世界の薬物遺伝子検査市場の規模、シェア、トレンド分析レポート
Market Size in USD Billion
CAGR :
% 
 USD
610.34 Million
USD
1,346.87 Million
2024
2032
USD
610.34 Million
USD
1,346.87 Million
2024
2032
| 2025 –2032 | |
| USD 610.34 Million | |
| USD 1,346.87 Million | |
|
|
|
|
世界の薬物遺伝子検査市場のセグメンテーション、タイプ別(不安、うつ病、気分障害、双極性障害、摂食障害、精神病)、製品別(消耗品、機器、ソフトウェアおよびサービス)、検査タイプ別(全ゲノムシーケンシングおよび染色体アレイベースの検査)、遺伝子タイプ別(CYP2C19、CYP2C9およびVKORC1、CYP2D6、HLA-B、HTR2A/C、HLA-A、CYP3A4、SLC6A4、MTHFR、COMT、その他)、患者タイプ別(成人、高齢者、小児)、エンドユーザー別(病院および診療所、診断研究所、学術研究機関、その他)、流通チャネル別(直接入札、第三者流通、病院薬局、その他) - 2032年までの業界動向および予測
薬物遺伝子検査市場規模
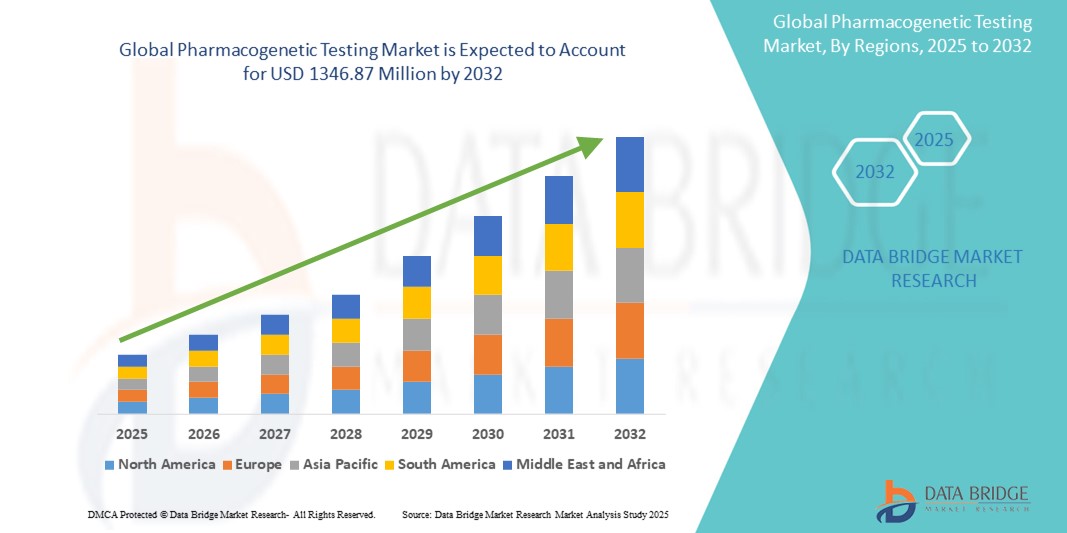
- 世界の薬物遺伝子検査市場は2024年に6億1,034万米ドルと評価され、 2032年までに1億3,4687万米ドルに達すると予想されています。
- 2025年から2032年の予測期間中、市場は主にパーソナライズ医療の採用増加により、年平均成長率10.4%で成長すると予想されます。
- この成長は、ゲノム技術の進歩、慢性疾患の罹患率の増加、個別化治療への意識の高まりなどの要因によって推進されている。
薬物遺伝子検査市場分析
- 消耗品セグメントが市場を支配すると予想されており、この優位性は、薬物遺伝子検査用の特殊な消耗品を必要とするカスタマイズされた医薬品の需要の増加に起因しています。
- この優位性は、薬物反応に影響を与える遺伝子変異を包括的に分析する能力によるもので、個別化された治療計画のための貴重な洞察を提供します。
- この遺伝子の変異はいくつかの抗うつ薬の代謝に重大な影響を及ぼすため、うつ病治療における薬理遺伝子検査の重要なターゲットとなっている。
- 病院と診療所が主要なエンドユーザーになると予想されます。その大きなシェアは、精神疾患患者の治療成績向上のために、薬物遺伝子検査が臨床診療にますます統合されていることに起因しています。
- たとえば、カナダのブリティッシュコロンビアで実施された研究では、抗うつ薬の薬理ゲノム検査により、患者にとって最も効果的な薬を見つけるのにかかる時間とコストを削減できることが実証されました。
レポートの範囲と薬物遺伝子検査市場のセグメンテーション
|
属性 |
薬理遺伝子検査の主要市場洞察 |
|
対象セグメント |
|
|
対象国 |
北米
ヨーロッパ
アジア太平洋
中東およびアフリカ
南アメリカ
|
|
主要な市場プレーヤー |
|
|
市場機会 |
|
|
付加価値データ情報セット |
Data Bridge Market Research がまとめた市場レポートには、市場価値、成長率、セグメンテーション、地理的範囲、主要プレーヤーなどの市場シナリオに関する洞察に加えて、専門家による詳細な分析、患者の疫学、パイプライン分析、価格分析、規制の枠組みも含まれています。 |
薬物遺伝子検査市場の動向
「薬物遺伝学的検査におけるAIとMLの統合の拡大」
- 薬物遺伝子検査市場では、遺伝子検査プラットフォームへの人工知能(AI)と機械学習(ML)の統合が進んでいます。これらの技術は、膨大な遺伝子データをより正確かつ効率的に分析するために利用されており、薬物遺伝子検査の迅速化とアクセス性の向上に役立っています。
- AIとMLアルゴリズムは、ゲノム検査から得られる大規模なデータセットを迅速に処理し、従来の分析手法では見逃される可能性のあるパターンや相関関係を特定することができます。これにより、薬理遺伝子検査の予測精度が向上し、うつ病や不安症などの精神疾患の患者に対するより効果的な治療戦略の策定に役立ちます。
- Tempusなどの企業は、AIを活用したプラットフォームを既に活用し、ゲノム配列を含む患者データを分析し、精神科患者に個別化された薬剤推奨を提供しています。薬物遺伝子検査を支援するAIプラットフォームの開発が進むにつれて、この傾向は拡大すると予想されます。
- AIのリアルタイムデータ解析能力により、薬物遺伝子検査は臨床試験や研究調査の最新の知見を取り入れて継続的に更新することができる。
- 例えば、イルミナのAI搭載ゲノムシーケンシングツールは臨床ワークフローに統合されており、医療提供者が患者への投薬処方に関してより迅速でデータに基づいた決定を下すのに役立っています。
- 薬物遺伝学的検査におけるAIとMLの使用は、遺伝子データの分析と解釈に関連するコストを削減する可能性を秘めている。
- 例えば、自動化されたデータ処理は、多くの場合大きなコスト要因となる手作業の必要性を減らし、さまざまな市場でテストをより手頃な価格で利用できるようにします。
薬物遺伝子検査市場の動向
ドライバ
「個別化医療の導入拡大」
- 個別化医療は、個人の遺伝子プロファイルに合わせて治療をカスタマイズすることに重点を置き、薬効の向上と副作用の軽減を図ることから、注目を集めています。これは特に精神医学において重要です。精神医学では、遺伝子変異のために標準的な治療がすべての人に有効とは限らないからです。
- 薬理遺伝子検査は、薬物代謝と反応に影響を与える遺伝子マーカーを特定するのに役立ちます。例えば、CYP2D6遺伝子の変異は、患者の抗うつ薬の代謝に影響を及ぼす可能性があります。これらの変異を特定することで、医師は副作用が少なく、患者に効果を発揮する可能性が高い薬を処方することができます。
- 次世代シーケンシング (NGS) などのゲノム技術の進歩により、薬物遺伝子検査はよりアクセスしやすく手頃な価格になり、臨床現場でのより広範な導入が促進されています。
- 例えば、米国では、サーモフィッシャーやイルミナなどの企業が遺伝子検査のコストを削減し、より多くの患者に個別化医療を届けようと取り組んでいます。
- うつ病や不安症といった精神疾患では、薬剤の効果が大きく異なるため、薬理遺伝子検査の必要性が高まっています。研究では、遺伝子検査によってうつ病患者が適切な抗うつ薬をより早く見つけられるようになり、治療における試行錯誤を減らすことができることが示されています。
- 例えば、ジェネレックスと米国の医療提供者との提携では、薬理遺伝子検査によって精神疾患の患者が遺伝子プロファイルに基づいて最適な薬を見つけるのを支援しています。
機会
「新興市場への進出」
- 新興市場、特にアジア太平洋地域とラテンアメリカでは、経済発展と政府の取り組みにより、医療へのアクセスが急速に拡大しています。その結果、薬物遺伝子検査などの高度な診断ツールのニーズが高まっています。
- 中国やインドなどの国では、うつ病や双極性障害といった精神疾患の増加が大きな人口を占めています。薬物遺伝子検査は、これらの地域における個別化治療戦略の必要性に応えるのに役立ちます。
- 新興市場の政府は、遺伝子検査技術への資金提供を含め、医療インフラへの投資を増やしています。
- 技術の進歩と官民連携の拡大により検査コストが削減され、薬物遺伝子検査は新興市場でより利用しやすくなってきています。
- 例えば、イルミナはゲノムシーケンシングサービスをアジア諸国に拡大し、個別化医療をより手頃な価格にしている。
- 例えば、QIAGENはインドと中国に進出し、精神疾患の個別治療を強化する取り組みの一環として薬物遺伝子検査をサポートし、十分なサービスが提供されていない市場に参入しています。
抑制/挑戦
「テストコストの高さとインフラの限界」
- 薬理遺伝子検査の普及における最も重大な課題の一つは、遺伝子検査の高コストである。
- 例えば、全ゲノム配列解析の費用は、特に発展途上国では多くの患者にとって依然として法外なほど高額である可能性がある。
- 多くの新興市場では、薬物遺伝子検査を実施するための専門研究所や訓練を受けた人員といった必要なインフラが不足しています。そのため、複雑な精神疾患の管理に最も必要とされる農村部や資源の乏しい地域では、検査の実施が制限されています。
- 多くの国では、保険会社が薬物遺伝子検査の費用を全額負担しておらず、患者は自己負担となっています。これは、医療制度の資金が不足している国では特に問題となります。
- 例えば、米国では薬物遺伝子検査への関心が高まっているにもかかわらず、多くの保険会社はうつ病などの精神疾患の費用を未だにカバーしていない。
- 遺伝子検査が利用可能であっても、その結果を解釈し、治療計画に組み込むことは複雑なプロセスです。これには高度な訓練を受けた専門家が必要であり、一部の地域ではそのような専門家の不足が、薬物遺伝子検査の効果的な活用を妨げている可能性があります。
- 例えば、オーストラリアのソニックヘルスケアは、コストの高さと、検査プロセスを処理するための追加のインフラストラクチャと人員の必要性のために、すべての施設で薬物遺伝子検査を導入することに限界を感じていました。
薬理遺伝子検査市場の展望
市場は、基本タイプ、製品、テストタイプ、遺伝子タイプ、患者タイプ、エンドユーザー、流通チャネルによって分割されています。
|
セグメンテーション |
サブセグメンテーション |
|
タイプ別 |
|
|
製品別 |
|
|
テストの種類別 |
|
|
遺伝子型別
|
|
|
患者タイプ別 |
|
|
エンドユーザー別
|
|
|
流通チャネル別 |
|
薬物遺伝子検査市場の地域分析
「北米は薬物遺伝子検査市場において主要な地域である」
- 北米は精神医学とうつ病の薬物遺伝子検査市場において優位性を維持すると予測されている。
- この地域は高度な医療インフラと研究開発への多額の投資を誇り、薬物遺伝子検査を臨床診療に統合することを促進している。
- 支援的な規制枠組みと償還政策は、薬物遺伝子検査を含む個別化医療アプローチの採用を促進する上で重要な役割を果たしてきた。
- 北米の病院や診療所では、精神科治療をカスタマイズし、患者の転帰を向上させ、薬物の有害反応を減らすために、薬物遺伝子検査を取り入れるケースが増えています。
- これらの要因を考慮すると、北米は今後数年間、世界市場をリードし続けると予想されます。
「アジア太平洋地域は最も高い成長率を記録すると予測される」
- アジア太平洋地域は、予測期間中に精神医学およびうつ病の薬物遺伝子検査市場において最も高い成長率を経験すると予測されています。
- 中国やインドなどの国では急速な経済成長が見られ、医療へのアクセスが向上し、高度な診断サービスの需要が高まっています。
- この地域の高齢者人口の増加は精神疾患の有病率の上昇に寄与しており、個別化された治療オプションの必要性が高まっている。
- 地元の診断企業と世界的リーダーとの協力、そして医療インフラへの投資の増加により、薬物遺伝子検査の導入が加速している。
- 政府の支援政策と償還制度により、地域全体で薬物遺伝子検査の精神科医療への統合がさらに促進されている。
薬物遺伝子検査市場シェア
市場競争環境は、競合他社ごとに詳細な情報を提供します。企業概要、財務状況、収益、市場ポテンシャル、研究開発投資、新規市場への取り組み、グローバルプレゼンス、生産拠点・設備、生産能力、強みと弱み、製品投入、製品群の幅広さ、アプリケーションにおける優位性などの詳細が含まれます。上記のデータは、各社の市場への注力分野にのみ関連しています。
市場で活動している主要なマーケットリーダーは次のとおりです。
- サーモフィッシャーサイエンティフィック株式会社(米国)
- イルミナ社(米国)
- ミリアド・ジェネティクス社(米国)
- ソニック・ヘルスケア・リミテッド(オーストラリア)
- QIAGEN(ドイツ)
- AB-BIOTICS SA(スペイン)
- バイオジェニック社(インド)
- キャッスル・バイオサイエンス社(米国)
- コリエルライフサイエンス(米国)
- ダイナミックDNAラボラトリーズ(米国)
- Eurofins Scientific (ルクセンブルク)
- ジェネレックス(米国)
- ジェネビス(米国)
- ジェノマインド株式会社(米国)
- GenXys(カナダ)
- ヘルススペック(米国)
- ハドソンアルファ(米国)
- MDラボ(米国)
- ONEOME LLC(米国)
- PacBio(米国)
世界の薬物遺伝子検査市場の最新動向
- 2024年2月、遺伝子検査と精密医療のパイオニアであるMyriad Genetics , Inc.は、Intermountain HealthのPrecision Genomics(IPG)研究所から厳選された資産を買収しました。これには、Precise Tumor Test、Precise Liquid Test、そしてユタ州セントジョージにあるIPGのCLIA認定研究所が含まれます。この買収により、Myriadは腫瘍プロファイリングサービスを強化し、遺伝性癌やコンパニオン診断検査オプションを含む腫瘍学ポートフォリオを拡大することができます。
- 2024年2月、QIAGENは、科学研究における持続可能性の促進に注力する非営利団体My Green Labから、持続可能性への取り組みが認められたことを発表しました。これにより、QIAGENの持続可能性に関する評判が向上し、環境意識の高い顧客やパートナーを引き付けることができる可能性があります。
- 2023年11月、QIAGENとDNA Labs Internationalは、QIAGENの法医学遺伝子系譜学向けForenSeq KintelligenceシステムとGEDmatch PROデータベースを連携させ、20年前の未解決事件の解決に協力しました。これは、このアプローチが人物特定に有効であり、採用が拡大していることを示唆しています。これにより、QIAGENの法医学遺伝子系譜学ソリューションの有効性が高まり、法医学市場における採用と売上が拡大する可能性があります。
- 2022年6月、患者ケアを導く革新的な検査を通じて健康を改善する企業であるCastle Biosciences , Incは本日、 AltheaDx , Inc.を買収する正式契約を締結したことを発表しました。この買収により、現在うつ病のメディケア償還を受けている検査から始めて、メンタルヘルスフランチャイズを開発できるようになります。
- 2022年5月、コリエル・ ライフ サイエンスは、薬理ゲノミクスの力を活用して医療を改善する先駆者として、第2回メドテック・ブレークスルー・アワードのゲノミクス・イノベーション部門を受賞しました。同社のコリゲン・メディケーション・セーフティ・プログラムは、2022年メドテック・ブレークスルー・アワードにおいて「最優秀ゲノミクス・ソリューション」に選出されました。この受賞により、検査の信頼性がユーザーの間でさらに高まります。
SKU-
世界初のマーケットインテリジェンスクラウドに関するレポートにオンラインでアクセスする
- インタラクティブなデータ分析ダッシュボード
- 成長の可能性が高い機会のための企業分析ダッシュボード
- カスタマイズとクエリのためのリサーチアナリストアクセス
- インタラクティブなダッシュボードによる競合分析
- 最新ニュース、更新情報、トレンド分析
- 包括的な競合追跡のためのベンチマーク分析のパワーを活用
調査方法
データ収集と基準年分析は、大規模なサンプル サイズのデータ収集モジュールを使用して行われます。この段階では、さまざまなソースと戦略を通じて市場情報または関連データを取得します。過去に取得したすべてのデータを事前に調査および計画することも含まれます。また、さまざまな情報ソース間で見られる情報の不一致の調査も含まれます。市場データは、市場統計モデルと一貫性モデルを使用して分析および推定されます。また、市場シェア分析と主要トレンド分析は、市場レポートの主要な成功要因です。詳細については、アナリストへの電話をリクエストするか、お問い合わせをドロップダウンしてください。
DBMR 調査チームが使用する主要な調査方法は、データ マイニング、データ変数が市場に与える影響の分析、および一次 (業界の専門家) 検証を含むデータ三角測量です。データ モデルには、ベンダー ポジショニング グリッド、市場タイムライン分析、市場概要とガイド、企業ポジショニング グリッド、特許分析、価格分析、企業市場シェア分析、測定基準、グローバルと地域、ベンダー シェア分析が含まれます。調査方法について詳しくは、お問い合わせフォームから当社の業界専門家にご相談ください。
カスタマイズ可能
Data Bridge Market Research は、高度な形成的調査のリーダーです。当社は、既存および新規のお客様に、お客様の目標に合致し、それに適したデータと分析を提供することに誇りを持っています。レポートは、対象ブランドの価格動向分析、追加国の市場理解 (国のリストをお問い合わせください)、臨床試験結果データ、文献レビュー、リファービッシュ市場および製品ベース分析を含めるようにカスタマイズできます。対象競合他社の市場分析は、技術ベースの分析から市場ポートフォリオ戦略まで分析できます。必要な競合他社のデータを、必要な形式とデータ スタイルでいくつでも追加できます。当社のアナリスト チームは、粗い生の Excel ファイル ピボット テーブル (ファクト ブック) でデータを提供したり、レポートで利用可能なデータ セットからプレゼンテーションを作成するお手伝いをしたりすることもできます。