世界の尿素呼気検査市場規模、シェア、トレンド分析レポート
Market Size in USD Billion
CAGR :
% 
 USD
129.98 Million
USD
199.48 Million
2024
2032
USD
129.98 Million
USD
199.48 Million
2024
2032
| 2025 –2032 | |
| USD 129.98 Million | |
| USD 199.48 Million | |
|
|
|
|
世界の尿素呼気検査市場のセグメンテーション、製品タイプ別(尿素呼気検査キット、尿素呼気検査分析装置)、検査タイプ別(ポイントオブケア検査(POCT)、検査室ベースの検査)、機器別(質量分析計、赤外線分光計、レーザー支援合理化装置)、用途別(病院、専門クリニック、検査室、その他) - 2032年までの業界動向と予測
尿素呼気検査市場規模
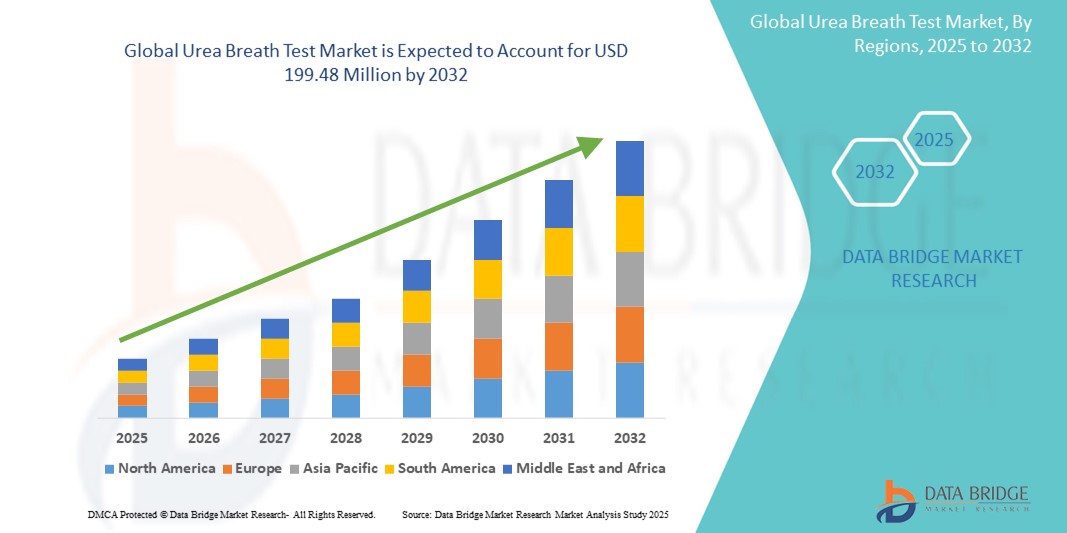
- 世界の尿素呼気検査市場規模は2024年に1億2,998万米ドルと評価され、予測期間中に5.50%のCAGRで成長し、2032年には1億9,948万米ドル に達すると予想されています 。
- 市場の成長は、ヘリコバクター・ピロリ(H. pylori)感染症の蔓延と非侵襲的診断オプションへの意識の高まりに大きく牽引されており、病院と外来の両方で尿素呼気試験(UBT)の普及が進んでいます。UBTは、内視鏡検査や生検に代わる迅速で正確、かつ患者に優しい診断方法であるため、特にH. pylori関連の胃腸疾患の発生率が高い地域では、医療提供者の間で好まれる選択肢となっています。
- さらに、同位体標識技術の進歩と携帯型・自動呼気分析装置の開発により、検査の精度とアクセス性が向上しています。これらの革新は、先進国市場と新興国市場の両方で診断効率を大幅に向上させ、尿素呼気検査ソリューションの普及を促進しています。
尿素呼気検査市場分析
- 尿素呼気試験は、主にヘリコバクター・ピロリ感染症の診断に用いられ、非侵襲性、高い精度、迅速な結果により、大きな注目を集めています。これらの診断ツールは、臨床現場とPOC(ポイントオブケア)現場の両方において、消化器疾患管理においてますます重要な要素となりつつあります。
- 尿素呼気検査の需要の高まりは、主にH.ピロリ菌感染の世界的な負担の増加、胃疾患の早期診断に関する意識の高まり、そして非侵襲的で患者に優しい診断ソリューションへの関心の高まりによって促進されている。
- 尿素呼気検査市場は、先進的な医療インフラ、患者の高い認知度、そして有利な償還政策を背景に、北米が2024年に38.6%という最大の収益シェアを獲得し、市場を席巻しました。特に米国は、技術の進歩、外来診断件数の増加、そして非侵襲性検査の普及により、力強い成長を遂げています。
- アジア太平洋地域は、医療アクセスの拡大、可処分所得の増加、そして中国、インド、日本などの国々における診断技術への投資増加に牽引され、予測期間(2025~2032年)において11.4%という最も高いCAGRを記録すると予想されています。消化器疾患の罹患率の上昇と集団検診の取り組みは、この地域の需要をさらに押し上げています。
- ポイントオブケア検査(POCT)分野は、特に外来診療や遠隔診療環境において、迅速かつアクセスしやすい診断ソリューションへの需要の高まりに牽引され、2024年には59.5%の収益シェアで市場を牽引しました。その利便性、迅速な処理時間、そして使いやすさは、世界中の様々な医療現場での導入を大幅に促進しています。
レポートの範囲と尿素呼気検査市場のセグメンテーション
|
属性 |
尿素呼気検査の主要市場洞察 |
|
対象セグメント |
|
|
対象国 |
北米
ヨーロッパ
アジア太平洋
中東およびアフリカ
南アメリカ
|
|
主要な市場プレーヤー |
|
|
市場機会 |
|
|
付加価値データ情報セット |
データブリッジマーケットリサーチがまとめた市場レポートには、市場価値、成長率、セグメンテーション、地理的範囲、主要プレーヤーなどの市場シナリオに関する洞察に加えて、専門家による詳細な分析、価格設定分析、ブランドシェア分析、消費者調査、人口統計分析、サプライチェーン分析、バリューチェーン分析、原材料/消耗品の概要、ベンダー選択基準、PESTLE分析、ポーター分析、規制の枠組みも含まれています。 |
尿素呼気検査市場の動向
「非侵襲性、迅速診断ソリューションへの移行」
- 世界の尿素呼気検査市場を形作る顕著な傾向は、ヘリコバクター・ピロリ(H.ピロリ)検出のための非侵襲的で患者に優しい診断方法への関心が高まっていることである。
- 尿素呼気試験は、従来の内視鏡検査に比べて感度と特異性が高く、患者の不快感が最小限で、結果が迅速に得られることから、ゴールドスタンダードの診断方法として注目を集めています。
- 世界中の医療システムでは、患者の負担を軽減し、診断の所要時間を改善するために、外来診療やポイントオブケアサービスの一環として呼吸に基づく診断を採用するケースが増えています。
- 最小限の準備で済むという利便性と、活動性感染と治療後の除菌の両方を検出できる検査能力により、消化器科クリニックでは好まれる選択肢となっています。
- さらに、胃潰瘍、胃炎、胃がんとH.ピロリ菌の関連性についての認識が高まり、先進地域と発展途上地域の両方で定期的な検査が促進されている。
- この傾向は、ポータブルUBTキットや分析装置の統合など、都市部と農村部の両方の医療インフラに対応する検査形式の継続的な革新によっても支えられています。
尿素呼気検査市場の動向
ドライバ
「H.ピロリ菌感染症の蔓延と早期発見の必要性」
- 世界人口の50%以上が感染しているヘリコバクターピロリ感染の世界的な負担は、尿素呼気検査市場を牽引する重要な要因です。
- この感染症は消化性潰瘍の主な原因であり、胃がんの危険因子でもあるため、医療従事者は早期診断と根絶にますます重点を置いている。
- 尿素呼気試験は、臨床医が活動性感染症を正確に検出し、抗生物質治療の成功を監視し、不必要な治療を回避することを可能にするため、治療ワークフローに不可欠なものとなっています。
- さらに、日本、中国、韓国などの国では、胃腸の健康と癌予防に焦点を当てた政府と公衆衛生の取り組みにより、UBTを使用した大規模なスクリーニングプログラムが推進されています。
- 外来診療所、薬局、プライマリケアの現場でのUBTの利用が増加し、携帯可能で使いやすい検査キットがサポートされ、導入がさらに加速しています。
抑制/挑戦
「低・中所得国における認知度とアクセシビリティの限界」
- ヘリコバクター・ピロリ感染の症状に関する認識の欠如と、尿素呼気試験(UBT)のような非侵襲的診断オプションの利用可能性は、多くの発展途上地域で依然として大きな障壁となっている。
- いくつかの低・中所得国(LMIC)では、医療従事者と患者は、UBT技術に関する研修や知識が限られているため、内視鏡検査や便抗原検査などの従来の侵襲的な診断方法に依然として大きく依存している。
- さらに、呼気検査分析装置の初期設定コストと訓練を受けた人員の必要性は、特に診断インフラがほとんど整っていない地方や資源の乏しい医療施設での導入を阻む可能性がある。
- さらに、償還ポリシーが限定的であることと、公衆衛生プログラムに含まれていないことから、特に公立病院や診療所では、UBT への患者のアクセスがさらに制限されています。
- この制約を克服するには、対象を絞った教育キャンペーン、診断のアクセシビリティに対する政府の支援、そしてサービスが行き届いていない市場における尿素呼気検査ソリューションの補助と配布のための戦略的パートナーシップが必要である。
尿素呼気検査市場の展望
市場は、製品タイプ、テストタイプ、機器、およびアプリケーションに基づいて分割されています。
- 製品タイプ別
製品タイプ別に見ると、尿素呼気検査市場は尿素呼気検査キットと尿素呼気検査分析装置に分類されます。尿素呼気検査キットセグメントは、ヘリコバクター・ピロリ菌の検出のために外来診療で広く採用されていることから、2024年には64.3%という最大の収益シェアで市場を席巻しました。これらのキットは、非侵襲性、使いやすさ、そして費用対効果に優れています。
尿素呼気検査分析装置セグメントは、病院や診断ラボにおける正確でリアルタイムの分析に対する需要の高まりを背景に、2025年から2032年にかけて7.8%という最速のCAGRで成長すると予想されています。
- テストの種類別
検査の種類に基づいて、尿素呼気検査市場は、ポイントオブケア検査(POCT)とラボベースの検査に分類されます。ポイントオブケア検査セグメントは、特に外来診療や遠隔診療における迅速診断の需要の高まりにより、2024年には59.5%と最大の市場シェアを占めました。
実験室ベースの検査セグメントは、包括的な感染モニタリングとフォローアップ検査のための中央実験室への統合の増加により、予測期間中に 8.4% の CAGR で成長すると予想されます。
- 楽器別
機器別に見ると、尿素呼気検査市場は質量分析計、赤外線分光計、レーザー支援合理化装置に分類されます。赤外線分光計セグメントは、その信頼性、手頃な価格、そして携帯性の高さから、2024年には52.7%の収益シェアで市場をリードするでしょう。
質量分析計セグメントは、その優れた精度と研究および高精度診断における導入の増加により、2025年から2032年にかけて9.1%という最高のCAGRで成長すると予測されています。
- アプリケーション別
用途別に見ると、尿素呼気検査市場は病院、専門クリニック、検査機関、その他に分類されます。病院分野は、強力なインフラ、患者数の増加、そして診断処置に対する保険適用により、2024年には48.6%という最大の市場シェアを獲得しました。
専門クリニック部門は、胃腸科ケアと個別感染管理への注目が高まることにより、予測期間中に8.9%という最も高いCAGRを記録すると予想されています。
尿素呼気検査市場の地域分析
- 2024年には、北米が世界の尿素呼気検査市場を独占し、最大の収益シェアの38.6%を占めた。
- この優位性は、主にこの地域の強力な医療インフラ、ヘリコバクターピロリ感染の増加、そして胃腸疾患の早期診断に関する意識の高まりに起因しています。
- 市場は、高い保険適用率、高度な診断ツールの広範な普及、そして強力な規制枠組みによって支えられています。さらに、非侵襲的な診断代替手段への需要の高まりが、地域全体で尿素呼気検査の導入を促進しています。
米国尿素呼気検査市場の洞察
米国の尿素呼気検査市場は、2024年に81%という最大の収益シェアを獲得しました。米国市場は、POC診断の普及、患者の認知度の高さ、そして尿素呼気検査サービスを提供する消化器科クリニックや病院の多さといった恩恵を受けています。有利な償還政策と、サーモフィッシャーサイエンティフィックやメリディアンバイオサイエンスといった主要メーカーの存在が、この分野における米国のリーダーシップを強化しています。さらに、H. pylori感染の正確かつ迅速な検出を求める医師の意識の高まりも、市場の成長を牽引し続けています。
欧州尿素呼気検査市場の洞察
欧州の尿素呼気検査市場は世界市場の大きな部分を占め、2024年には28.4%の収益シェアを獲得しました。この地域の成長は、消化器疾患の負担増加と、非侵襲的で信頼性の高い診断に対する高い需要によって牽引されています。ドイツ、英国、フランス、イタリアなどの国々は、外来診療や予防医療への移行を背景に、呼気検査技術の導入をリードしています。強力な政府による保健政策と早期発見法への投資も、この地域の市場規模の拡大に貢献しています。
英国尿素呼気検査市場の洞察
英国の尿素呼気検査市場は、2024年に欧州市場の21.6%を占めました。この成長は、公衆衛生意識の高まり、国民保健サービス(NHS)による全国的なスクリーニングプログラムの実施、そして費用対効果の高い診断方法への関心の高まりが主な要因です。英国の堅牢なデジタルヘルスインフラは、病院や診療所におけるPOC呼気検査キットの導入を容易にしています。
ドイツの尿素呼気検査市場の洞察
ドイツの尿素呼気検査市場は、高度な診断サービスへの需要の高まりと確立されたヘルスケアエコシステムにより、2024年には欧州市場シェアの約25.1%を占めると予測されています。同国では、消化器疾患の早期発見と外来診療への呼気検査ソリューションの統合を重視しており、これが市場の成長に大きく貢献しています。さらに、ドイツのイノベーションへの取り組みと医療費支出の多さも、尿素呼気検査の利用を促進しています。
アジア太平洋地域の尿素呼気検査市場に関する洞察
アジア太平洋地域の尿素呼気検査市場は、尿素呼気検査市場において最も急成長している地域として浮上しており、2025年から2032年にかけて11.4%の年平均成長率(CAGR)が予測されています。この地域は、2024年には世界全体の収益シェアの20.7%を占めました。この成長は、都市化の進展、中流階級人口の増加、そして胃腸の健康に対する意識の高まりによって牽引されています。中国、日本、インドなどの国々は、政府主導のスクリーニング・イニシアチブ、診断技術の進歩、そして都市部および準都市部における医療アクセスの拡大を通じて、市場拡大を牽引しています。
日本における尿素呼気検査市場の洞察
日本の尿素呼気検査市場は、2024年にアジア太平洋地域の尿素呼気検査市場の約29.4%を占めました。イノベーション主導の医療システムで知られる日本では、特に高齢化社会において、尿素呼気検査技術を日常的なスクリーニングに導入するケースが増えています。非侵襲的で正確な診断への日本の注力は患者の希望と一致しており、病院と在宅ケアの両方の現場で需要を促進しています。
中国尿素呼気検査市場の洞察
中国の尿素呼気検査市場はアジア太平洋地域で最大のシェアを占め、2024年には地域売上高の38.2%を占めました。中国における市場成長は、急速な都市化、医療保険の普及、そしてH.ピロリ菌関連疾患の罹患率の増加に起因しています。同国の堅固な製造能力と競争力のある価格設定により、公立病院、診療所、薬局などにおいて呼気検査キットが広く入手可能となっています。さらに、スマートヘルスケアへの取り組みや政府によるデジタル診断への注力も、市場の成長を支えています。
尿素呼気検査の市場シェア
尿素呼気検査業界は、主に、次のような定評のある企業によって牽引されています。
- セルコングループ(英国)
- Kibion GmbH(スウェーデン)
- アドバケア・ファーマ(米国)
- キズロン社(英国)
- メリディアン・バイオサイエンス社(米国)
- サーモフィッシャーサイエンティフィック社(米国)
- バイオ・ラッド・ラボラトリーズ社(米国)
- パラディンファーマ社(カナダ)
- Eurofins Scientific (ルクセンブルク)
- 北京理辰力科技有限公司(中国)
- 深セン中和ヘッドウェイバイオサイエンス&テクノロジー株式会社(中国)
世界の尿素呼気検査市場の最新動向
- 2021年、大塚アメリカファーマシューティカル社は、北米におけるBreathTek事業を、診断検査ソリューションおよびライフサイエンス原料の世界的リーディングプロバイダーであるメリディアン・バイオサイエンス社に譲渡しました。この戦略的買収により、メリディアンは特に消化器系診断分野において、市場における地位を強化することが期待されます。BreathTekポートフォリオを統合することで、メリディアンは提供内容を拡大し、この分野における既存の専門知識を活用することができます。この買収により、メリディアンは消化器系診断ソリューション分野における成長と競争力の強化を図ることができます。
SKU-
世界初のマーケットインテリジェンスクラウドに関するレポートにオンラインでアクセスする
- インタラクティブなデータ分析ダッシュボード
- 成長の可能性が高い機会のための企業分析ダッシュボード
- カスタマイズとクエリのためのリサーチアナリストアクセス
- インタラクティブなダッシュボードによる競合分析
- 最新ニュース、更新情報、トレンド分析
- 包括的な競合追跡のためのベンチマーク分析のパワーを活用
調査方法
データ収集と基準年分析は、大規模なサンプル サイズのデータ収集モジュールを使用して行われます。この段階では、さまざまなソースと戦略を通じて市場情報または関連データを取得します。過去に取得したすべてのデータを事前に調査および計画することも含まれます。また、さまざまな情報ソース間で見られる情報の不一致の調査も含まれます。市場データは、市場統計モデルと一貫性モデルを使用して分析および推定されます。また、市場シェア分析と主要トレンド分析は、市場レポートの主要な成功要因です。詳細については、アナリストへの電話をリクエストするか、お問い合わせをドロップダウンしてください。
DBMR 調査チームが使用する主要な調査方法は、データ マイニング、データ変数が市場に与える影響の分析、および一次 (業界の専門家) 検証を含むデータ三角測量です。データ モデルには、ベンダー ポジショニング グリッド、市場タイムライン分析、市場概要とガイド、企業ポジショニング グリッド、特許分析、価格分析、企業市場シェア分析、測定基準、グローバルと地域、ベンダー シェア分析が含まれます。調査方法について詳しくは、お問い合わせフォームから当社の業界専門家にご相談ください。
カスタマイズ可能
Data Bridge Market Research は、高度な形成的調査のリーダーです。当社は、既存および新規のお客様に、お客様の目標に合致し、それに適したデータと分析を提供することに誇りを持っています。レポートは、対象ブランドの価格動向分析、追加国の市場理解 (国のリストをお問い合わせください)、臨床試験結果データ、文献レビュー、リファービッシュ市場および製品ベース分析を含めるようにカスタマイズできます。対象競合他社の市場分析は、技術ベースの分析から市場ポートフォリオ戦略まで分析できます。必要な競合他社のデータを、必要な形式とデータ スタイルでいくつでも追加できます。当社のアナリスト チームは、粗い生の Excel ファイル ピボット テーブル (ファクト ブック) でデータを提供したり、レポートで利用可能なデータ セットからプレゼンテーションを作成するお手伝いをしたりすることもできます。