFRICA DEEP COVERAGE WGS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 88 SOUTH AFRICA MICROARRAY IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 89 SOUTH AFRICA CHROMOSOMAL ARRAY BASED TESTS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 90 SOUTH AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY GENE TYPE, 2020-2029 (USD MILLION)
TABLE 91 SOUTH AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 92 SOUTH AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 93 SOUTH AFRICA HOSPITAL AND CLINICS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 94 SOUTH AFRICA DIAGNOSTIC LABORATORIES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 95 SOUTH AFRICA ACADEMIC AND RESEARCH INSTITUTES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 96 SOUTH AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 97 SAUDI ARABIA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 98 SAUDI ARABIA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 99 SAUDI ARABIA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 VOLUME (UNITS)
TABLE 100 SAUDI ARABIA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 ASP (USD)
TABLE 101 SAUDI ARABIA CONSUMABLES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 102 SAUDI ARABIA CONSUMABLES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 VOLUME (UNITS)
TABLE 103 SAUDI ARABIA CONSUMABLES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 ASP (USD)
TABLE 104 SAUDI ARABIA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 105 SAUDI ARABIA WHOLE GENOME SEQUENCING IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 106 SAUDI ARABIA EXOME SEQUENCING IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 107 SAUDI ARABIA KARYOTYPE IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 108 SAUDI ARABIA LOW COVERAGE WGS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 109 SAUDI ARABIA DEEP COVERAGE WGS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 110 SAUDI ARABIA MICROARRAY IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 111 SAUDI ARABIA CHROMOSOMAL ARRAY BASED TESTS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 112 SAUDI ARABIA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY GENE TYPE, 2020-2029 (USD MILLION)
TABLE 113 SAUDI ARABIA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 114 SAUDI ARABIA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 115 SAUDI ARABIA HOSPITAL AND CLINICS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 116 SAUDI ARABIA DIAGNOSTIC LABORATORIES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 117 SAUDI ARABIA ACADEMIC AND RESEARCH INSTITUTES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 118 SAUDI ARABIA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 119 UAE PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 120 UAE PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 121 UAE PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 VOLUME (UNITS)
TABLE 122 UAE PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 ASP (USD)
TABLE 123 UAE CONSUMABLES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 124 UAE CONSUMABLES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 VOLUME (UNITS)
TABLE 125 UAE CONSUMABLES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 ASP (USD)
TABLE 126 UAE PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 127 UAE WHOLE GENOME SEQUENCING IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 128 UAE EXOME SEQUENCING IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 129 UAE KARYOTYPE IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 130 UAE LOW COVERAGE WGS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 131 UAE DEEP COVERAGE WGS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 132 UAE MICROARRAY IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 133 UAE CHROMOSOMAL ARRAY BASED TESTS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 134 UAE PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY GENE TYPE, 2020-2029 (USD MILLION)
TABLE 135 UAE PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 136 UAE PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 137 UAE HOSPITAL AND CLINICS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 138 UAE DIAGNOSTIC LABORATORIES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 139 UAE ACADEMIC AND RESEARCH INSTITUTES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 140 UAE PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 141 ISRAEL PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 142 ISRAEL PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 143 ISRAEL PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 VOLUME (UNITS)
TABLE 144 ISRAEL PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 ASP (USD)
TABLE 145 ISRAEL CONSUMABLES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 146 ISRAEL CONSUMABLES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 VOLUME (UNITS)
TABLE 147 ISRAEL CONSUMABLES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 ASP (USD)
TABLE 148 ISRAEL PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 149 ISRAEL WHOLE GENOME SEQUENCING IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 150 ISRAEL EXOME SEQUENCING IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 151 ISRAEL KARYOTYPE IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 152 ISRAEL LOW COVERAGE WGS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 153 ISRAEL DEEP COVERAGE WGS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 154 ISRAEL MICROARRAY IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 155 ISRAEL CHROMOSOMAL ARRAY BASED TESTS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 156 ISRAEL PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY GENE TYPE, 2020-2029 (USD MILLION)
TABLE 157 ISRAEL PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 158 ISRAEL PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 159 ISRAEL HOSPITAL AND CLINICS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 160 ISRAEL DIAGNOSTIC LABORATORIES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 161 ISRAEL ACADEMIC AND RESEARCH INSTITUTES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 162 ISRAEL PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 163 EGYPT PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 164 EGYPT PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 165 EGYPT PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 VOLUME (UNITS)
TABLE 166 EGYPT PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 ASP (USD)
TABLE 167 EGYPT CONSUMABLES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 168 EGYPT CONSUMABLES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 VOLUME (UNITS)
TABLE 169 EGYPT CONSUMABLES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 ASP (USD)
TABLE 170 EGYPT PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 171 EGYPT WHOLE GENOME SEQUENCING IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 172 EGYPT EXOME SEQUENCING IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 173 EGYPT KARYOTYPE IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 174 EGYPT LOW COVERAGE WGS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 175 EGYPT DEEP COVERAGE WGS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 176 EGYPT MICROARRAY IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 177 EGYPT CHROMOSOMAL ARRAY BASED TESTS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 178 EGYPT PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY GENE TYPE, 2020-2029 (USD MILLION)
TABLE 179 EGYPT PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 180 EGYPT PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 181 EGYPT HOSPITAL AND CLINICS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 182 EGYPT DIAGNOSTIC LABORATORIES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 183 EGYPT ACADEMIC AND RESEARCH INSTITUTES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 184 EGYPT PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 185 REST OF MIDDLE EAST AND AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TYPE, 2020-2029 (USD MILLION)
図表一覧
FIGURE 1 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: SEGMENTATION
FIGURE 2 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: DATA TRIANGULATION
FIGURE 3 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: DROC ANALYSIS
FIGURE 4 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: MIDDLE EAST & AFRICA VS REGIONAL MARKET ANALYSIS
FIGURE 5 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: MARKET APPLICATION COVERAGE GRID
FIGURE 8 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: DBMR MARKET POSITION GRID
FIGURE 9 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: SEGMENTATION
FIGURE 11 THE INCREASING ADOPTION OF PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION DEVICES AND PROCEDURES AND RISING PREFERENCE FOR NON-SURGICAL PROCEDURES ARE EXPECTED TO DRIVE THE MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET IN THE FORECAST PERIOD OF 2022 TO 2029
FIGURE 12 SOFTWARE IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET IN 2022 & 2029
FIGURE 13 PATIENT FLOW DIAGRAM
FIGURE 14 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF THE MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN THE PSYCHIATRY/DEPRESSION MARKET
FIGURE 15 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY TYPE, 2021
FIGURE 16 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY TYPE, 2022-2029 (USD MILLION)
FIGURE 17 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY TYPE, CAGR (2022-2029)
FIGURE 18 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY TYPE, LIFELINE CURVE
FIGURE 19 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY PRODUCT, 2021
FIGURE 20 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY PRODUCT, 2022-2029 (USD MILLION)
FIGURE 21 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY PRODUCT, CAGR (2022-2029)
FIGURE 22 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY PRODUCT, LIFELINE CURVE
FIGURE 23 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY TEST TYPE, 2021
FIGURE 24 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY TEST TYPE, 2022-2029 (USD MILLION)
FIGURE 25 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY TEST TYPE, CAGR (2022-2029)
FIGURE 26 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY TEST TYPE, LIFELINE CURVE
FIGURE 27 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY GENE TYPE, 2021
FIGURE 28 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY GENE TYPE, 2022-2029 (USD MILLION)
FIGURE 29 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY GENE TYPE, CAGR (2022-2029)
FIGURE 30 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY GENE TYPE, LIFELINE CURVE
FIGURE 31 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY PATIENT TYPE, 2021
FIGURE 32 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY PATIENT TYPE, 2022-2029 (USD MILLION)
FIGURE 33 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY PATIENT TYPE, CAGR (2022-2029)
FIGURE 34 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY PATIENT TYPE, LIFELINE CURVE
FIGURE 35 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET : BY END USER, 2021
FIGURE 36 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET : BY END USER, 2022-2029 (USD MILLION)
FIGURE 37 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET : BY END USER, CAGR (2022-2029)
FIGURE 38 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET : BY END USER, LIFELINE CURVE
FIGURE 39 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY DISTRIBUTION CHANNEL, 2021
FIGURE 40 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY DISTRIBUTION CHANNEL, 2022-2029 (USD MILLION)
FIGURE 41 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET : BY DISTRIBUTION CHANNEL, CAGR (2022-2029)
FIGURE 42 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET : BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 43 MIDDLE EAST AND AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: SNAPSHOT (2021)
FIGURE 44 MIDDLE EAST AND AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY COUNTRY (2021)
FIGURE 45 MIDDLE EAST AND AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY COUNTRY (2022 & 2029)
FIGURE 46 MIDDLE EAST AND AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY COUNTRY (2021 & 2029)
FIGURE 47 MIDDLE EAST AND AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY TYPE (2022-2029)
FIGURE 48 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: COMPANY SHARE 2021 (%)

調査方法
データ収集と基準年分析は、大規模なサンプル サイズのデータ収集モジュールを使用して行われます。この段階では、さまざまなソースと戦略を通じて市場情報または関連データを取得します。過去に取得したすべてのデータを事前に調査および計画することも含まれます。また、さまざまな情報ソース間で見られる情報の不一致の調査も含まれます。市場データは、市場統計モデルと一貫性モデルを使用して分析および推定されます。また、市場シェア分析と主要トレンド分析は、市場レポートの主要な成功要因です。詳細については、アナリストへの電話をリクエストするか、お問い合わせをドロップダウンしてください。
DBMR 調査チームが使用する主要な調査方法は、データ マイニング、データ変数が市場に与える影響の分析、および一次 (業界の専門家) 検証を含むデータ三角測量です。データ モデルには、ベンダー ポジショニング グリッド、市場タイムライン分析、市場概要とガイド、企業ポジショニング グリッド、特許分析、価格分析、企業市場シェア分析、測定基準、グローバルと地域、ベンダー シェア分析が含まれます。調査方法について詳しくは、お問い合わせフォームから当社の業界専門家にご相談ください。
カスタマイズ可能
Data Bridge Market Research は、高度な形成的調査のリーダーです。当社は、既存および新規のお客様に、お客様の目標に合致し、それに適したデータと分析を提供することに誇りを持っています。レポートは、対象ブランドの価格動向分析、追加国の市場理解 (国のリストをお問い合わせください)、臨床試験結果データ、文献レビュー、リファービッシュ市場および製品ベース分析を含めるようにカスタマイズできます。対象競合他社の市場分析は、技術ベースの分析から市場ポートフォリオ戦略まで分析できます。必要な競合他社のデータを、必要な形式とデータ スタイルでいくつでも追加できます。当社のアナリスト チームは、粗い生の Excel ファイル ピボット テーブル (ファクト ブック) でデータを提供したり、レポートで利用可能なデータ セットからプレゼンテーションを作成するお手伝いをしたりすることもできます。













