欧州多発性遺伝性骨腫市場の規模、シェア、動向分析レポート
Market Size in USD Billion
CAGR :
% 
 USD
37.42 Million
USD
49.65 Million
2024
2032
USD
37.42 Million
USD
49.65 Million
2024
2032
| 2025 –2032 | |
| USD 37.42 Million | |
| USD 49.65 Million | |
|
|
|
|
欧州多発性遺伝性骨腫市場のセグメンテーション、タイプ別(無柄性および有茎性)、治療(手術、薬物療法、その他)、診断(X線、コンピューター断層撮影(CT)スキャン、磁気共鳴画像(MRI)、遺伝子検査、その他)、部位別(脚、腕、肩、骨盤、指、つま先)、年齢層別(小児および成人)、エンドユーザー別(病院、専門クリニック、外来手術センター、その他) - 2032年までの業界動向と予測
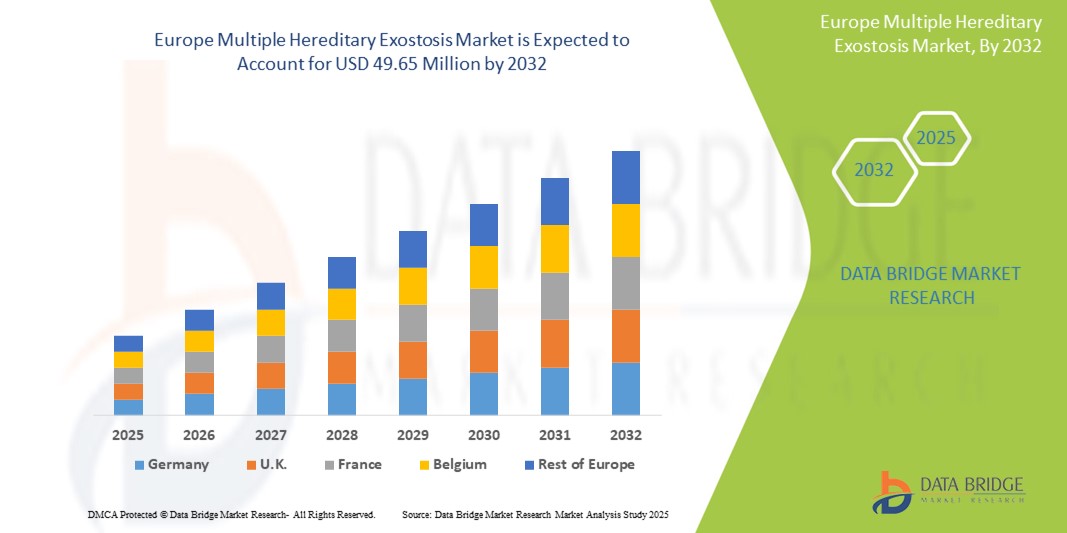
欧州多発性遺伝性骨腫市場規模
- ヨーロッパの多発性遺伝性骨腫市場規模は2024年に3,742万米ドルと評価され、予測期間中に3.60%のCAGRで成長し、2032年には4,965万米ドル に達すると予想されています 。
- 市場の成長は、主に希少遺伝性疾患に対する認知度と診断率の上昇、そして欧州諸国における高度な遺伝子検査へのアクセス向上によって推進されている。
- さらに、希少疾病用医薬品開発プログラムの拡大、患者支援、そして希少疾患研究に対する規制当局の支援が相まって、治療革新が推進されています。これらの要因が重なり合い、よりターゲットを絞った医療環境が促進され、欧州における多発性遺伝性骨腫市場の成長を大きく支えています。
欧州多発性遺伝性骨腫市場分析
- 多発性遺伝性骨腫症(MHE)は、良性の骨腫瘍を特徴とする稀な遺伝性の筋骨格疾患であり、遺伝子診断の進歩と小児および成人集団における臨床監視の改善により、欧州の医療現場でますます認知度が高まっています。
- 早期診断と患者固有の治療アプローチに対する需要の高まりは、主に希少疾患登録の拡大、医師の意識の向上、そしてヨーロッパ全土の患者擁護団体と研究機関の積極的な協力によって促進されている。
- ドイツは、堅牢な医療インフラ、十分に発達した希少疾患ネットワーク、専門的な遺伝子研究センターや臨床試験拠点の存在に支えられ、2024年には24.8%という最大の収益シェアでヨーロッパの多発性遺伝性骨腫市場を支配しました。
- ポーランドは、公的医療サービスの大幅な改善、希少疾患診断への投資の増加、高度な遺伝子スクリーニングプログラムへのアクセスの拡大により、予測期間中にヨーロッパの多発性遺伝性骨腫市場で最も急速に成長する国になると予想されています。
- ヨーロッパの多発性遺伝性骨腫市場では、骨付着部の基盤が広く、臨床的有病率が高く、放射線学的検出が容易なことから、2024年には62.5%の市場シェアを獲得し、無柄骨セグメントが市場を支配した。
レポートの範囲とヨーロッパの多発性遺伝性骨腫市場のセグメンテーション
|
属性 |
欧州における多発性遺伝性骨腫症の主要市場分析 |
|
対象セグメント |
|
|
対象国 |
ヨーロッパ
|
|
主要な市場プレーヤー |
|
|
市場機会 |
|
|
付加価値データ情報セット |
データブリッジマーケットリサーチがまとめた市場レポートには、市場価値、成長率、セグメンテーション、地理的範囲、主要プレーヤーなどの市場シナリオに関する洞察に加えて、専門家による詳細な分析、価格設定分析、ブランドシェア分析、消費者調査、人口統計分析、サプライチェーン分析、バリューチェーン分析、原材料/消耗品の概要、ベンダー選択基準、PESTLE分析、ポーター分析、規制の枠組みも含まれています。 |
欧州における多発性遺伝性骨腫市場の動向
「遺伝子診断と個別化医療の進歩」
- 欧州の多発性遺伝性骨腫(MHE)市場における重要なトレンドとして、早期発見と長期的な疾患管理の改善を目的とした高度な遺伝子診断と個別化治療経路の利用増加が挙げられます。この進歩は、ゲノム医療の技術進歩と、欧州の医療システム全体における次世代シーケンシングへのアクセス拡大によって推進されています。
- 例えば、ドイツとフランスのいくつかの大学病院では、小児骨格疾患の標準的な診断プロトコルに全エクソーム配列解析を統合し、リスクのある家族におけるMHEの早期かつ正確な特定を可能にしています。
- 個別化ケアモデルも登場しており、各医療機関は個々の遺伝子プロファイルと腫瘍の増殖パターンに基づいて手術計画と術後リハビリテーションをカスタマイズしています。欧州希少骨疾患レジストリ(EuRR-Bone)などの希少疾患レジストリの普及により、疾患の進行、治療成果、ケアの標準化に関するデータに基づく洞察がさらに可能になっています。
- これらの診断と治療の革新により、MHEは従来診断が不十分だった状態から、精密医療のアプローチで管理される状態へと変化しています。
- その結果、欧州の医療提供者は、整形外科、遺伝学、放射線学、理学療法を統合した多科医療の枠組みを採用しています。その結果、企業や研究機関は、MHEの早期管理に特化したツールや治療プロトコルの開発に向けた共同研究に投資しています。
- 積極的な遺伝子スクリーニングへの傾向と、希少疾患治療における汎欧州的調和の推進により、地域全体のMHE患者の生活の質と臨床結果が改善されると期待されます。
欧州における多発性遺伝性骨腫市場の動向
ドライバ
「認知度の高まり、早期診断、そして希少疾病用医薬品の開発」
- 臨床医と一般の人々の両方における希少遺伝性疾患に対する意識の高まりと早期診断能力の向上は、ヨーロッパのMHE市場の成長の重要な原動力となっている。
- 例えば、英国やドイツなどの国では、国家的な取り組みとして、特に骨格異常の既往歴のある家族を対象に早期スクリーニングプログラムを積極的に支援し、タイムリーな介入を通じて患者の転帰を改善しています。
- さらに、欧州医薬品庁(EMA)の優遇措置による希少疾病用医薬品の開発拡大は、これまで標的治療の選択肢がなかったMHEなどの疾患への製薬投資を促進している。
- 政府が支援する希少疾患の枠組みと超希少疾患の治療薬を開発する企業に対する財政的インセンティブは、好ましい研究環境を育んでいる。
- さらに、Eurodisなどの患者擁護団体と臨床研究センターとの連携により、より広範なケアへのアクセスが促進され、登録や臨床研究への患者の参加が増加し、疾患管理に関するより深い洞察がもたらされています。
抑制/挑戦
「限られた治療選択肢と専門治療の高額な費用」
- 認知度の高まりと診断の進歩にもかかわらず、ヨーロッパの多発性遺伝性骨腫市場は、治療薬の入手が限られていることと、特に外科的介入と長期フォローアップのための専門治療の費用が高いことから、課題に直面しています。
- 例えば、骨腫の外科的除去が依然として主な治療法である一方、腫瘍の形成や進行を防ぐための承認された薬理学的治療法はなく、大きな治療ギャップが浮き彫りになっている。
- 繰り返しの画像診断、遺伝子検査、整形外科手術に伴う経済的負担は、特に希少疾患の償還政策がまだ発展途上にある国では、相当なものとなる可能性がある。
- さらに、ヨーロッパ全域における専門医療センターへのアクセスの格差は、都市化が遅れている地域や低所得地域の患者が診断の遅れや多科医療へのアクセスの制限に直面する可能性があることを意味している。
- 希少疾患治療への資金提供の改善、非侵襲的治療オプションの開発、資源共有における汎欧州協力を通じてこれらの障壁に対処することは、公平なアクセスと持続的な市場発展を確保する上で不可欠です。
欧州における多発性遺伝性骨腫市場の展望
市場は、タイプ、治療、診断、場所、年齢層、エンドユーザーに基づいてセグメント化されています。
- タイプ別
欧州の多発性遺伝性骨腫市場は、種類別に無柄型と有柄型の2つに分類されます。無柄型セグメントは、骨付着基盤が広いため臨床発見率が高く、医療処置を必要とする合併症の発生率も高いことから、2024年には62.5%という最大の市場収益シェアを獲得し、市場を席巻しました。無柄型骨腫は、X線画像でより一般的に観察され、骨への影響が大きい場合が多いため、早期診断と早期治療が求められます。
有柄セグメントは、予測期間中に最も急速に成長すると予想されており、外部から目立つことが多く、サイズや解剖学的位置に応じて個別の外科的アプローチを必要とする骨の突起などの柄が含まれます。
- 治療別
欧州の多発性遺伝性骨腫市場は、治療方法に基づいて、外科手術、薬物療法、その他に分類されます。2024年には、薬物療法が市場シェアの55.0%を占め、最大の収益シェアを占めました。これは、薬物療法が確立されていないこと、そして症状のある骨腫や変形性骨腫の管理において外科的切除への依存度が高いことが原因です。小児患者においては、長期的な運動機能障害の予防や骨格のずれの矯正のために、外科的介入が必要となる場合が多くあります。
医薬品分野は、鎮痛および炎症抑制に関する研究の増加に支えられ、2025年から2032年にかけて最も急速な成長を遂げると予想されています。医薬品は治癒を目的としたものではありませんが、手術の補助として、あるいは保存的治療計画の一環として頻繁に使用されます。
- 診断別
診断に基づいて、欧州の多発性遺伝性骨腫市場は、X線、コンピュータ断層撮影(CT)、磁気共鳴画像(MRI)、遺伝子検査、その他に分類されます。X線検査は、アクセス性、費用対効果、そして骨増殖の検出における有効性から、依然として主要な診断ツールであり、2024年には45.0%という最大の市場収益シェアを獲得しました。X線検査は、初期評価と継続的なモニタリングに広く利用されています。
遺伝子検査セグメントは、分子診断の進歩、次世代シーケンシングの利用可能性の向上、および特にMHEの病歴が知られている家族に対する積極的な遺伝カウンセリングにより、2025年から2032年にかけて最も速い速度で成長すると予想されています。
- サイト別
欧州の多発性遺伝性骨腫市場は、部位別に、脚、腕、肩、骨盤、指、つま先に区分されています。脚部セグメントは、大腿骨や脛骨などの長骨が最も多く罹患し、歩行困難、脚長差、早期外科的介入につながることが多いため、2024年には48.0%という最大のシェアを占め、市場を席巻しました。
腕と肩の有病率は次に高く、予測期間中に最も急速に増加すると予想され、罹患率に大きく寄与し、手足の機能と可動域を回復するために矯正整形外科手術が必要になる場合が多い。
- 年齢別
年齢層に基づいて、欧州の多発性遺伝性骨腫市場は小児と成人に区分されています。小児セグメントは2024年に68.0%と最大の市場シェアを占めました。これは、この疾患が通常、幼少期に診断され、成長期を通して継続的なモニタリングと介入が必要となるためです。早期発見は、骨格発達における合併症や変形を最小限に抑える上で非常に重要です。
成人セグメントは予測期間中に最も急速な成長が見込まれ、末期合併症、残存変形、または二次手術のフォローアップを継続する患者が含まれ、ヘルスケアサービスの長期的需要に貢献します。
- エンドユーザー別
エンドユーザーに基づいて、欧州の多発性遺伝性骨腫市場は、病院、専門クリニック、外来手術センター、その他に分類されます。病院セグメントは、診断、外科治療、遺伝カウンセリング、そして長期ケアの主要拠点としての役割を担っていることから、2024年には58.0%と最大の収益シェアを占めました。多職種連携チームと高度な画像診断および外科的リソースへのアクセスにより、病院はMHEの標準的なケア環境となっています。
整形外科や遺伝学の専門センターが特に都市部の医療拠点で普及するにつれ、専門クリニック部門は予測期間中に最も急速に成長すると予想されています。
欧州多発性遺伝性骨腫市場の地域分析
- ドイツは、堅牢な医療インフラ、十分に発達した希少疾患ネットワーク、専門的な遺伝子研究センターや臨床試験拠点の存在に支えられ、2024年には24.8%という最大の収益シェアでヨーロッパの多発性遺伝性骨腫市場を支配しました。
- 国内の患者と医療提供者は、包括的な償還政策と希少疾患登録および臨床試験への積極的な参加の恩恵を受けており、これらはMHEの早期診断と長期にわたる調整されたケアをサポートしています。
- ドイツのリーダーシップは、大学病院、整形外科専門医、遺伝子研究機関の強力なネットワークによってさらに強化され、ヨーロッパ全体の遺伝性骨格疾患の分野における革新と治療の中心地となっています。
ドイツにおける多発性遺伝性骨腫市場の洞察
ドイツの多発性遺伝性骨腫(MHE)市場は、2024年にヨーロッパで最大の収益シェアを獲得しました。これは、同国の充実した医療制度、遺伝性疾患に対する高い意識、そして先進的な診断ツールの早期導入に牽引されています。一流の大学医療センターや整形外科研究機関の存在が、早期診断と包括的な治療を促進しています。希少疾患登録への政府支援とオーファンドラッグ開発への資金提供は、遺伝性筋骨格疾患の管理におけるドイツのリーダーとしての地位をさらに強化しています。
英国における多発性遺伝性骨腫市場の洞察
英国のMHE市場は、希少疾患のスクリーニングと多科医療を重視する統合的な公的医療制度(NHS)に支えられ、予測期間を通じて着実に成長すると予測されています。国民の意識向上と患者支援活動、そして欧州および世界の希少疾患ネットワークへの積極的な参加が、早期診断と医療へのアクセス向上を促進しています。小児整形外科サービスにおけるイノベーションと、標準診療への遺伝カウンセリングの導入は、市場拡大に大きく貢献すると期待されます。
ポーランドにおける多発性遺伝性骨腫市場の洞察
ポーランドの多発性遺伝性骨腫症(MHE)市場は、医療インフラの継続的な改善と希少疾患診断への公的投資の増加に支えられ、予測期間中にヨーロッパで最も高いCAGRで成長すると予想されています。特に大学付属病院や地域の診断センターにおける遺伝子検査へのアクセス拡大は、遺伝性骨疾患の早期発見に貢献しています。EUの希少疾患戦略への国家的な取り組みと、専門整形外科医療提供者の増加は、国全体でより広範な治療へのアクセスと患者管理を支えています。
欧州における多発性遺伝性骨腫の市場シェア
欧州の多発性遺伝性骨腫業界は、主に以下の企業を含む定評ある企業によって牽引されています。
- バイエルAG(ドイツ)
- ハレオングループ(英国)
- BASF(ドイツ)
- ビアトリス社(米国)
- マリンクロット(米国)
- ProQR Therapeutics NV(オランダ)
- オクタファーマAG(スイス)
- SOMバイオテック(スペイン)
- TiGenix NV(ベルギー)
- ウィルソン・セラピューティクスAB(スウェーデン)
- ファーネクスト(フランス)
- アルケマブ・セラピューティクス(英国)
- iOnctura(スイス)
- ジェネトン(フランス)
- ジェノサイエンスファーマ(フランス)
- ジェンサイト・バイオロジクス(フランス)
- ブーストファーマ(スウェーデン)
- キエージ・ファルマチェウティチ(イタリア)
- スウィックス・バイオファーマ(スイス)
- フェイト・セラピューティクス(米国)
ヨーロッパの多発性遺伝性骨腫市場の最近の動向は何ですか?
- 2024年5月、ドイツ小児整形外科学会は、シャリテ・ベルリン大学医学部と共同研究プログラムを開始しました。このプログラムは、小児における複雑な多発性遺伝性骨腫(MHE)症例に対する外科手術技術の向上を目指しています。この取り組みは、低侵襲手術の開発、術後ケアプロトコルの改善、可動性の向上、再発率の低減に重点を置いています。この開発は、小児希少疾患治療におけるドイツのリーダーシップと、MHE治療における外科的イノベーションへの献身を浮き彫りにするものです。
- 2024年4月、フランスのキュリー研究所は、術後MHE患者を対象とした個別化理学療法プログラムの長期的な有効性を評価する多施設共同臨床試験を開始しました。このプログラムでは、AI駆動型動作追跡ツールを統合し、回復と機能改善のモニタリングを行います。この研究は、遺伝性骨格疾患の管理において、患者中心のケアアプローチとテクノロジーを統合するというフランスの取り組みを強調するものです。
- 英国国民保健サービス(NHS)は2024年3月、多発性遺伝性骨腫症をゲノム医療サービスの希少疾患診断パスウェイに追加しました。この新しい政策により、MHEの疑いのある患者とその家族は全ゲノムシークエンシングを無料で受けられるようになり、早期診断と精密医療が支援されます。この戦略的な追加は、希少疾患ゲノミクスの推進と医療の公平性向上に向けた英国の積極的な姿勢を反映しています。
- 2024年2月、イタリアのリッツォーリ整形外科研究所は、小児のMHEの早期発見に特化した画像診断プロトコルの開発を発表しました。高解像度3D画像診断技術を活用したこのプロトコルは、診断の曖昧さを軽減し、術前計画の改善を目指しています。イタリアの画像診断技術への投資は、整形外科研究と個別化医療におけるイタリアの役割の拡大を反映しています。
- 2024年1月、スペインのFundación Española de Enfermedades Raras(FEDER)は、地方保健当局と提携し、MHEを含む遺伝性骨疾患に関する全国的な啓発キャンペーンを開始しました。このキャンペーンは、早期発見の改善、遺伝子検査の促進、診断遅延の削減を目指しています。この取り組みは、スペインにおける地域密着型の教育と希少疾患擁護への取り組みの強化を象徴しています。
SKU-
世界初のマーケットインテリジェンスクラウドに関するレポートにオンラインでアクセスする
- インタラクティブなデータ分析ダッシュボード
- 成長の可能性が高い機会のための企業分析ダッシュボード
- カスタマイズとクエリのためのリサーチアナリストアクセス
- インタラクティブなダッシュボードによる競合分析
- 最新ニュース、更新情報、トレンド分析
- 包括的な競合追跡のためのベンチマーク分析のパワーを活用
目次
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF THE EUROPE MULTIPLE HEREDITARY EXOSTOSIS MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATIONS
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 DBMR TRIPOD DATA VALIDATION MODEL
2.5 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.6 MULTIVARIATE MODELLING
2.7 MARKET END USER COVERAGE GRID
2.8 PRODUCT LIFELINE CURVE
2.9 DBMR MARKET POSITION GRID
2.1 VENDOR SHARE ANALYSIS
2.11 SECONDARY SOURCES
2.12 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PORTER’S FIVE FORCES
4.2 PESTEL ANALYSIS
4.3 PIPELINE ANALYSIS - OBSERVATORY DATA
4.4 EPIDEMIOLOGY
5 EUROPE MULTIPLE HEREDITARY EXOSTOSIS MARKET: REGULATIONS
5.1 REGULATION IN UNITED STATES: U.S. FOOD AND DRUG ADMINISTRATION (FDA)
5.1.1 REGULATION IN EUROPE: EUROPEAN MEDICINES AGENCY (EMA)
5.2 REGULATION IN ASIA-PACIFIC (JAPAN): PHARMACEUTICAL AND MEDICAL DEVICES AGENCY (PMDA)
5.3 MEDICAL DEVICE STANDARDS
6 MARKET OVERVIEW
6.1 DRIVERS
6.1.1 RISING PREVALENCE OF GENETIC DISORDERS
6.1.2 GROWING PEDIATRIC POPULATION
6.1.3 DEVELOPMENT OF NOVEL THERAPIES
6.2 RESTRAINTS
6.2.1 HIGH COST OF ADVANCED THERAPIES
6.2.2 LIMITED AVAILABILITY OF THERAPIES
6.3 OPPORTUNITIES
6.3.1 INCREASE IN PATIENT CARE AND SUPPORT SYSTEMS
6.3.2 INCREASE IN THE NUMBER OF COLLABORATIONS AND PARTNERSHIPS
6.4 CHALLENGES
6.4.1 LIMITED AWARENESS ABOUT THE DISORDER
6.4.2 LACK OF DRUG APPROVALS ASSOCIATED WITH THE DISORDER
7 EUROPE MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE
7.1 OVERVIEW
7.2 SESSILE
7.3 PEDUNCULATED
8 EUROPE MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TREATMENT
8.1 OVERVIEW
8.2 SURGERY
8.2.1 REMOVE THE TUMOR
8.2.2 LENGTHEN LIMBS
8.3 MEDICATION
8.3.1 HOSPITAL PHARMACIES
8.3.2 DRUGS STORES AND RETAIL PHARMACIES
8.3.3 ONLINE PHARMACIES
8.4 OTHERS
9 EUROPE MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DIAGNOSIS
9.1 OVERVIEW
9.2 X-RAY
9.2.1 SESSILE
9.2.2 PEDUNCULATED
9.3 COMPUTED TOMOGRAPHY (CT) SCAN
9.3.1 SESSILE
9.3.2 PEDUNCULATED
9.4 MAGNETIC RESONANCE IMAGING (MRI)
9.4.1 SESSILE
9.4.2 PEDUNCULATED
9.5 GENETIC TESTS
9.5.1 SESSILE
9.5.2 PEDUNCULATED
9.6 OTHERS
9.6.1 SESSILE
9.6.2 PEDUNCULATED
10 EUROPE MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY SITE
10.1 OVERVIEW
10.2 LEGS
10.3 ARMS
10.4 SHOULDERS
10.5 PELVIS
10.6 FINGERS
10.7 TOES
11 EUROPE MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY AGE GROUP
11.1 OVERVIEW
11.2 PEDIATRIC
11.3 ADULT
12 EUROPE MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY END USER
12.1 OVERVIEW
12.2 HOSPITAL
12.2.1 PRIVATE
12.2.2 GOVERNMENT
12.3 SPECIALTY CLINICS
12.4 AMBULATORY SURGICAL CENTERS
12.5 OTHERS
13 EUROPE MULTIPLE HEREDITY EXOSTOSIS MARKET, BY REGION
13.1 EUROPE
13.1.1 GERMANY
13.1.2 U.K
13.1.3 FRANCE
13.1.4 ITALY
13.1.5 SPAIN
13.1.6 RUSSIA
13.1.7 TURKEY
13.1.8 SWITZERLAND
13.1.9 BELGIUM
13.1.10 NETHERLAND
13.1.11 DENMARK
13.1.12 SWEDEN
13.1.13 POLAND
13.1.14 NORWAY
13.1.15 FINLAND
13.1.16 REST OF EUROPE
14 EUROPE MULTIPLE HEREDITARY EXOSTOSIS MARKET: COMPANY LANDSCAPE
14.1 COMPANY SHARE ANALYSIS: EUROPE
15 SWOT ANALYSIS
16 COMPANY PROFILES
16.1 BAYERS AG
16.1.1 COMPANY SNAPSHOT
16.1.2 REVENUE ANALYSIS
16.1.3 COMPANY SHARE ANALYSIS
16.1.4 PRODUCT PORTFOLIO
16.1.5 RECENT UPDATES
16.2 HALEON GROUP OF COMPANIES
16.2.1 COMPANY SNAPSHOT
16.2.2 REVENUE ANALYSIS
16.2.3 COMPANY SHARE ANALYSIS
16.2.4 PRODUCT PORTFOLIO
16.2.5 RECENT DEVELOPMENTS
16.3 BASF
16.3.1 COMPANY SNAPSHOT
16.3.2 REVENUE ANALYSIS
16.3.3 COMPANY SHARE ANALYSIS
16.3.4 PRODUCT PORTFOLIO
16.3.5 RECENT UPDATES
16.4 VIATRIS INC.
16.4.1 COMPANY SNAPSHOT
16.4.2 REVENUE ANALYSIS
16.4.3 COMPANY SHARE ANALYSIS
16.4.4 PRODUCT PORTFOLIO
16.4.5 RECENT UPDATES
16.5 ACTIZAPHARMA
16.5.1 COMPANY SNAPSHOT
16.5.2 PRODUCT PORTFOLIO
16.5.3 RECENT UPDATES
16.6 ADVACARE PHARMA
16.6.1 COMPANY SNAPSHOT
16.6.2 PRODUCT PORTFOLIO
16.6.3 RECENT UPDATES
16.7 AUROBINDO PHARMA
16.7.1 COMPANY SNAPSHOT
16.7.2 REVENUE ANALYSIS
16.7.3 PRODUCT PORTFOLIO
16.7.4 RECENT TABLETS
16.8 HALEON GROUP OF COMPANIES
16.8.1 COMPANY SNAPSHOT
16.8.2 REVENUE ANALYSIS
16.8.3 PRODUCT PORTFOLIO
16.8.4 RECENT UPDATES
16.9 IPSEN BIOPHARMACEUTICALS, INC.
16.9.1 COMPANY SNAPSHOT
16.9.2 REVENUE ANALYSIS
16.9.3 PRODUCT PORTFOLIO
16.9.4 RECENT UPDATES
16.1 MALLINCKRODT
16.10.1 COMPANY SNAPSHOT
16.10.2 REVENUE ANALYSIS
16.10.3 PRODUCT PORTFOLIO
16.10.4 RECENT UPDATES
16.11 TEVA PHARMACEUTICAL INDUSTRIES LTD.
16.11.1 COMPANY SNAPSHOT
16.11.2 REVENUE ANALYSIS
16.11.3 PRODUCT PORTFOLIO
16.11.4 RECENT UPDATES
16.12 TAJ PHARMACEUTICALS LIMITED
16.12.1 COMPANY SNAPSHOT
16.12.2 PRODUCT PORTFOLIO
16.12.3 RECENT UPDATES
17 QUESTIONNAIRE
18 RELATED REPORTS
表のリスト
TABLE 1 PIPELINE ANALYSIS - OBSERVATORY DATA
TABLE 2 PIPELINE ANALYSIS - INTERVENTIONAL DATA
TABLE 3 SALES DATA - 2024
TABLE 4 SALES DATA - 2023
TABLE 5 SALES DATA - 2022
TABLE 6 SALES DATA - 2021
TABLE 7 SALES DATA - 2020
TABLE 8 SALES DATA - 2019
TABLE 9 SALES DATA - 2018
TABLE 10 COST OF PALOVAROTENE
TABLE 11 EUROPE MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 12 EUROPE SESSILE IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 13 EUROPE PEDUNCULATED IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 14 EUROPE MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TREATMENT, 2022-2031 (USD THOUSAND)
TABLE 15 EUROPE SURGERY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 16 EUROPE SURGERY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 17 EUROPE MEDICATION IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 18 EUROPE MEDICATION IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD THOUSAND)
TABLE 19 EUROPE MEDICATION IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 20 EUROPE MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DIAGNOSIS, 2022-2031 (USD THOUSAND)
TABLE 21 EUROPE X-RAY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 22 EUROPE X-RAY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 23 EUROPE COMPUTED TOMOGRAPHY (CT) SCAN IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 24 EUROPE COMPUTED TOMOGRAPHY (CT) SCAN IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 25 EUROPE MAGNETIC RESONANCE IMAGING (MRI) IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 26 EUROPE MAGNETIC RESONANCE IMAGING (MRI) IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 27 EUROPE GENETIC TESTS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 28 EUROPE GENETIC TESTS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 29 EUROPE OTHERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 30 EUROPE OTHERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 31 EUROPE MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY SITE, 2022-2031 (USD THOUSAND)
TABLE 32 EUROPE LEGS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 33 EUROPE ARMS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 34 EUROPE SHOULDERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 35 EUROPE PELVIS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 36 EUROPE FINGERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 37 EUROPE TOES IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 38 EUROPE MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY AGE GROUP, 2022-2031 (USD THOUSAND)
TABLE 39 EUROPE PEDIATRIC IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 40 EUROPE ADULT IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 41 EUROPE MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY END USER, 2022-2031 (USD THOUSAND)
TABLE 42 EUROPE HOSPITAL IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 43 EUROPE HOSPITAL IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 44 EUROPE SPECIALTY CLINICS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 45 EUROPE AMBULATORY SURGICAL CENTERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 46 EUROPE OTHERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 47 EUROPE MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY COUNTRY, 2022-2031 (USD THOUSAND)
TABLE 48 EUROPE MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 49 EUROPE MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TREATMENT, 2022-2031 (USD THOUSAND)
TABLE 50 EUROPE SURGERY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 51 EUROPE MEDICATION IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD THOUSAND)
TABLE 52 EUROPE MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DIAGNOSIS, 2022-2031 (USD THOUSAND)
TABLE 53 EUROPE X-RAY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 54 EUROPE COMPUTED TOMOGRAPHY (CT) SCAN IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 55 EUROPE MAGNETIC RESONANCE IMAGING (MRI) IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 56 EUROPE GENETIC TESTS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 57 EUROPE OTHERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 58 EUROPE MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY SITE, 2022-2031 (USD THOUSAND)
TABLE 59 EUROPE MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY AGE GROUP, 2022-2031 (USD THOUSAND)
TABLE 60 EUROPE MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY END USER, 2022-2031 (USD THOUSAND)
TABLE 61 EUROPE HOSPITAL IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 62 GERMANY MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 63 GERMANY MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TREATMENT, 2022-2031 (USD THOUSAND)
TABLE 64 GERMANY SURGERY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 65 GERMANY MEDICATION IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD THOUSAND)
TABLE 66 GERMANY MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DIAGNOSIS, 2022-2031 (USD THOUSAND)
TABLE 67 GERMANY X-RAY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 68 GERMANY COMPUTED TOMOGRAPHY (CT) SCAN IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 69 GERMANY MAGNETIC RESONANCE IMAGING (MRI) IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 70 GERMANY GENETIC TESTS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 71 GERMANY OTHERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 72 GERMANY MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY SITE, 2022-2031 (USD THOUSAND)
TABLE 73 GERMANY MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY AGE GROUP, 2022-2031 (USD THOUSAND)
TABLE 74 GERMANY MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY END USER, 2022-2031 (USD THOUSAND)
TABLE 75 GERMANY HOSPITAL IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 76 U.K MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 77 U.K MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TREATMENT, 2022-2031 (USD THOUSAND)
TABLE 78 U.K SURGERY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 79 U.K MEDICATION IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD THOUSAND)
TABLE 80 U.K MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DIAGNOSIS, 2022-2031 (USD THOUSAND)
TABLE 81 U.K X-RAY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 82 U.K COMPUTED TOMOGRAPHY (CT) SCAN IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 83 U.K MAGNETIC RESONANCE IMAGING (MRI) IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 84 U.K GENETIC TESTS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 85 U.K OTHERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 86 U.K MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY SITE, 2022-2031 (USD THOUSAND)
TABLE 87 U.K MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY AGE GROUP, 2022-2031 (USD THOUSAND)
TABLE 88 U.K MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY END USER, 2022-2031 (USD THOUSAND)
TABLE 89 U.K HOSPITAL IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 90 FRANCE MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 91 FRANCE MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TREATMENT, 2022-2031 (USD THOUSAND)
TABLE 92 FRANCE SURGERY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 93 FRANCE MEDICATION IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD THOUSAND)
TABLE 94 FRANCE MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DIAGNOSIS, 2022-2031 (USD THOUSAND)
TABLE 95 FRANCE X-RAY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 96 FRANCE COMPUTED TOMOGRAPHY (CT) SCAN IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 97 FRANCE MAGNETIC RESONANCE IMAGING (MRI) IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 98 FRANCE GENETIC TESTS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 99 FRANCE OTHERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 100 FRANCE MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY SITE, 2022-2031 (USD THOUSAND)
TABLE 101 FRANCE MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY AGE GROUP, 2022-2031 (USD THOUSAND)
TABLE 102 FRANCE MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY END USER, 2022-2031 (USD THOUSAND)
TABLE 103 FRANCE HOSPITAL IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 104 ITALY MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 105 ITALY MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TREATMENT, 2022-2031 (USD THOUSAND)
TABLE 106 ITALY SURGERY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 107 ITALY MEDICATION IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD THOUSAND)
TABLE 108 ITALY MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DIAGNOSIS, 2022-2031 (USD THOUSAND)
TABLE 109 ITALY X-RAY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 110 ITALY COMPUTED TOMOGRAPHY (CT) SCAN IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 111 ITALY MAGNETIC RESONANCE IMAGING (MRI) IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 112 ITALY GENETIC TESTS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 113 ITALY OTHERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 114 ITALY MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY SITE, 2022-2031 (USD THOUSAND)
TABLE 115 ITALY MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY AGE GROUP, 2022-2031 (USD THOUSAND)
TABLE 116 ITALY MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY END USER, 2022-2031 (USD THOUSAND)
TABLE 117 ITALY HOSPITAL IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 118 SPAIN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 119 SPAIN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TREATMENT, 2022-2031 (USD THOUSAND)
TABLE 120 SPAIN SURGERY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 121 SPAIN MEDICATION IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD THOUSAND)
TABLE 122 SPAIN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DIAGNOSIS, 2022-2031 (USD THOUSAND)
TABLE 123 SPAIN X-RAY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 124 SPAIN COMPUTED TOMOGRAPHY (CT) SCAN IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 125 SPAIN MAGNETIC RESONANCE IMAGING (MRI) IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 126 SPAIN GENETIC TESTS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 127 SPAIN OTHERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 128 SPAIN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY SITE, 2022-2031 (USD THOUSAND)
TABLE 129 SPAIN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY AGE GROUP, 2022-2031 (USD THOUSAND)
TABLE 130 SPAIN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY END USER, 2022-2031 (USD THOUSAND)
TABLE 131 SPAIN HOSPITAL IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 132 RUSSIA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 133 RUSSIA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TREATMENT, 2022-2031 (USD THOUSAND)
TABLE 134 RUSSIA SURGERY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 135 RUSSIA MEDICATION IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD THOUSAND)
TABLE 136 RUSSIA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DIAGNOSIS, 2022-2031 (USD THOUSAND)
TABLE 137 RUSSIA X-RAY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 138 RUSSIA COMPUTED TOMOGRAPHY (CT) SCAN IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 139 RUSSIA MAGNETIC RESONANCE IMAGING (MRI) IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 140 RUSSIA GENETIC TESTS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 141 RUSSIA OTHERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 142 RUSSIA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY SITE, 2022-2031 (USD THOUSAND)
TABLE 143 RUSSIA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY AGE GROUP, 2022-2031 (USD THOUSAND)
TABLE 144 RUSSIA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY END USER, 2022-2031 (USD THOUSAND)
TABLE 145 RUSSIA HOSPITAL IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 146 TURKEY MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 147 TURKEY MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TREATMENT, 2022-2031 (USD THOUSAND)
TABLE 148 TURKEY SURGERY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 149 TURKEY MEDICATION IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD THOUSAND)
TABLE 150 TURKEY MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DIAGNOSIS, 2022-2031 (USD THOUSAND)
TABLE 151 TURKEY X-RAY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 152 TURKEY COMPUTED TOMOGRAPHY (CT) SCAN IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 153 TURKEY MAGNETIC RESONANCE IMAGING (MRI) IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 154 TURKEY GENETIC TESTS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 155 TURKEY OTHERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 156 TURKEY MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY SITE, 2022-2031 (USD THOUSAND)
TABLE 157 TURKEY MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY AGE GROUP, 2022-2031 (USD THOUSAND)
TABLE 158 TURKEY MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY END USER, 2022-2031 (USD THOUSAND)
TABLE 159 TURKEY HOSPITAL IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 160 SWITZERLAND MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 161 SWITZERLAND MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TREATMENT, 2022-2031 (USD THOUSAND)
TABLE 162 SWITZERLAND SURGERY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 163 SWITZERLAND MEDICATION IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD THOUSAND)
TABLE 164 SWITZERLAND MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DIAGNOSIS, 2022-2031 (USD THOUSAND)
TABLE 165 SWITZERLAND X-RAY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 166 SWITZERLAND COMPUTED TOMOGRAPHY (CT) SCAN IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 167 SWITZERLAND MAGNETIC RESONANCE IMAGING (MRI) IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 168 SWITZERLAND GENETIC TESTS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 169 SWITZERLAND OTHERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 170 SWITZERLAND MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY SITE, 2022-2031 (USD THOUSAND)
TABLE 171 SWITZERLAND MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY AGE GROUP, 2022-2031 (USD THOUSAND)
TABLE 172 SWITZERLAND MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY END USER, 2022-2031 (USD THOUSAND)
TABLE 173 SWITZERLAND HOSPITAL IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 174 BELGIUM MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 175 BELGIUM MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TREATMENT, 2022-2031 (USD THOUSAND)
TABLE 176 BELGIUM SURGERY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 177 BELGIUM MEDICATION IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD THOUSAND)
TABLE 178 BELGIUM MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DIAGNOSIS, 2022-2031 (USD THOUSAND)
TABLE 179 BELGIUM X-RAY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 180 BELGIUM COMPUTED TOMOGRAPHY (CT) SCAN IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 181 BELGIUM MAGNETIC RESONANCE IMAGING (MRI) IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 182 BELGIUM GENETIC TESTS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 183 BELGIUM OTHERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 184 BELGIUM MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY SITE, 2022-2031 (USD THOUSAND)
TABLE 185 BELGIUM MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY AGE GROUP, 2022-2031 (USD THOUSAND)
TABLE 186 BELGIUM MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY END USER, 2022-2031 (USD THOUSAND)
TABLE 187 BELGIUM HOSPITAL IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 188 NETHERLAND MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 189 NETHERLAND MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TREATMENT, 2022-2031 (USD THOUSAND)
TABLE 190 NETHERLAND SURGERY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 191 NETHERLAND MEDICATION IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD THOUSAND)
TABLE 192 NETHERLAND MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DIAGNOSIS, 2022-2031 (USD THOUSAND)
TABLE 193 NETHERLAND X-RAY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 194 NETHERLAND COMPUTED TOMOGRAPHY (CT) SCAN IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 195 NETHERLAND MAGNETIC RESONANCE IMAGING (MRI) IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 196 NETHERLAND GENETIC TESTS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 197 NETHERLAND OTHERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 198 NETHERLAND MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY SITE, 2022-2031 (USD THOUSAND)
TABLE 199 NETHERLAND MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY AGE GROUP, 2022-2031 (USD THOUSAND)
TABLE 200 NETHERLAND MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY END USER, 2022-2031 (USD THOUSAND)
TABLE 201 NETHERLAND HOSPITAL IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 202 DENMARK MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 203 DENMARK MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TREATMENT, 2022-2031 (USD THOUSAND)
TABLE 204 DENMARK SURGERY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 205 DENMARK MEDICATION IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD THOUSAND)
TABLE 206 DENMARK MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DIAGNOSIS, 2022-2031 (USD THOUSAND)
TABLE 207 DENMARK X-RAY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 208 DENMARK COMPUTED TOMOGRAPHY (CT) SCAN IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 209 DENMARK MAGNETIC RESONANCE IMAGING (MRI) IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 210 DENMARK GENETIC TESTS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 211 DENMARK OTHERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 212 DENMARK MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY SITE, 2022-2031 (USD THOUSAND)
TABLE 213 DENMARK MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY AGE GROUP, 2022-2031 (USD THOUSAND)
TABLE 214 DENMARK MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY END USER, 2022-2031 (USD THOUSAND)
TABLE 215 DENMARK HOSPITAL IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 216 SWEDEN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 217 SWEDEN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TREATMENT, 2022-2031 (USD THOUSAND)
TABLE 218 SWEDEN SURGERY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 219 SWEDEN MEDICATION IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD THOUSAND)
TABLE 220 SWEDEN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DIAGNOSIS, 2022-2031 (USD THOUSAND)
TABLE 221 SWEDEN X-RAY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 222 SWEDEN COMPUTED TOMOGRAPHY (CT) SCAN IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 223 SWEDEN MAGNETIC RESONANCE IMAGING (MRI) IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 224 SWEDEN GENETIC TESTS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 225 SWEDEN OTHERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 226 SWEDEN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY SITE, 2022-2031 (USD THOUSAND)
TABLE 227 SWEDEN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY AGE GROUP, 2022-2031 (USD THOUSAND)
TABLE 228 SWEDEN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY END USER, 2022-2031 (USD THOUSAND)
TABLE 229 SWEDEN HOSPITAL IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 230 POLAND MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 231 POLAND MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TREATMENT, 2022-2031 (USD THOUSAND)
TABLE 232 POLAND SURGERY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 233 POLAND MEDICATION IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD THOUSAND)
TABLE 234 POLAND MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DIAGNOSIS, 2022-2031 (USD THOUSAND)
TABLE 235 POLAND X-RAY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 236 POLAND COMPUTED TOMOGRAPHY (CT) SCAN IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 237 POLAND MAGNETIC RESONANCE IMAGING (MRI) IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 238 POLAND GENETIC TESTS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 239 POLAND OTHERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 240 POLAND MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY SITE, 2022-2031 (USD THOUSAND)
TABLE 241 POLAND MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY AGE GROUP, 2022-2031 (USD THOUSAND)
TABLE 242 POLAND MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY END USER, 2022-2031 (USD THOUSAND)
TABLE 243 POLAND HOSPITAL IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 244 NORWAY MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 245 NORWAY MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TREATMENT, 2022-2031 (USD THOUSAND)
TABLE 246 NORWAY SURGERY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 247 NORWAY MEDICATION IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD THOUSAND)
TABLE 248 NORWAY MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DIAGNOSIS, 2022-2031 (USD THOUSAND)
TABLE 249 NORWAY X-RAY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 250 NORWAY COMPUTED TOMOGRAPHY (CT) SCAN IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 251 NORWAY MAGNETIC RESONANCE IMAGING (MRI) IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 252 NORWAY GENETIC TESTS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 253 NORWAY OTHERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 254 NORWAY MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY SITE, 2022-2031 (USD THOUSAND)
TABLE 255 NORWAY MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY AGE GROUP, 2022-2031 (USD THOUSAND)
TABLE 256 NORWAY MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY END USER, 2022-2031 (USD THOUSAND)
TABLE 257 NORWAY HOSPITAL IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 258 FINLAND MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 259 FINLAND MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TREATMENT, 2022-2031 (USD THOUSAND)
TABLE 260 FINLAND SURGERY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 261 FINLAND MEDICATION IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD THOUSAND)
TABLE 262 FINLAND MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DIAGNOSIS, 2022-2031 (USD THOUSAND)
TABLE 263 FINLAND X-RAY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 264 FINLAND COMPUTED TOMOGRAPHY (CT) SCAN IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 265 FINLAND MAGNETIC RESONANCE IMAGING (MRI) IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 266 FINLAND GENETIC TESTS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 267 FINLAND OTHERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 268 FINLAND MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY SITE, 2022-2031 (USD THOUSAND)
TABLE 269 FINLAND MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY AGE GROUP, 2022-2031 (USD THOUSAND)
TABLE 270 FINLAND MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY END USER, 2022-2031 (USD THOUSAND)
TABLE 271 FINLAND HOSPITAL IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 272 REST OF EUROPE MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
図表一覧
FIGURE 1 EUROPE MULTIPLE HEREDITARY EXOSTOSIS MARKET: SEGMENTATION
FIGURE 2 EUROPE MULTIPLE HEREDITARY EXOSTOSIS MARKET: DATA TRIANGULATION
FIGURE 3 EUROPE MULTIPLE HEREDITARY EXOSTOSIS MARKET: DROC ANALYSIS
FIGURE 4 EUROPE MULTIPLE HEREDITARY EXOSTOSIS MARKET: EUROPE VS REGIONAL MARKET ANALYSIS
FIGURE 5 EUROPE MULTIPLE HEREDITARY EXOSTOSIS MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 EUROPE MULTIPLE HEREDITARY EXOSTOSIS MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 EUROPE MULTIPLE HEREDITARY EXOSTOSIS MARKET: MARKET END USER COVERAGE GRID
FIGURE 8 PRODUCT LIFELINE CURVE
FIGURE 9 EUROPE MULTIPLE HEREDITARY EXOSTOSIS MARKET: DBMR MARKET POSITION GRID
FIGURE 10 EUROPE MULTIPLE HEREDITARY EXOSTOSIS MARKET: VENDOR SHARE ANALYSIS
FIGURE 11 EUROPE MULTIPLE HEREDITARY EXOSTOSIS MARKET: SEGMENTATION
FIGURE 12 EXECUTIVE SUMMARY
FIGURE 13 STRATEGIC DECISIONS
FIGURE 14 TWO SEGMENTS COMPRISE THE EUROPE MULTIPLE HEREDITY EXOSTOSIS MARKET, BY TYPE
FIGURE 15 RISING PREVALENCE OF GENETIC DISORDERS IS DRIVING THE GROWTH OF THE EUROPE MULTIPLE HEREDITARY EXOSTOSIS MARKET FROM 2024 TO 2031
FIGURE 16 THE TYPE SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE EUROPE MULTIPLE HEREDITARY EXOSTOSIS MARKET IN 2024 AND 2031
FIGURE 17 MARKET OVERVIEW
FIGURE 18 EUROPE MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY TYPE, 2023
FIGURE 19 EUROPE MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY TYPE, 2024-2031 (USD THOUSAND)
FIGURE 20 EUROPE MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY TYPE, CAGR (2024-2031)
FIGURE 21 EUROPE MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY TYPE, LIFELINE CURVE
FIGURE 22 EUROPE MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY TREATMENT, 2023
FIGURE 23 EUROPE MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY TREATMENT, 2024-2031 (USD THOUSAND)
FIGURE 24 EUROPE MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY TREATMENT, CAGR (2024-2031)
FIGURE 25 EUROPE MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY TREATMENT, LIFELINE CURVE
FIGURE 26 EUROPE MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY DIAGNOSIS, 2023
FIGURE 27 EUROPE MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY DIAGNOSIS, 2024-2031 (USD THOUSAND)
FIGURE 28 EUROPE MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY DIAGNOSIS, CAGR (2024-2031)
FIGURE 29 EUROPE MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY DIAGNOSIS, LIFELINE CURVE
FIGURE 30 EUROPE MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY SITE, 2023
FIGURE 31 EUROPE MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY SITE, 2024-2031 (USD THOUSAND)
FIGURE 32 EUROPE MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY SITE, CAGR (2024-2031)
FIGURE 33 EUROPE MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY SITE, LIFELINE CURVE
FIGURE 34 EUROPE MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY AGE GROUP, 2023
FIGURE 35 EUROPE MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY AGE GROUP, 2024-2031 (USD THOUSAND)
FIGURE 36 EUROPE MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY AGE GROUP, CAGR (2024-2031)
FIGURE 37 EUROPE MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY AGE GROUP, LIFELINE CURVE
FIGURE 38 EUROPE MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY END USER, 2023
FIGURE 39 EUROPE MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY END USER, 2024-2031 (USD THOUSAND)
FIGURE 40 EUROPE MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY END USER, CAGR (2024-2031)
FIGURE 41 EUROPE MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY END USER, LIFELINE CURVE
FIGURE 42 EUROPE MULTIPLE HEREDITY EXOSTOSIS MARKET: SNAPSHOT (2023)
FIGURE 43 EUROPE MULTIPLE HEREDITARY EXOSTOSIS MARKET: COMPANY SHARE 2023 (%)

調査方法
データ収集と基準年分析は、大規模なサンプル サイズのデータ収集モジュールを使用して行われます。この段階では、さまざまなソースと戦略を通じて市場情報または関連データを取得します。過去に取得したすべてのデータを事前に調査および計画することも含まれます。また、さまざまな情報ソース間で見られる情報の不一致の調査も含まれます。市場データは、市場統計モデルと一貫性モデルを使用して分析および推定されます。また、市場シェア分析と主要トレンド分析は、市場レポートの主要な成功要因です。詳細については、アナリストへの電話をリクエストするか、お問い合わせをドロップダウンしてください。
DBMR 調査チームが使用する主要な調査方法は、データ マイニング、データ変数が市場に与える影響の分析、および一次 (業界の専門家) 検証を含むデータ三角測量です。データ モデルには、ベンダー ポジショニング グリッド、市場タイムライン分析、市場概要とガイド、企業ポジショニング グリッド、特許分析、価格分析、企業市場シェア分析、測定基準、グローバルと地域、ベンダー シェア分析が含まれます。調査方法について詳しくは、お問い合わせフォームから当社の業界専門家にご相談ください。
カスタマイズ可能
Data Bridge Market Research は、高度な形成的調査のリーダーです。当社は、既存および新規のお客様に、お客様の目標に合致し、それに適したデータと分析を提供することに誇りを持っています。レポートは、対象ブランドの価格動向分析、追加国の市場理解 (国のリストをお問い合わせください)、臨床試験結果データ、文献レビュー、リファービッシュ市場および製品ベース分析を含めるようにカスタマイズできます。対象競合他社の市場分析は、技術ベースの分析から市場ポートフォリオ戦略まで分析できます。必要な競合他社のデータを、必要な形式とデータ スタイルでいくつでも追加できます。当社のアナリスト チームは、粗い生の Excel ファイル ピボット テーブル (ファクト ブック) でデータを提供したり、レポートで利用可能なデータ セットからプレゼンテーションを作成するお手伝いをしたりすることもできます。